Renoprotective Effects of Alpha-1 Antitrypsin against Tacrolimus-Induced Renal Injury
Abstract
1. Introduction
2. Results
2.1. Effect of AAT on the Basic Parameters
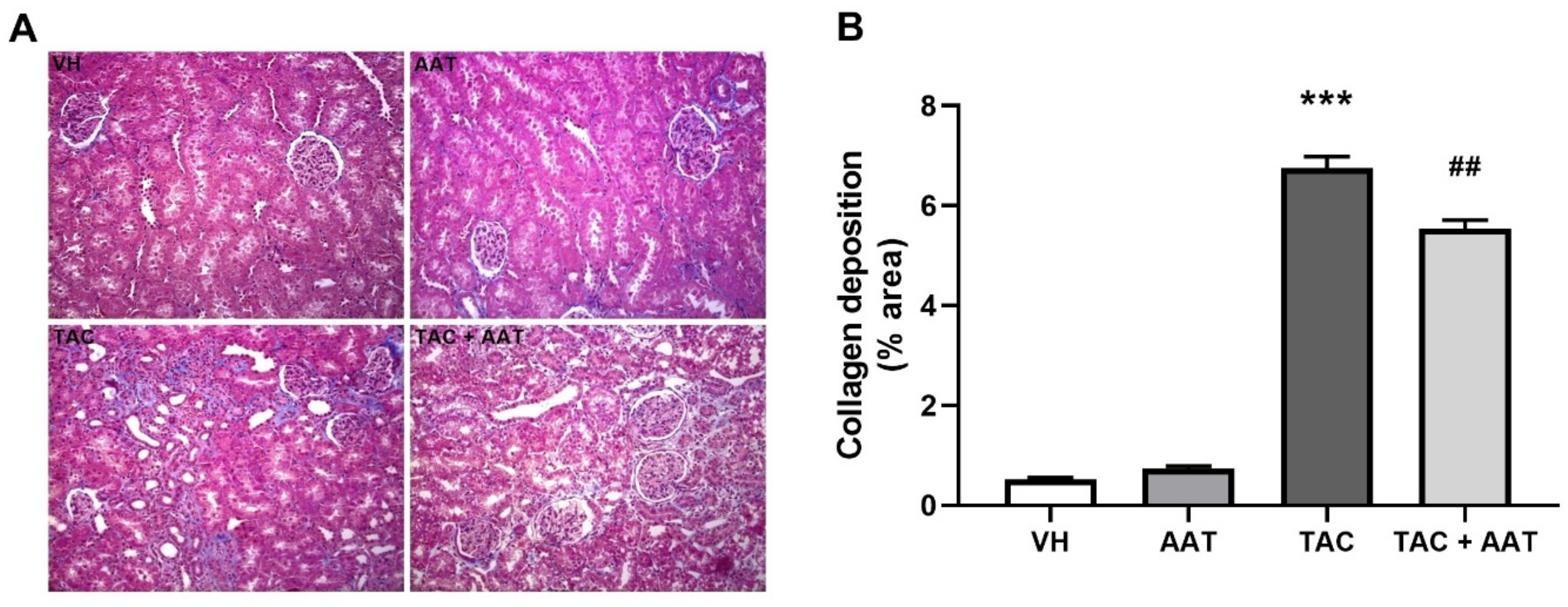
2.2. Effect of AAT on Renal Fibrosis in TAC-Induced Renal Injury
2.3. Effect of AAT on Inflammation in TAC-Induced Renal Injury
2.4. Effect of AAT on Apoptosis in TAC-Induced Renal Injury
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Quantification of Tacrolimus
4.3. Renal Function and Histopathological Studies
4.4. Immunohistochemistry
4.5. Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick-End Labeling (TUNEL) Assay
4.6. Measurement of Caspase-3 Activity
4.7. Western Blotting Assay
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Luisa, R.; Monroy Funes, M.A. Clinical Pharmacokinetics of Triple Immunosuppression Scheme in Kidney Transplant (Tacrolimus, Mycophenolate Mofetil and Corticosteroids); InTech: London, UK, 2011. [Google Scholar]
- Axelrod, D.A.; Naik, A.S.; Schnitzler, M.A.; Segev, D.L.; Dharnidharka, V.R.; Brennan, D.C.; Bae, S.; Chen, J.; Massie, A.; Lentine, K.L. National Variation in Use of Immunosuppression for Kidney Transplantation: A Call for Evidence-Based Regimen Selection. Am. J. Transplant. 2016, 16, 2453–2462. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Jin, J.; Luo, K.; Piao, S.G.; Zheng, H.L.; Jin, J.Z.; Lim, S.W.; Choi, B.S.; Yang, C.W.; Li, C. Shen-Kang protects against tacrolimus-induced renal injury. Korean J. Intern Med. 2019, 34, 1078–1090. [Google Scholar] [CrossRef]
- Shihab, F.S.; Bennett, W.M.; Tanner, A.M.; Andoh, T.F. Mechanism of fibrosis in experimental tacrolimus nephrotoxicity. Transplantation 1997, 64, 1829–1837. [Google Scholar] [CrossRef]
- Wang, L.; Kubodera, S.; Ueno, A.; Takeda, M. Effects of nitric oxide synthesis inhibition on FK506-induced nephrotoxicity in rats. Ren. Fail. 2001, 23, 11–19. [Google Scholar] [CrossRef][Green Version]
- Jeong, H.J. Diagnosis of renal transplant rejection: Banff classification and beyond. Kidney Res. Clin. Pract. 2020, 39, 17–31. [Google Scholar] [CrossRef]
- Jin, J.; Lim, S.W.; Jin, L.; Yu, J.H.; Kim, H.S.; Chung, B.H.; Yang, C.W. Effects of metformin on hyperglycemia in an experimental model of tacrolimus- and sirolimus-induced diabetic rats. Korean J. Intern Med. 2017, 32, 314–322. [Google Scholar] [CrossRef]
- Jin, J.; Jin, L.; Luo, K.; Lim, S.W.; Chung, B.H.; Yang, C.W. Effect of Empagliflozin on Tacrolimus-Induced Pancreas Islet Dysfunction and Renal Injury. Am. J. Transplant. 2017, 17, 2601–2616. [Google Scholar] [CrossRef]
- Lim, S.W.; Jin, L.; Piao, S.G.; Chung, B.H.; Yang, C.W. Inhibition of dipeptidyl peptidase IV protects tacrolimus-induced kidney injury. Lab. Investig. 2015, 95, 1174–1185. [Google Scholar] [CrossRef]
- Jedicke, N.; Struever, N.; Aggrawal, N.; Welte, T.; Manns, M.P.; Malek, N.P.; Zender, L.; Janciauskiene, S.; Wuestefeld, T. α-1-antitrypsin inhibits acute liver failure in mice. Hepatology 2014, 59, 2299–2308. [Google Scholar] [CrossRef]
- Toldo, S.; Seropian, I.M.; Mezzaroma, E.; Van Tassell, B.W.; Salloum, F.N.; Lewis, E.C.; Voelkel, N.; Dinarello, C.A.; Abbate, A. Alpha-1 antitrypsin inhibits caspase-1 and protects from acute myocardial ischemia-reperfusion injury. J. Mol. Cell Cardiol. 2011, 51, 244–251. [Google Scholar] [CrossRef]
- Jonigk, D.; Al-Omari, M.; Maegel, L.; Müller, M.; Izykowski, N.; Hong, J.; Hong, K.; Kim, S.-H.; Dorsch, M.; Mahadeva, R.; et al. Anti-inflammatory and immunomodulatory properties of α1-antitrypsin without inhibition of elastase. Proc. Natl. Acad. Sci. USA 2013, 110, 15007–15012. [Google Scholar] [CrossRef]
- Lim, J.H.; Park, S.M.; Yook, J.M.; Ahn, J.S.; Choi, S.Y.; Oh, S.H.; Jung, H.Y.; Choi, J.Y.; Cho, J.H.; Park, S.H.; et al. Alpha-1 antitrypsin inhibits formaldehyde-induced apoptosis of human peritoneal mesothelial cells. Perit. Dial. Int. 2020, 40, 124–131. [Google Scholar] [CrossRef]
- Zhang, B.; Lu, Y.; Campbell-Thompson, M.; Spencer, T.; Wasserfall, C.; Atkinson, M.; Song, S. Alpha1-antitrypsin protects beta-cells from apoptosis. Diabetes 2007, 56, 1316–1323. [Google Scholar] [CrossRef]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Jevnikar, A.M.; Mannon, R.B. Late kidney allograft loss: What we know about it, and what we can do about it. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. 2), S56–S67. [Google Scholar] [CrossRef]
- Al-Harbi, N.O.; Imam, F.; Al-Harbi, M.M.; Iqbal, M.; Nadeem, A.; Al-Shahrah, O.A.; Korashy, H.M.; Al-Hosaini, K.A.; Ahmed, M.; Bahashwar, S. Treatment with aliskiren ameliorates tacrolimus-induced nephrotoxicity in rats. J. Renin. Angiotensin Aldosterone Syst. 2015, 16, 1329–1336. [Google Scholar] [CrossRef]
- Luo, K.; Lim, S.W.; Jin, J.; Jin, L.; Gil, H.W.; Im, D.S.; Hwang, H.S.; Yang, C.W. Cilastatin protects against tacrolimus-induced nephrotoxicity via anti-oxidative and anti-apoptotic properties. BMC Nephrol. 2019, 20, 221. [Google Scholar] [CrossRef]
- Marcondes, A.M.; Karoopongse, E.; Lesnikova, M.; Margineantu, D.; Welte, T.; Dinarello, C.A.; Hockenbery, D.; Janciauskiene, S.; Deeg, H.J. α-1-Antitrypsin (AAT)-modified donor cells suppress GVHD but enhance the GVL effect: A role for mitochondrial bioenergetics. Blood 2014, 124, 2881–2891. [Google Scholar] [CrossRef]
- Lewis, E.C.; Mizrahi, M.; Toledano, M.; Defelice, N.; Wright, J.L.; Churg, A.; Shapiro, L.; Dinarello, C.A. alpha1-Antitrypsin monotherapy induces immune tolerance during islet allograft transplantation in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 16236–16241. [Google Scholar] [CrossRef]
- Elshikha, A.S.; Lu, Y.; Chen, M.-J.; Akbar, M.; Zeumer, L.; Ritter, A.; Elghamry, H.; Mahdi, M.A.; Morel, L.; Song, S. Alpha 1 Antitrypsin Inhibits Dendritic Cell Activation and Attenuates Nephritis in a Mouse Model of Lupus. PLoS ONE 2016, 11, e0156583. [Google Scholar] [CrossRef]
- Emtiazjoo, A.M.; Hu, H.; Lu, L.; Brantly, M.L. Alpha-1 Antitrypsin Attenuates Acute Lung Allograft Injury in a Rat Lung Transplant Model. Transplant. Direct. 2019, 5, e458. [Google Scholar] [CrossRef] [PubMed]
- Bata, J.; Revillard, J.P. Interaction between alpha 1 antitrypsin and lymphocyte surface proteases: Immunoregulatory effects. Agents Actions 1981, 11, 614–616. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Shapiro, L.; Fellingham, G.; Willardson, B.M.; Burton, G.F. HIV replication in CD4+ T lymphocytes in the presence and absence of follicular dendritic cells: Inhibition of replication mediated by α-1-antitrypsin through altered IκBα ubiquitination. J. Immunol. 2011, 186, 3148–3155. [Google Scholar] [CrossRef] [PubMed]
- Shahaf, G.; Moser, H.; Ozeri, E.; Mizrahi, M.; Abecassis, A.; Lewis, E.C. α-1-antitrypsin gene delivery reduces inflammation, increases T-regulatory cell population size and prevents islet allograft rejection. Mol. Med. 2011, 17, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Liu, M.; Uknis, M.E.; Koulmanda, M. Alpha-1-antitrypsin in cell and organ transplantation. Am. J. Transplant. 2018, 18, 1589–1595. [Google Scholar] [CrossRef]
- Farris, A.B.; Alpers, C.E. What is the best way to measure renal fibrosis? A pathologist’s perspective. Kidney Int. Suppl. 2014, 4, 9–15. [Google Scholar] [CrossRef]
- Nath, K.A. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am. J. Kidney Dis. 1992, 20, 1–17. [Google Scholar] [CrossRef]
- Risdon, R.A.; Sloper, J.C.; De Wardener, H.E. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet 1968, 2, 363–366. [Google Scholar] [CrossRef]
- Yokoi, H.; Mukoyama, M.; Sugawara, A.; Mori, K.; Nagae, T.; Makino, H.; Suganami, T.; Yahata, K.; Fujinaga, Y.; Tanaka, I.; et al. Role of connective tissue growth factor in fibronectin expression and tubulointerstitial fibrosis. Am. J. Physiol. Renal. Physiol. 2002, 282, F933–F942. [Google Scholar] [CrossRef]
- Hinz, B. Myofibroblasts. Exp. Eye Res. 2016, 142, 56–70. [Google Scholar] [CrossRef]
- Bochaton-Piallat, M.-L.; Gabbiani, G.; Hinz, B. The myofibroblast in wound healing and fibrosis: Answered and unanswered questions. F1000Research 2016, 5. F1000 Faculty Rev-752. [Google Scholar] [CrossRef]
- Cho, J.-H.; Ryu, H.-M.; Oh, E.-J.; Yook, J.-M.; Ahn, J.-S.; Jung, H.-Y.; Choi, J.-Y.; Park, S.-H.; Kim, Y.-L.; Kwak, I.S.; et al. Alpha1-Antitrypsin Attenuates Renal Fibrosis by Inhibiting TGF-β1-Induced Epithelial Mesenchymal Transition. PLoS ONE 2016, 11, e0162186. [Google Scholar] [CrossRef]
- Jeong, K.H.; Lim, J.H.; Lee, K.H.; Kim, M.J.; Jung, H.Y.; Choi, J.Y.; Cho, J.H.; Park, S.H.; Kim, Y.L.; Kim, C.D. Protective Effect of Alpha 1-Antitrypsin on Renal Ischemia-Reperfusion Injury. Transplant. Proc. 2019, 51, 2814–2822. [Google Scholar] [CrossRef]
- González-Guerrero, C.; Cannata-Ortiz, P.; Guerri, C.; Egido, J.; Ortiz, A.; Ramos, A.M. TLR4-mediated inflammation is a key pathogenic event leading to kidney damage and fibrosis in cyclosporine nephrotoxicity. Arch Toxicol. 2017, 91, 1925–1939. [Google Scholar] [CrossRef]
- Bucurenci, N.; Blake, D.R.; Chidwick, K.; Winyard, P.G. Inhibition of neutrophil superoxide production by human plasma alpha 1-antitrypsin. FEBS Lett. 1992, 300, 21–24. [Google Scholar] [CrossRef]
- Ralston, D.R.; Marsh, C.B.; Lowe, M.P.; Wewers, M.D. Antineutrophil cytoplasmic antibodies induce monocyte IL-8 release. Role of surface proteinase-3, alpha1-antitrypsin, and Fcgamma receptors. J. Clin. Investig. 1997, 100, 1416–1424. [Google Scholar] [CrossRef]
- Denhardt, D.T.; Guo, X. Osteopontin: A protein with diverse functions. FASEB J. 1993, 7, 1475–1482. [Google Scholar] [CrossRef]
- Giachelli, C.M.; Schwartz, S.M.; Liaw, L. Molecular and cellular biology of osteopontin: Potential role in cardiovascular disease. Trends Cardiovas Med. 1995, 5, 88–95. [Google Scholar] [CrossRef]
- Ophascharoensuk, V.; Giachelli, C.M.; Gordon, K.; Hughes, J.; Pichler, R.; Brown, P.; Liaw, L.; Schmidt, R.; Shankland, S.J.; Alpers, C.E.; et al. Obstructive uropathy in the mouse: Role of osteopontin in interstitial fibrosis and apoptosis. Kidney Int. 1999, 56, 571–580. [Google Scholar] [CrossRef]
- Giachelli, C.M.; Lombardi, D.; Johnson, R.J.; Murry, C.E.; Almeida, M. Evidence for a role of osteopontin in macrophage infiltration in response to pathological stimuli in vivo. Am. J. Pathol. 1998, 152, 353–358. [Google Scholar]
- Khanna, A. Tacrolimus and cyclosporinein vitro and in vivo induce osteopontin mRNA and protein expression in renal tissues. Nephron Exp. Nephrol. 2005, 101, e119–e126. [Google Scholar] [CrossRef] [PubMed]
- Damoiseaux, J.G.; Döpp, E.A.; Calame, W.; Chao, D.; MacPherson, G.G.; Dijkstra, C.D. Rat macrophage lysosomal membrane antigen recognized by monoclonal antibody ED1. Immunology 1994, 83, 140–147. [Google Scholar] [PubMed]
- Hisamura, F.; Kojima-Yuasa, A.; Kennedy, D.O.; Matsui-Yuasa, I. Protective effect of green tea extract and tea polyphenols against FK506-induced cytotoxicity in renal cells. Basic Clin. Pharmacol. Toxicol. 2006, 98, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Jin, L.; Lim, S.; Yang, C. Klotho Deficiency Aggravates Tacrolimus-Induced Renal Injury via the Phosphatidylinositol 3-Kinase-Akt-Forkhead Box Protein O Pathway. Am. J. Nephrol. 2016, 43, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lim, S.W.; Jin, L.; Jin, J.; Chung, B.H.; Yang, C.W. The Protective Effect of Febuxostat on Chronic Tacrolimus-Induced Nephrotoxicity in Rats. Nephron 2017, 135, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Petrache, I.; Fijalkowska, I.; Medler, T.R.; Skirball, J.; Cruz, P.; Zhen, L.; Petrache, H.I.; Flotte, T.R.; Tuder, R.M. Alpha-1 antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis. Am. J. Pathol. 2006, 169, 1155–1166. [Google Scholar] [CrossRef]
- Gross, A.; McDonnell, J.M.; Korsmeyer, S.J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999, 13, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Bogdał, M.N.; Hat, B.; Kochańczyk, M.; Lipniacki, T. Levels of pro-apoptotic regulator Bad and anti-apoptotic regulator Bcl-xL determine the type of the apoptotic logic gate. BMC Syst. Biol. 2013, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Holzscheiter, L.; Beck, C.; Rutz, S.; Manuilova, E.; Domke, I.; Guder, W.G.; Hofmann, W. NGAL, L-FABP, and KIM-1 in comparison to established markers of renal dysfunction. Clin. Chem. Lab. Med. 2014, 52, 537–546. [Google Scholar] [PubMed]
- Eisner, C.; Faulhaber-Walter, R.; Wang, Y.; Leelahavanichkul, A.; Yuen, P.; Mizel, D.; Star, R.; Briggs, J.; Levine, M.; Schnermann, J. Major contribution of tubular secretion to creatinine clearance in mice. Kidney Int. 2010, 77, 519–526. [Google Scholar] [CrossRef]







| VH (n = 6) | AAT (n = 6) | TAC (n = 6) | TAC + AAT (n = 6) | |
|---|---|---|---|---|
| Baseline BW, g | 254.3 ± 5.0 | 253.0 ± 7.0 | 253.0 ± 5.2 | 256.7 ± 5.5 |
| Last BW, g | 400.3 ± 8.9 | 397.7 ± 14.7 | 364.3 ± 6.4 *** | 358.7 ± 12.8 *** |
| BW gain, g | 146.0 ± 10.8 | 144.7 ± 18.4 | 111.3 ± 7.2 *** | 102.0 ± 14.4 *** |
| Serum BUN, mg/dL | 16.1 ± 1.0 | 15.5 ± 1.4 | 61.1 ± 8.9 *** | 45.0 ± 4.8 ***, ## |
| Serum creatinine, mg/dL | 0.40 ± 0.04 | 0.39 ± 0.03 | 0.67 ± 0.11 *** | 0.53 ± 0.05 ***, ## |
| TAC con, ng/mL | NA | NA | 26.3 ± 4.8 | 25.7 ± 5.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.-H.; Oh, E.-J.; Oh, S.-H.; Jung, H.-Y.; Choi, J.-Y.; Cho, J.-H.; Park, S.-H.; Kim, Y.-L.; Kim, C.-D. Renoprotective Effects of Alpha-1 Antitrypsin against Tacrolimus-Induced Renal Injury. Int. J. Mol. Sci. 2020, 21, 8628. https://doi.org/10.3390/ijms21228628
Lim J-H, Oh E-J, Oh S-H, Jung H-Y, Choi J-Y, Cho J-H, Park S-H, Kim Y-L, Kim C-D. Renoprotective Effects of Alpha-1 Antitrypsin against Tacrolimus-Induced Renal Injury. International Journal of Molecular Sciences. 2020; 21(22):8628. https://doi.org/10.3390/ijms21228628
Chicago/Turabian StyleLim, Jeong-Hoon, Eun-Joo Oh, Se-Hyun Oh, Hee-Yeon Jung, Ji-Young Choi, Jang-Hee Cho, Sun-Hee Park, Yong-Lim Kim, and Chan-Duck Kim. 2020. "Renoprotective Effects of Alpha-1 Antitrypsin against Tacrolimus-Induced Renal Injury" International Journal of Molecular Sciences 21, no. 22: 8628. https://doi.org/10.3390/ijms21228628
APA StyleLim, J.-H., Oh, E.-J., Oh, S.-H., Jung, H.-Y., Choi, J.-Y., Cho, J.-H., Park, S.-H., Kim, Y.-L., & Kim, C.-D. (2020). Renoprotective Effects of Alpha-1 Antitrypsin against Tacrolimus-Induced Renal Injury. International Journal of Molecular Sciences, 21(22), 8628. https://doi.org/10.3390/ijms21228628








