Sustained Exposure of Substance P Causes Tendinopathy
Abstract
:1. Introduction
2. Results
2.1. In Vitro Experiments
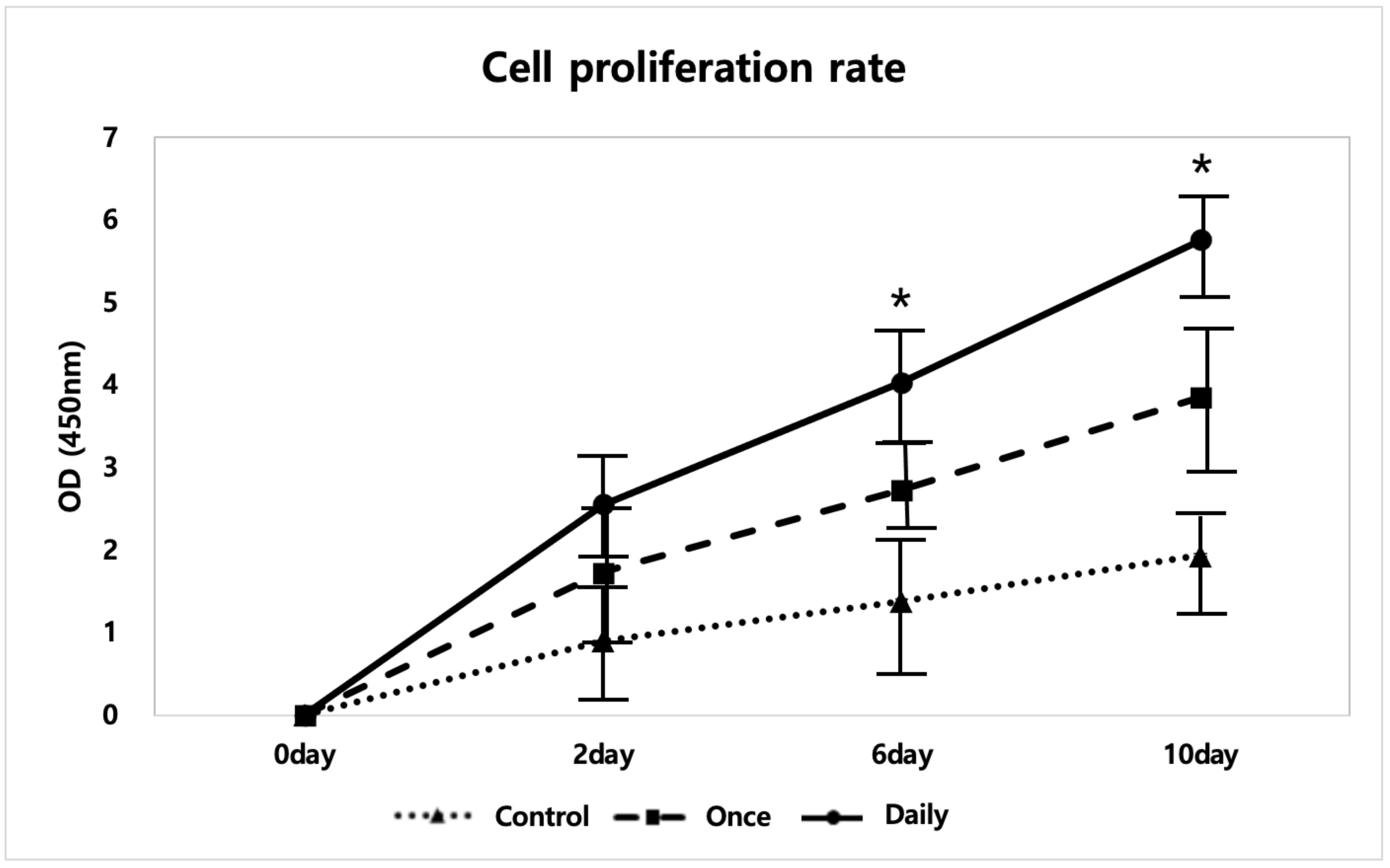
2.1.1. SP Increased Tenocyte Proliferation
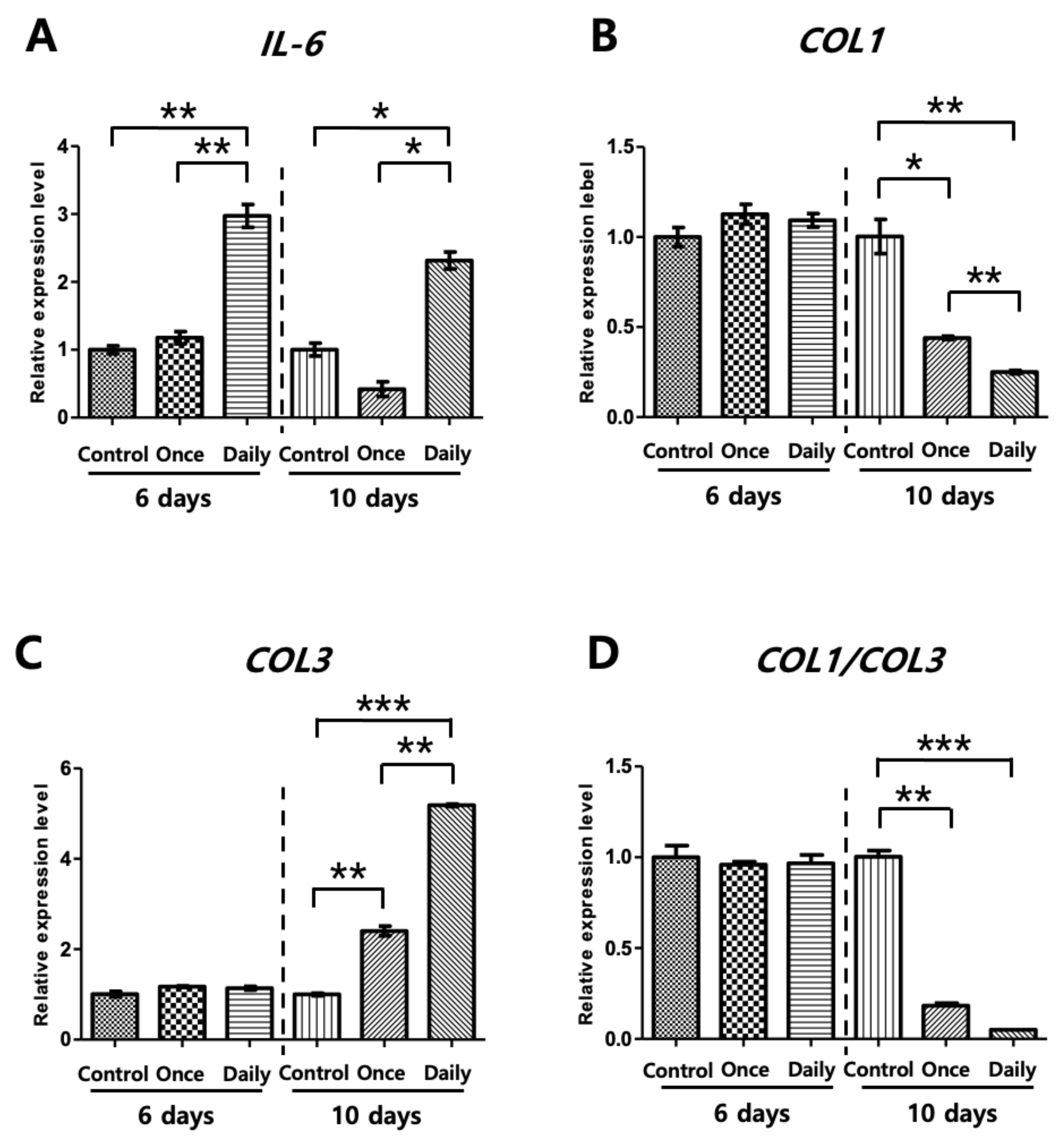
2.1.2. Continuous SP Exposure Induced Inflammation and Deteriorates Collagen Synthesis In Vitro
2.2. In Vivo Experiments
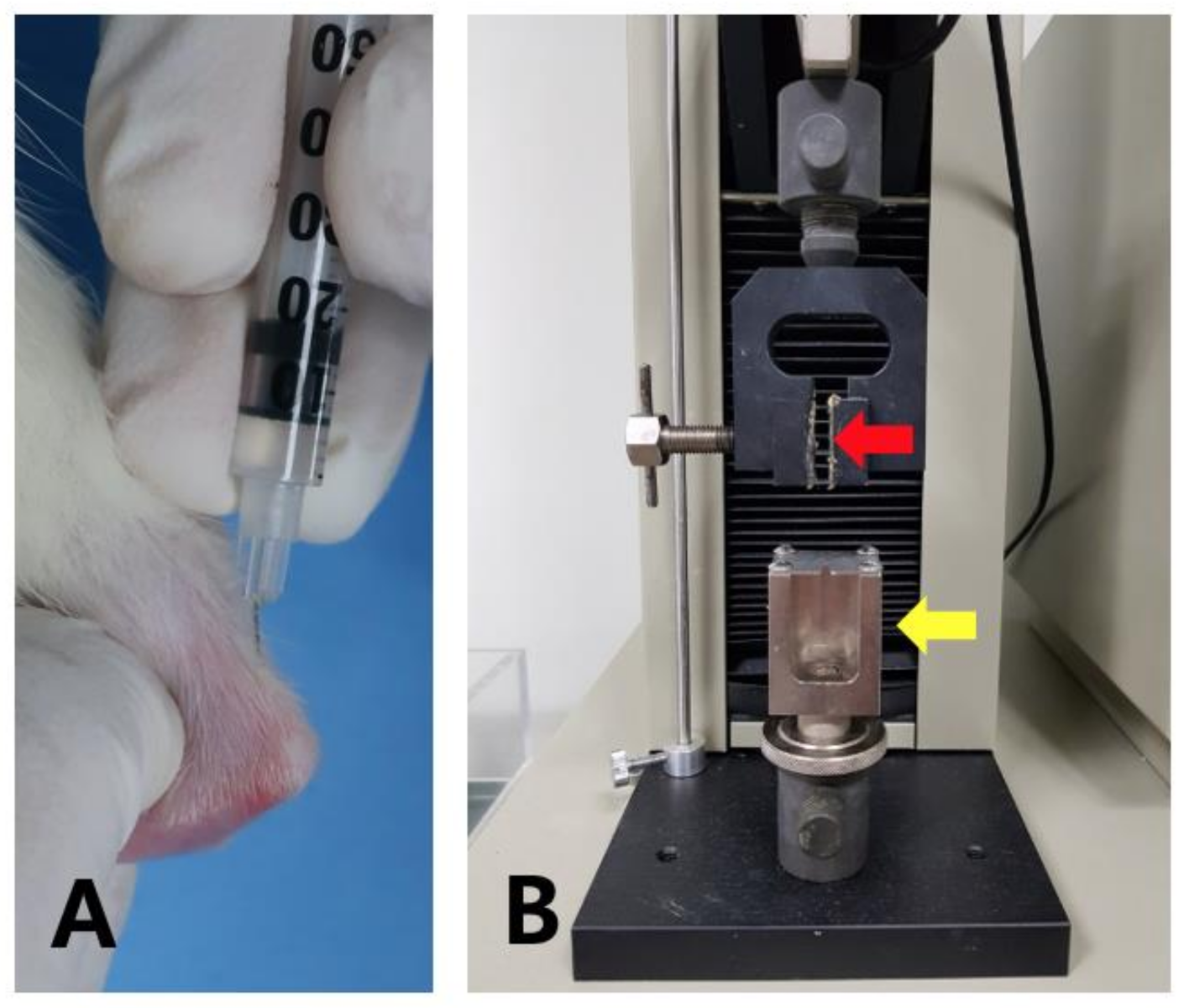
2.2.1. SP Injection Deteriorated the Tendon Strength
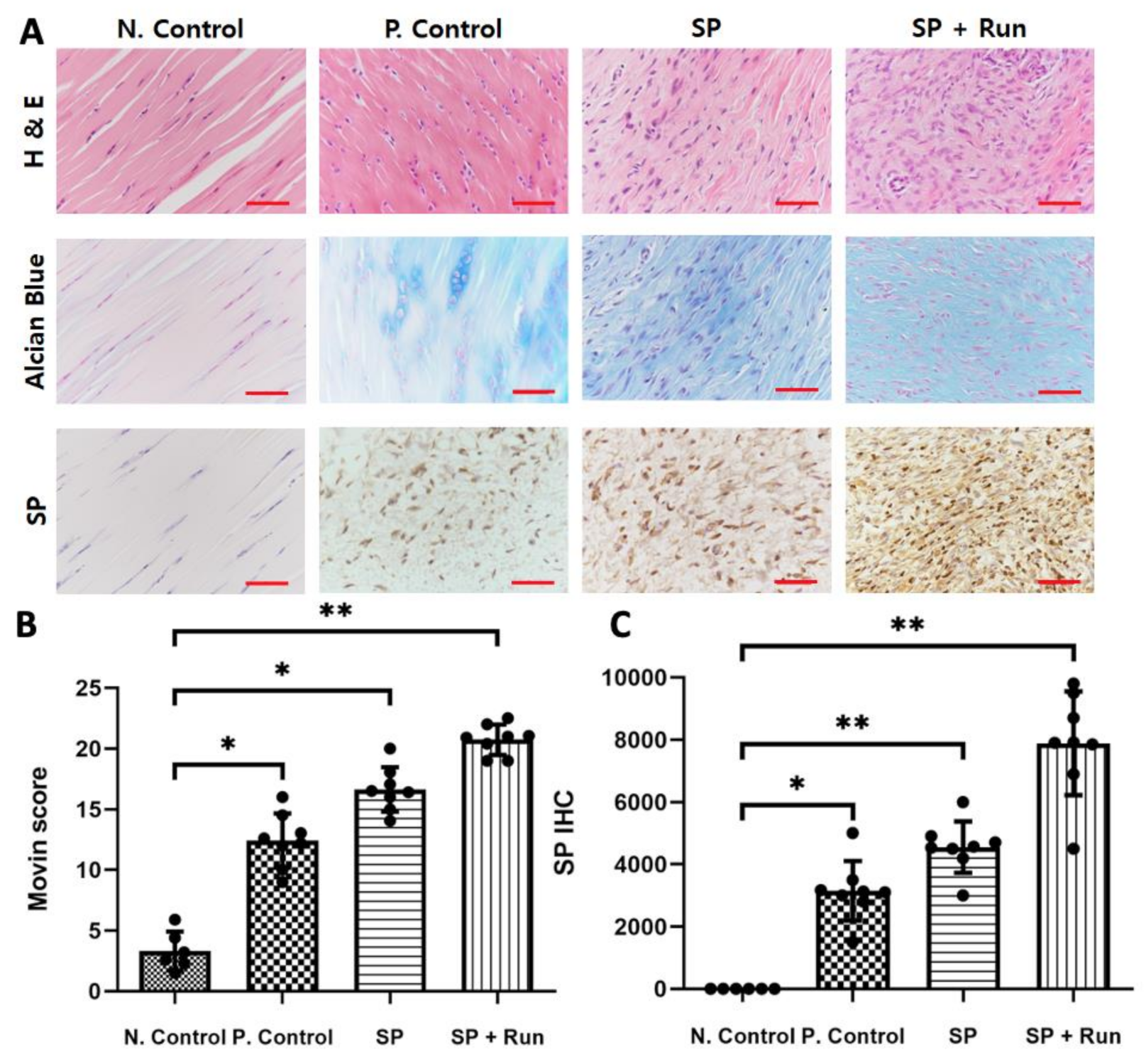
2.2.2. Multiple SP Injections Induced Histological Tendinopathic Changes
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Viability Test
4.3. RNA Isolation and qRT-PCR
4.4. ELISA of IL-6 and TNMD
4.5. ICC of Tenocytes
4.6. In Vivo Experiment Design
4.7. Injection of SP and Treadmill Running
4.8. Mechanical Test of Harvested Tendons
4.9. Histology
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SP | Substance P |
| IL-6 | Interleukin-6 |
| COL3 | Collagen type 3 |
| COL1 | Collagen type 1 |
| PBS | Phosphate-buffered saline |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| ELISA | Enzyme-linked immunosorbent assay |
| ICC | Immunocytochemistry |
| TNMD | Tenomodulin |
| cDNA | Complementary DNA |
| H and E | Hematoxylin and eosin |
References
- Xu, Y.; Murrell, G.A. The Basic Science of Tendinopathy. Clin. Orthop. Relat. Res. 2008, 466, 1528–1538. [Google Scholar] [CrossRef] [Green Version]
- Riley, G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology 2004, 43, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Maffulli, N. Tendon Injury and Tendinopathy: Healing and Repair. J. Bone Jt. Surg. AM 2005, 87, 17–19. [Google Scholar]
- LoIacono, C.; Palermi, S.; Massa, B.; Belviso, I.; Romano, V.; Gregorio, A.D.; Sirico, F.; Sacco, A.M. Gregorio Tendinopathy: Pathophysiology, Therapeutic Options, and Role of Nutraceutics. A Narrative Literature Review. Medicina 2019, 55, 447. [Google Scholar] [CrossRef] [Green Version]
- Lopez, R.G.L.; Jung, H.-G. Achilles Tendinosis: Treatment Options. Clin. Orthop. Surg. 2015, 7, 1–7. [Google Scholar] [CrossRef]
- Wu, F.; Nerlich, M.; Docheva, D. Tendon injuries: Basic science and new repair proposals. EFFORT Open Rev. 2017, 2, 332–342. [Google Scholar] [CrossRef]
- Bass, L.E. Tendinopathy: Why the Difference Between Tendinitis and Tendinosis Matters. Int. J. Ther. Massage Bodyw. Res. Educ. Pract. 2012, 5, 14–17. [Google Scholar] [CrossRef]
- Yoshinao, Z.H.; Takehiro, U.; Hiromi, U.; Masato, U.; Kazushige, T. Comparative study of the properties of tendinocytes derived from three different sites in the equine superficial digital flexor tendon. Biomed. Res. 2010, 31, 35–44. [Google Scholar]
- Durgam, S.S.; Stewart, M. Cellular and Molecular Factors Influencing Tendon Repair. Tissue Eng. Part B Rev. 2016, 23, 307–317. [Google Scholar] [CrossRef]
- Arnoczky, S.P.; Lavagnino, M.; Egerbacher, M. The mechanobiological aetiopathogenesis of tendinopathy: Is it the over-stimulation or the under-stimulation of tendon cells? Int. J. Exp. Pathol. 2007, 88, 217–226. [Google Scholar] [CrossRef]
- Arnoczky, S.P.; Lavagnino, M.; Egerbacher, M.; Caballero, O.; Gardner, K. Matrix Metalloproteinase Inhibitors Prevent a Decrease in the Mechanical Properties of Stress-Deprived Tendons an In Vitro Experimental Study. Am. J. Sports Med. 2007, 35, 763–769. [Google Scholar] [CrossRef]
- Öhberg, L. Is vasculo-neural ingrowth the cause of pain in chronic Achilles tendinosis? An investigation using ultrasonography and colour Doppler, immunohistochemistry. Sports Med. 2003, 11, 334–338. [Google Scholar]
- Ackermann, P.W. Neuronal regulation of tendon homoeostasis. Int. J. Exp. Pathol. 2013, 94, 271–286. [Google Scholar] [CrossRef] [PubMed]
- D’Addona, A.; Maffulli, N.; Formisano, S.; Rosa, D. Inflammation in tendinopathy. Surgeon 2017, 15, 297–302. [Google Scholar] [CrossRef]
- Khatib, M.E.; Mauro, A.; Mattia, M.D.; Wyrwa, R.; Schweder, M.; Ancora, M.; Lazzaro, F.; Berardinelli, P.; Valbonetti, L.; Giacinto, O.D.; et al. Electrospun PLGA Fiber Diameter and Alignment of Tendon Biomimetic Fleece Potentiate Tenogenic Differentiation and Immunomodulatory Function of Amniotic Epithelial Stem Cells. Cells 2020, 9, 1207. [Google Scholar] [CrossRef]
- Tang, C.; Chen, Y.; Huang, J.; Zhao, K.; Chen, X.; Yin, Z.; Heng, B.C.; Chen, W.; Shen, W. The roles of inflammatory mediators and immunocytes in tendinopathy. J. Orthop. Transl. 2018, 14, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, O.; Schizas, N.; Li, J.; Ackermann, P.W. Substance P injections enhance tissue proliferation and regulate sensory nerve ingrowth in rat tendon repair. Scand. J. Med. Sci. Sports 2011, 21, 562–569. [Google Scholar] [CrossRef]
- Fong, G.; Backman, L.J.; Hart, D.A.; Danielson, P.; McCormack, B.; Scott, A. Substance P enhances collagen remodeling and MMP-3 expression by human tenocytes. J. Orthop. Res. 2012, 31, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Zhou, Y.; Tang, K. The effects of substance P on pluripotent tendon cells: An in vitro and in vivo study. J. Musculoskelet. Neuronal Interact. 2014, 14, 349–358. [Google Scholar]
- Andersson, G.; Backman, L.J.; Scott, A.; Lorentzon, R.; Forsgren, S.; Danielson, P. Substance P accelerates hypercellularity and angiogenesis in tendon tissue and enhances paratendinitis in response to Achilles tendon overuse in a tendinopathy model. Br. J. Sports Med. 2011, 45, 1017–1022. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.K.; Kim, J.I.; Hwang, T.I.; Sim, B.R.; Khang, G. Bioengineered Osteoinductive Broussonetia kazinoki/Silk Fibroin Composite Scaffolds for Bone Tissue Regeneration. ACS Appl. Matter. Interfaces 2017, 9, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-H.; Choi, W.; Song, J.; Kim, J.; Lee, S.; Choi, Y.; Byun, S.-E.; Ahn, T.-K.; An, H.-J.; Ding, C.; et al. The Implication of Substance P in the Development of Tendinopathy: A Case Control Study. Int. J. Mol. Sci. 2017, 18, 1241. [Google Scholar] [CrossRef] [Green Version]
- Fong, G.; Backman, L.J.; Alfredson, H.; Scott, A.; Danielson, P. The effects of substance P and acetylcholine on human tenocyte proliferation converge mechanistically via TGF-β1. PLoS ONE 2017, 12, e0174101. [Google Scholar] [CrossRef] [PubMed]
- Lipman, K.; Wang, C.; Ting, K.; Soo, C.; Zheng, Z. Tendinopathy: Injury, repair, and current exploration. Drug Des. Dev. Ther. 2018, 12, 591–603. [Google Scholar] [CrossRef] [Green Version]
- Harrison, S.; Geppetti, P. Substance P. Int. J. Biochem. Cell Biol. 2001, 33, 555–576. [Google Scholar] [CrossRef]
- Andersson, G.; Forsgren, S.; Scott, A.; Gaida, J.E.; Stjernfeldt, J.E.; Lorentzon, R.; Alfredson, H.; Backman, C.; Danielson, P. Tenocyte hypercellularity and vascular proliferation in a rabbit model of tendinopathy: Contralateral effects suggest the involvement of central neuronal mechanisms. Br. J. Sports Med. 2011, 45, 399–406. [Google Scholar] [CrossRef]
- Millar, N.L.; Murrell, G.A.C.; Mcinnes, I.B. Inflammatory mechanisms in tendinopathy—Towards translation. Nat. Rev. Rheumatol. 2017, 13, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Barbe, M.F.; Hilliard, B.; Fisher, P.; White, A.; Delany, S.; Iannarone, V.; Harris, M.; Amin, M.; Cruz, G.; Popoff, S. Blocking substance P signaling reduces musculotendinous and dermal fibrosis and sensorimotor declines in a rat model of overuse injury. Connect. Tissue Res. 2020, 61, 604–619. [Google Scholar] [CrossRef] [Green Version]
- Fedorczyk, J.M.; Barr, A.E.; Rani, S.; Gao, H.G.; Amin, M.; Amin, S.; Litvin, J.; Barbe, M.F. Exposure-dependent increases in IL-1β, substance P, CTGF, and tendinosis in flexor digitorum tendons with upper extremity repetitive strain injury. J. Orthop. Res. 2010, 28, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Spang, C.; Chen, J.; Backman, L.J. The tenocyte phenotype of human primary tendon cells in vitro is reduced by glucocorticoids. BMC Musculoskelet. Disord. 2016, 17, 467. [Google Scholar] [CrossRef] [Green Version]
- Docheva, D.; Müller, S.A.; Majewski, M.; Evans, C.H. Biologics for tendon repair. Adv. Drug Deliv. Rev. 2015, 84, 222–239. [Google Scholar] [CrossRef] [Green Version]
- Netto, C.C.; Pereira, C.A.M.; Godoy-Santos, A.; Lima, F.D.O.; Pontin, P.A.; Zahoor, T.A.; Dein, E.J.; Schon, L.C.; Camargo, O.P.; Fernandes, T. Tendinopathy Induced by Serial Low-Dose Collagenase Injections. Foot Ankle Orthop. 2016, 1. [Google Scholar] [CrossRef] [Green Version]
- Jafari, L.; Vachon, P.; Beaudry, F.; Langelier, E. Histopathological, biomechanical, and behavioral pain findings of Achilles tendinopathy using an animal model of overuse injury. Physiol. Rep. 2015, 3, e12265. [Google Scholar] [CrossRef]
- Frara, N.; Fisher, P.W.; Zhao, Y.; Tarr, J.T.; Amin, M.; Popoff, S.N.; Barbe, M.F. Substance P increases CCN2 dependent on TGF-beta yet Collagen Type I via TGF-beta1 dependent and independent pathways in tenocytes. Connect. Tissue Res. 2018, 59, 30–44. [Google Scholar] [CrossRef]
- Warden, S.J. Animal models for the study of tendinopathy. Br. J. Sports Med. 2007, 41, 232–240. [Google Scholar] [CrossRef]
- Loppini, M.; Longo, U.G.; Niccoli, G.; Khan, W.S.; Maffulli, N.; Denaro, V. Histopathological Scores for Tissue-Engineered, Repaired and Degenerated Tendon: A Systematic Review of the Literature. Curr. Stem Cell Res. Ther. 2015, 10, 43–55. [Google Scholar] [CrossRef]
- Maffulli, N.; Longo, U.G.; Franceschi, F.; Rabitti, C.; Denaro, V. Movin and Bonar Scores Assess the Same Characteristics of Tendon Histology. Clin. Orthop. Relat. Res. 2008, 466, 1605–1611. [Google Scholar] [CrossRef] [Green Version]


| Group (n) | Treatment | Duration | Frequency | Dose |
|---|---|---|---|---|
| Negative Control | PBS injection | 2 weeks | EOD * | 20 µL |
| Positive Control | Running | 7 weeks | 1 h/day | |
| SP | SP † injection | 2 weeks | EOD | 20 µL |
| SP + Run | SP injection + Running | 2 weeks | EOD + 1 h/day | 20 µL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.Y.; Kim, D.K.; Han, S.H.; Lee, H.H.; Jeong, Y.; Baek, M.; Kim, H.; Ahn, W.; Lee, S. Sustained Exposure of Substance P Causes Tendinopathy. Int. J. Mol. Sci. 2020, 21, 8633. https://doi.org/10.3390/ijms21228633
Oh SY, Kim DK, Han SH, Lee HH, Jeong Y, Baek M, Kim H, Ahn W, Lee S. Sustained Exposure of Substance P Causes Tendinopathy. International Journal of Molecular Sciences. 2020; 21(22):8633. https://doi.org/10.3390/ijms21228633
Chicago/Turabian StyleOh, Seo Yoon, Do Kyung Kim, Soo Hong Han, Hyun Hae Lee, Yunhui Jeong, Minjung Baek, Hyeongkyung Kim, Wooyeol Ahn, and Soonchul Lee. 2020. "Sustained Exposure of Substance P Causes Tendinopathy" International Journal of Molecular Sciences 21, no. 22: 8633. https://doi.org/10.3390/ijms21228633
APA StyleOh, S. Y., Kim, D. K., Han, S. H., Lee, H. H., Jeong, Y., Baek, M., Kim, H., Ahn, W., & Lee, S. (2020). Sustained Exposure of Substance P Causes Tendinopathy. International Journal of Molecular Sciences, 21(22), 8633. https://doi.org/10.3390/ijms21228633