Abscisic Acid Mediates Drought and Salt Stress Responses in Vitis vinifera—A Review
Abstract
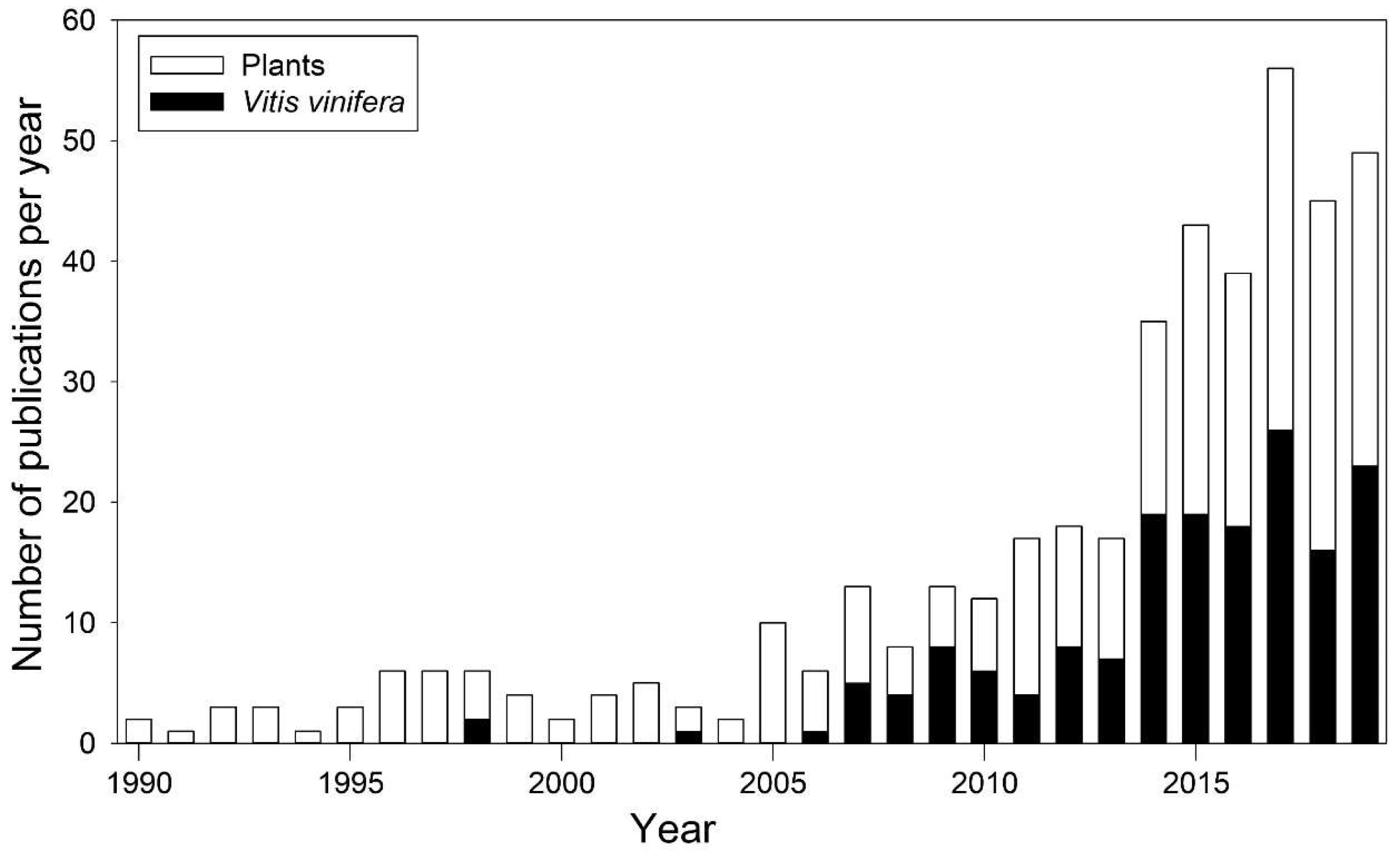
:1. Introduction
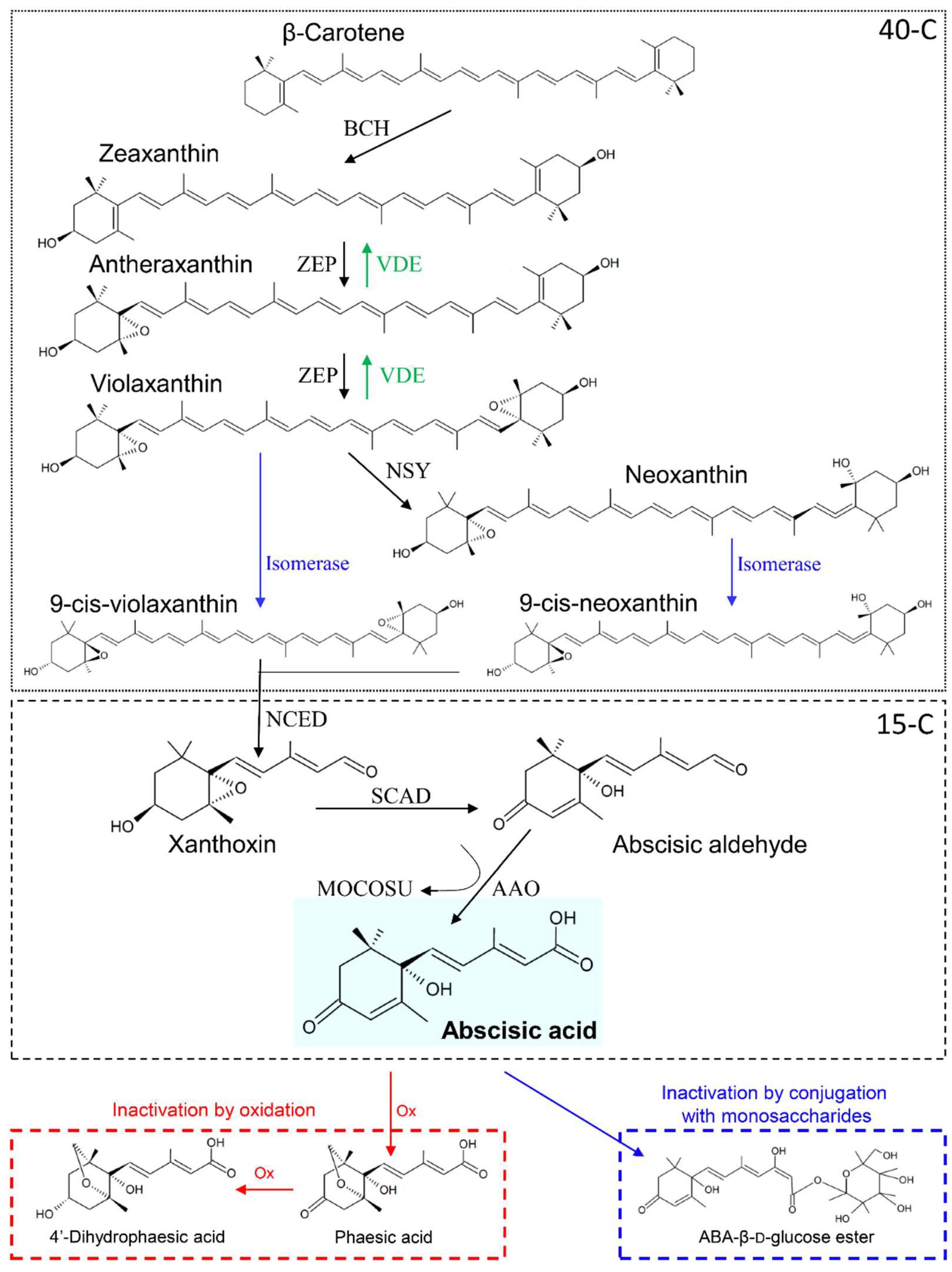
2. ABA Biosynthesis and Translocation
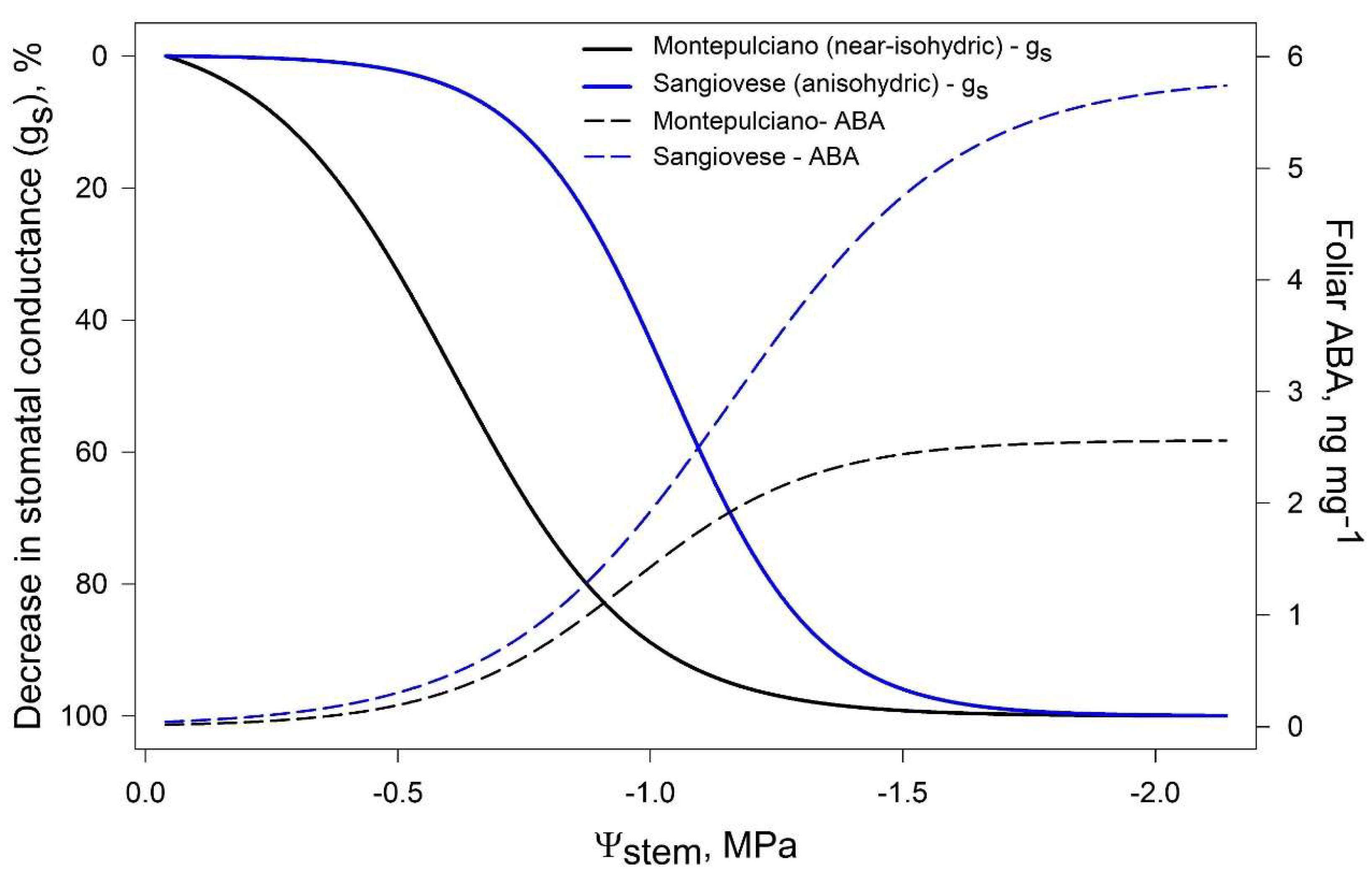
3. ABA Role in Regulating Stomatal Closure
4. ABA Role in Carbohydrates Mobilization
5. ABA in Salt-Stress Response
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. Climate Change 2013: The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Costa, J.M.; Vaz, M.; Escalona, J.; Egipto, R.; Lopes, C.; Medrano, H.; Chaves, M.M. Modern viticulture in southern Europe: Vulnerabilities and strategies for adaptation to water scarcity. Agric. Water Manag. 2016, 164, 5–18. [Google Scholar] [CrossRef]
- Duchêne, E.; Huard, F.; Dumas, V.; Schneider, C.; Merdinoglu, D. The challenge of adapting grapevine varieties to climate change. Clim. Res. 2010, 41, 193–204. [Google Scholar] [CrossRef] [Green Version]
- Gambetta, G.A.; Herrera, J.C.; Dayer, S.; Feng, Q.; Hochberg, U.; Castellarin, S.D. The physiology of drought stress in grapevine: Towards an integrative definition of drought tolerance. J. Exp. Bot. 2020, 71, 4658–4676. [Google Scholar] [CrossRef]
- Perrone, I.; Pagliarani, C.; Lovisolo, C.; Chitarra, W.; Roman, F.; Schubert, A. Recovery from water stress affects grape leaf petiole transcriptome. Planta 2012, 235, 1383–1396. [Google Scholar] [CrossRef]
- Savi, T.; Casolo, V.; Dal Borgo, A.; Rosner, S.; Torboli, V.; Stenni, B.; Bertoncin, P.; Martellos, S.; Pallavicini, A.; Nardini, A. Drought-induced dieback of Pinus nigra: A tale of hydraulic failure and carbon starvation. Conserv. Physiol. 2019, 7, coz012. [Google Scholar]
- Lovisolo, C.; Perrone, I.; Carra, A.; Ferrandino, A.; Flexas, J.; Medrano, H.; Schubert, A. Drought-induced changes in development and function of grapevine (Vitis spp.) organs and in their hydraulic and non-hydraulic interactions at the whole-plant level: A physiological and molecular update. Funct. Plant Biol. 2010, 37, 98–116. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic Acid Signaling and Abiotic Stress Tolerance in Plants: A Review on Current Knowledge and Future Prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef] [Green Version]
- Kempa, S.; Krasensky, J.; Santo, S.D.; Kopka, J.; Jonak, C. A Central Role of Abscisic Acid in Stress-Regulated Carbohydrate Metabolism. PLoS ONE 2008, 3, e3935. [Google Scholar] [CrossRef] [Green Version]
- Millar, A.A.; Jacobsen, J.V.; Ross, J.J.; Helliwell, C.A.; Poole, A.T.; Scofield, G.; Reid, J.B.; Gubler, F. Seed dormancy and ABA metabolism in Arabidopsis and barley: The role of ABA 8′-hydroxylase. Plant J. 2006, 45, 942–954. [Google Scholar] [CrossRef]
- Ding, Q.; Zeng, J.; He, X.-Q. MiR169 and its target PagHAP2-6 regulated by ABA are involved in poplar cambium dormancy. J. Plant Physiol. 2016, 198, 1–9. [Google Scholar] [CrossRef] [PubMed]
- De Smet, I.; Signora, L.; Beeckman, T.; Inzé, D.; Foyer, C.H.; Zhang, H. An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant J. 2003, 33, 543–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, H.-F.; Chai, Y.-M.; Li, C.-L.; Lu, D.; Luo, J.-J.; Qin, L.; Shen, Y.-Y. Abscisic Acid Plays an Important Role in the Regulation of Strawberry Fruit Ripening. Plant Physiol. 2011, 157, 188–199. [Google Scholar] [CrossRef] [Green Version]
- Downton, W.J.S.; Loveys, B.R.; Grant, W.J.R. Salinity effects on the stomatal behaviour of grapevine. New Phytol. 1990, 116, 499–503. [Google Scholar] [CrossRef]
- Dodd, I.C.; Egea, G.; Davies, W.J. Abscisic acid signalling when soil moisture is heterogeneous: Decreased photoperiod sap flow from drying roots limits abscisic acid export to the shoots. Plant Cell Environ. 2008, 31, 1263–1274. [Google Scholar] [CrossRef]
- Dodd, I.C.; Puértolas, J.; Huber, K.; Pérez-Pérez, J.G.; Wright, H.R.; Blackwell, M.S. The importance of soil drying and re-wetting in crop phytohormonal and nutritional responses to deficit irrigation. J. Exp. Bot. 2015, 66, 2239–2252. [Google Scholar] [CrossRef]
- Frioni, T.; Tombesi, S.; Sabbatini, P.; Squeri, C.; Lavado Rodas, N.; Palliotti, A.; Poni, S. Kaolin Reduces ABA Biosynthesis through the Inhibition of Neoxanthin Synthesis in Grapevines under Water Deficit. Int. J. Mol. Sci. 2020, 21, 4950. [Google Scholar] [CrossRef]
- Tombesi, S.; Nardini, A.; Frioni, T.; Soccolini, M.; Zadra, C.; Farinelli, D.; Poni, S.; Palliotti, A. Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevine. Sci. Rep. 2015, 5, 12449. [Google Scholar] [CrossRef]
- Falchi, R.; Petrussa, E.; Braidot, E.; Sivilotti, P.; Boscutti, F.; Vuerich, M.; Calligaro, C.; Filippi, A.; Herrera, J.C.; Sabbatini, P. Analysis of Non-Structural Carbohydrates and Xylem Anatomy of Leaf Petioles Offers New Insights in the Drought Response of Two Grapevine Cultivars. Int. J. Mol. Sci. 2020, 21, 1457. [Google Scholar] [CrossRef] [Green Version]
- Saleh, B.; Alshehadah, E.; Slaman, H. Abscisic Acid (ABA) and Salicylic Acid (SA) Content in Relation to Transcriptional Patterns in Grapevine (Vitis vinifera L.) under Salt Stress. J. Plant Biochem. Physiol. 2020, 8, 245. [Google Scholar]
- Banerjee, A.; Sharkey, T.D. Methylerythritol 4-phosphate (MEP) pathway metabolic regulation. Nat. Prod. Rep. 2014, 31, 1043–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loveys, B.R.; Kriedemann, P.E. Internal Control of Stomatal Physiology and Photosynthesis. I. Stomatal Regulation and Associated Changes in Endogenous Levels of Abscisic and Phaseic Acids. Funct. Plant Biol. 1974, 1, 407–415. [Google Scholar] [CrossRef]
- Loveys, B.R. The Intracellular Location of Abscisic Acid in Stressed and Non-Stressed Leaf Tissue. Physiol. Plant. 1977, 40, 6–10. [Google Scholar] [CrossRef]
- Loveys, B.R.; Düring, H. Diurnal Changes in Water Relations and Abscisic Acid in Field-Grown Vitis Vinifera Cultivars. New Phytol. 1984, 97, 37–47. [Google Scholar] [CrossRef]
- Pierce, M.; Raschke, K. Correlation between loss of turgor and accumulation of abscisic acid in detached leaves. Planta 1980, 148, 174–182. [Google Scholar] [CrossRef]
- Zhang, J.; Davies, W.J. Increased Synthesis of ABA in Partially Dehydrated Root Tips and ABA Transport from Roots to Leaves. J. Exp. Bot. 1987, 38, 2015–2023. [Google Scholar] [CrossRef]
- Zhang, J.; Davies, W.J. Changes in the concentration of ABA in xylem sap as a function of changing soil water status can account for changes in leaf conductance and growth. Plant Cell Environ. 1990, 13, 277–285. [Google Scholar] [CrossRef]
- Soar, C.J.; Dry, P.R.; Loveys, B.R. Scion photosynthesis and leaf gas exchange in Vitis vinifera L. cv. Shiraz: Mediation of rootstock effects via xylem sap ABA. Aust. J. Grape Wine Res. 2006, 12, 82–96. [Google Scholar] [CrossRef]
- Speirs, J.; Binney, A.; Collins, M.; Edwards, E.; Loveys, B. Expression of ABA synthesis and metabolism genes under different irrigation strategies and atmospheric VPDs is associated with stomatal conductance in grapevine (Vitis vinifera L. cv Cabernet Sauvignon). J. Exp. Bot. 2013, 64, 1907–1916. [Google Scholar] [CrossRef] [Green Version]
- Dry, P.R.; Loveys, B.R.; During, H. Partial drying of the rootzone of grape I. Transient changes in shoot growth and gas exchange. Vitis-Geilweilerhof- 2000, 39, 3–8. [Google Scholar]
- Poni, S.; Bernizzoni, F.; Civardi, S. Response of “Sangiovese” grapevines to partial root-zone drying: Gas-exchange, growth and grape composition. Sci. Hortic. 2007, 114, 96–103. [Google Scholar] [CrossRef]
- Romero, P.; Dodd, I.C.; Martinez-Cutillas, A. Contrasting physiological effects of partial root zone drying in field-grown grapevine (Vitis vinifera L. cv. Monastrell) according to total soil water availability. J. Exp. Bot. 2012, 63, 4071–4083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, P.; Pérez-Pérez, J.G.; Amor, F.M.D.; Martinez-Cutillas, A.; Dodd, I.C.; Botía, P. Partial root zone drying exerts different physiological responses on field-grown grapevine (Vitis vinifera cv. Monastrell) in comparison to regulated deficit irrigation. Funct. Plant Biol. 2014, 41, 1087–1106. [Google Scholar] [CrossRef] [PubMed]
- Schachtman, D.P.; Goodger, J.Q.D. Chemical root to shoot signaling under drought. Trends Plant Sci. 2008, 13, 281–287. [Google Scholar] [CrossRef]
- Dodd, I.C.; Theobald, J.C.; Bacon, M.A.; Davies, W.J. Alternation of wet and dry sides during partial rootzone drying irrigation alters root-to-shoot signalling of abscisic acid. Funct. Plant Biol. 2006, 33, 1081–1089. [Google Scholar] [CrossRef]
- Kochhar, S.L.; Gujral, S.K. Plant Physiology: Theory and Applications: Theory and Applications; Cambridge University Press: Cambridge, UK, 2020; pp. 491–495. [Google Scholar]
- Pérez-Pérez, J.G.; Puertolas, J.; Albacete, A.; Dodd, I.C. Alternation of wet and dry sides during partial rootzone drying irrigation enhances leaf ethylene evolution. Environ. Exp. Bot. 2020, 176, 104095. [Google Scholar] [CrossRef]
- Soar, C.J.; Speirs, J.; Maffei, S.M.; Penrose, A.B.; Mccarthy, M.G.; Loveys, B.R. Grape vine varieties Shiraz and Grenache differ in their stomatal response to VPD: Apparent links with ABA physiology and gene expression in leaf tissue. Aust. J. Grape Wine Res. 2006, 12, 2–12. [Google Scholar] [CrossRef]
- Ikegami, K.; Okamoto, M.; Seo, M.; Koshiba, T. Activation of abscisic acid biosynthesis in the leaves of Arabidopsis thaliana in response to water deficit. J. Plant Res. 2008, 122, 235. [Google Scholar] [CrossRef]
- Manzi, M.; Lado, J.; Rodrigo, M.J.; Zacarías, L.; Arbona, V.; Gómez-Cadenas, A. Root ABA Accumulation in Long-Term Water-Stressed Plants is Sustained by Hormone Transport from Aerial Organs. Plant Cell Physiol. 2015, 56, 2457–2466. [Google Scholar] [CrossRef]
- McAdam, S.A.M.; Brodribb, T.J. Mesophyll Cells Are the Main Site of Abscisic Acid Biosynthesis in Water-Stressed Leaves. Plant Physiol. 2018, 177, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Sack, L.; John, G.P.; Buckley, T.N. ABA Accumulation in Dehydrating Leaves Is Associated with Decline in Cell Volume, Not Turgor Pressure. Plant Physiol. 2018, 176, 489–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, P.; Puertolas, J.; Dodd, I.C. Stem girdling uncouples soybean stomatal conductance from leaf water potential by enhancing leaf xylem ABA concentration. Environ. Exp. Bot. 2019, 159, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yuan, H. Chromoplast biogenesis and carotenoid accumulation. Arch. Biochem. Biophys. 2013, 539, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Howitt, C.A.; Pogson, B.J. Carotenoid accumulation and function in seeds and non-green tissues. Plant Cell Environ. 2006, 29, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Kuromori, T.; Fujita, M.; Urano, K.; Tanabata, T.; Sugimoto, E.; Shinozaki, K. Overexpression of AtABCG25 enhances the abscisic acid signal in guard cells and improves plant water use efficiency. Plant Sci. 2016, 251, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Bota, B.J.; Flexas, J.; Medrano, H. Genetic variability of photosynthesis and water use in Balearic grapevine cultivars. Ann. Appl. Biol. 2001, 138, 353–361. [Google Scholar] [CrossRef]
- Levin, A.D.; Williams, L.E.; Matthews, M.A. A continuum of stomatal responses to water deficits among 17 wine grape cultivars (Vitis vinifera). Funct. Plant Biol. 2020, 47, 11–25. [Google Scholar] [CrossRef]
- Pagliarani, C.; Vitali, M.; Ferrero, M.; Vitulo, N.; Incarbone, M.; Lovisolo, C.; Valle, G.; Schubert, A. The Accumulation of miRNAs Differentially Modulated by Drought Stress Is Affected by Grafting in Grapevine. Plant Physiol. 2017, 173, 2180–2195. [Google Scholar] [CrossRef] [Green Version]
- Bota, J.; Tomás, M.; Flexas, J.; Medrano, H.; Escalona, J.M. Differences among grapevine cultivars in their stomatal behavior and water use efficiency under progressive water stress. Agric. Water Manag. 2016, 164, 91–99. [Google Scholar] [CrossRef]
- Tyree, M.T. The Cohesion-Tension theory of sap ascent: Current controversies. J. Exp. Bot. 1997, 48, 1753–1765. [Google Scholar] [CrossRef] [Green Version]
- Tyree, M.T.; Sperry, J.S. Vulnerability of Xylem to Cavitation and Embolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 19–36. [Google Scholar] [CrossRef]
- Hacke, U.G.; Sperry, J.S.; Pockman, W.T.; Davis, S.D.; McCulloh, K.A. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 2001, 126, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Buckley, T.N. How do stomata respond to water status? New Phytol. 2019, 224, 21–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mäser, P.; Leonhardt, N.; Schroeder, J. The Clickable Guard Cell: Electronically Linked Model of Guard Cell Signal Transduction Pathways. Arab. Book 2003, 32, 1–4. [Google Scholar]
- Wang, Y.; Chen, Z.-H.; Zhang, B.; Hills, A.; Blatt, M.R. PYR/PYL/RCAR Abscisic Acid Receptors Regulate K+ and Cl- Channels through Reactive Oxygen Species-Mediated Activation of Ca2+ Channels at the Plasma Membrane of Intact Arabidopsis Guard Cells. Plant Physiol. 2013, 163, 566–577. [Google Scholar] [CrossRef] [Green Version]
- Tardieu, F.; Simonneau, T. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: Modelling isohydric and anisohydric behaviours. J. Exp. Bot. 1998, 49, 419–432. [Google Scholar] [CrossRef] [Green Version]
- Ratzmann, G.; Meinzer, F.C.; Tietjen, B. Iso/Anisohydry: Still a Useful Concept. Trends Plant Sci. 2019, 24, 191–194. [Google Scholar] [CrossRef]
- Hochberg, U.; Rockwell, F.E.; Holbrook, N.M.; Cochard, H. Iso/Anisohydry: A Plant–Environment Interaction Rather Than a Simple Hydraulic Trait. Trends Plant Sci. 2018, 23, 112–120. [Google Scholar] [CrossRef]
- Coupel-Ledru, A.; Lebon, É.; Christophe, A.; Doligez, A.; Cabrera-Bosquet, L.; Péchier, P.; Hamard, P.; This, P.; Simonneau, T. Genetic variation in a grapevine progeny (Vitis vinifera L. cvs Grenache × Syrah) reveals inconsistencies between maintenance of daytime leaf water potential and response of transpiration rate under drought. J. Exp. Bot. 2014, 65, 6205–6218. [Google Scholar] [CrossRef] [Green Version]
- Nardini, A.; Salleo, S. Limitation of stomatal conductance by hydraulic traits: Sensing or preventing xylem cavitation? Trees 2000, 15, 14–24. [Google Scholar] [CrossRef]
- Christmann, A.; Weiler, E.W.; Steudle, E.; Grill, E. A hydraulic signal in root-to-shoot signalling of water shortage. Plant J. 2007, 52, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, R.M.; Bond, B.J.; Ryan, M.G. Evidence that hydraulic conductance limits photosynthesis in old Pinus ponderosa trees. Tree Physiol. 1999, 19, 165–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, T. The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct. Ecol. 2014, 28, 1313–1320. [Google Scholar] [CrossRef]
- Tombesi, S.; Nardini, A.; Farinelli, D.; Palliotti, A. Relationships between stomatal behavior, xylem vulnerability to cavitation and leaf water relations in two cultivars of Vitis vinifera. Physiol. Plant. 2014, 152, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Skelton, R.P.; West, A.G.; Dawson, T.E. Predicting plant vulnerability to drought in biodiverse regions using functional traits. Proc. Natl. Acad. Sci. USA 2015, 112, 5744–5749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochberg, U.; Windt, C.W.; Ponomarenko, A.; Zhang, Y.-J.; Gersony, J.; Rockwell, F.E.; Holbrook, N.M. Stomatal Closure, Basal Leaf Embolism, and Shedding Protect the Hydraulic Integrity of Grape Stems. Plant Physiol. 2017, 174, 764–775. [Google Scholar] [CrossRef] [Green Version]
- McAdam, S.A.M.; Brodribb, T.J. Separating Active and Passive Influences on Stomatal Control of Transpiration. Plant Physiol. 2014, 164, 1578–1586. [Google Scholar] [CrossRef] [Green Version]
- Degu, A.; Hochberg, U.; Wong, D.C.; Alberti, G.; Lazarovitch, N.; Peterlunger, E.; Castellarin, S.D.; Herrera, J.C.; Fait, A. Swift metabolite changes and leaf shedding are milestones in the acclimation process of grapevine under prolonged water stress. BMC Plant Biol. 2019, 19, 69. [Google Scholar] [CrossRef]
- Tramontini, S.; Döring, J.; Vitali, M.; Ferrandino, A.; Stoll, M.; Lovisolo, C. Soil water-holding capacity mediates hydraulic and hormonal signals of near-isohydric and near-anisohydric Vitis cultivars in potted grapevines. Funct. Plant Biol. 2014, 41, 1119–1128. [Google Scholar] [CrossRef]
- Coupel-Ledru, A.; Tyerman, S.D.; Masclef, D.; Lebon, E.; Christophe, A.; Edwards, E.J.; Simonneau, T. Abscisic acid down-regulates hydraulic conductance of grapevine leaves in isohydric genotypes only. Plant Physiol. 2017, 175, 1121–1134. [Google Scholar] [CrossRef] [Green Version]
- Dayer, S.; Scharwies, J.D.; Ramesh, S.A.; Sullivan, W.; Doerflinger, F.C.; Pagay, V.; Tyerman, S.D. Comparing hydraulics between two grapevine cultivars reveals differences in stomatal regulation under water stress and exogenous ABA applications. Front. Plant Sci. 2020, 11, 705. [Google Scholar] [CrossRef] [PubMed]
- Gambetta, G.A.; Knipfer, T.; Fricke, W.; McElrone, A.J. Aquaporins and Root Water Uptake. In Plant Aquaporins: From Transport to Signaling; Chaumont, F., Tyerman, S.D., Eds.; Signaling and Communication in Plants; Springer International Publishing: Cham, Switzerland, 2017; pp. 133–153. [Google Scholar]
- Dayer, S.; Reingwirtz, I.; McElrone, A.J.; Gambetta, G.A. Response and Recovery of Grapevine to Water Deficit: From Genes to Physiology. In The Grape Genome; Springer: Berlin/Heidelberg, Germany, 2019; pp. 223–245. [Google Scholar]
- McDowell, N.G.; Beerling, D.J.; Breshears, D.D.; Fisher, R.A.; Raffa, K.F.; Stitt, M. The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends Ecol. Evol. 2011, 26, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Tomasella, M.; Petrussa, E.; Petruzzellis, F.; Nardini, A.; Casolo, V. The Possible Role of Non-Structural Carbohydrates in the Regulation of Tree Hydraulics. Int. J. Mol. Sci. 2020, 21, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Secchi, F.; Pagliarani, C.; Cavalletto, S.; Petruzzellis, F.; Tonel, G.; Savi, T.; Tromba, G.; Obertino, M.M.; Lovisolo, C.; Nardini, A.; et al. Chemical inhibition of xylem cellular activity impedes the removal of drought-induced embolisms in poplar stems—New insights from micro-CT analysis. New Phytol. 2020. [Google Scholar] [CrossRef]
- Brodersen, C.R.; McElrone, A.J.; Choat, B.; Lee, E.F.; Shackel, K.A.; Matthews, M.A. In vivo visualizations of drought-induced embolism spread in Vitis vinifera. Plant Physiol. 2013, 161, 1820–1829. [Google Scholar] [CrossRef] [Green Version]
- Pan, Q.-H.; Li, M.-J.; Peng, C.-C.; Zhang, N.; Zou, X.; Zou, K.-Q.; Wang, X.-L.; Yu, X.-C.; Wang, X.-F.; Zhang, D.-P. Abscisic acid activates acid invertases in developing grape berry. Physiol. Plant. 2005, 125, 157–170. [Google Scholar] [CrossRef]
- Thalmann, M.; Pazmino, D.; Seung, D.; Horrer, D.; Nigro, A.; Meier, T.; Kölling, K.; Pfeifhofer, H.W.; Zeeman, S.C.; Santelia, D. Regulation of Leaf Starch Degradation by Abscisic Acid Is Important for Osmotic Stress Tolerance in Plants. Plant Cell 2016, 28, 1860–1878. [Google Scholar] [CrossRef] [Green Version]
- Secchi, F.; Perrone, I.; Chitarra, W.; Zwieniecka, A.K.; Lovisolo, C.; Zwieniecki, M.A. The Dynamics of Embolism Refilling in Abscisic Acid (ABA)-Deficient Tomato Plants. Int. J. Mol. Sci. 2013, 14, 359–377. [Google Scholar] [CrossRef] [Green Version]
- Brunetti, C.; Savi, T.; Nardini, A.; Loreto, F.; Gori, A.; Centritto, M. Changes in abscisic acid content during and after drought are related to carbohydrate mobilization and hydraulic recovery in poplar stems. Tree Physiol. 2020, 40, 1043–1057. [Google Scholar] [CrossRef]
- Cardoso, A.A.; Gori, A.; Da-Silva, C.J.; Brunetti, C. Abscisic Acid Biosynthesis and Signaling in Plants: Key Targets to Improve Water Use Efficiency and Drought Tolerance. Appl. Sci. 2020, 10, 6322. [Google Scholar] [CrossRef]
- Tomasella, M.; Häberle, K.-H.; Nardini, A.; Hesse, B.; Machlet, A.; Matyssek, R. Post-drought hydraulic recovery is accompanied by non-structural carbohydrate depletion in the stem wood of Norway spruce saplings. Sci. Rep. 2017, 7, 14308. [Google Scholar] [CrossRef]
- Tortosa, I.; Escalona, J.M.; Douthe, C.; Pou, A.; Garcia-Escudero, E.; Toro, G.; Medrano, H. The intra-cultivar variability on water use efficiency at different water status as a target selection in grapevine: Influence of ambient and genotype. Agric. Water Manag. 2019, 223, 105648. [Google Scholar] [CrossRef]
- Rogiers, S.Y.; Hardie, W.J.; Smith, J.P. Stomatal density of grapevine leaves (Vitis vinifera L.) responds to soil temperature and atmospheric carbon dioxide. Aust. J. Grape Wine Res. 2011, 17, 147–152. [Google Scholar] [CrossRef]
- Walter, J.; Nagy, L.; Hein, R.; Rascher, U.; Beierkuhnlein, C.; Willner, E.; Jentsch, A. Do plants remember drought? Hints towards a drought-memory in grasses. Environ. Exp. Bot. 2011, 71, 34–40. [Google Scholar] [CrossRef]
- Lukić, N.; Kukavica, B.; Davidović-Plavšić, B.; Hasanagić, D.; Walter, J. Plant stress memory is linked to high levels of anti-oxidative enzymes over several weeks. Environ. Exp. Bot. 2020, 178, 104166. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Wang, Z.; He, L.; Xu, K.; Wang, G. Glucose triggers stomatal closure mediated by basal signaling through HXK1 and PYR/RCAR receptors in Arabidopsis. J. Exp. Bot. 2018, 69, 1471–1484. [Google Scholar] [CrossRef]
- Nguyen, T.T.Q.; Trinh, L.T.H.; Pham, H.B.V.; Le, T.V.; Phung, T.K.H.; Lee, S.-H.; Cheong, J.-J. Evaluation of proline, soluble sugar and ABA content in soybean Glycine max (L.) under drought stress memory. AIMS Bioeng. 2020, 7, 114. [Google Scholar] [CrossRef]
- Netzer, Y.; Munitz, S.; Shtein, I.; Schwartz, A. Structural memory in grapevines: Early season water availability affects late season drought stress severity. Eur. J. Agron. 2019, 105, 96–103. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Laurenson, S.; Bolan, N.S.; Smith, E.; Mccarthy, M. Review: Use of recycled wastewater for irrigating grapevines. Aust. J. Grape Wine Res. 2012, 18, 1–10. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, W.; Yang, J.; Ismail, A.M. Role of ABA in integrating plant responses to drought and salt stresses. Field Crop. Res. 2006, 97, 111–119. [Google Scholar] [CrossRef]
- Chen, S.; Li, J.; Wang, S.; Hüttermann, A.; Altman, A. Salt, nutrient uptake and transport, and ABA of Populus euphratica; a hybrid in response to increasing soil NaCl. Trees 2001, 15, 186–194. [Google Scholar] [CrossRef]
- Gomez-Cadenas, A.; Arbona, V.; Jacas, J.; Primo-Millo, E.; Talon, M. Abscisic acid reduces leaf abscission and increases salt tolerance in citrus plants. J. Plant Growth Regul. 2002, 21, 234–240. [Google Scholar] [CrossRef]
- Popova, L.P.; Stoinova, Z.G.; Maslenkova, L.T. Involvement of abscisic acid in photosynthetic process in Hordeum vulgare L. during salinity stress. J. Plant Growth Regul. 1995, 14, 211. [Google Scholar] [CrossRef]
- Downton, W.J.S. Influence of rootstocks on the accumulation of chloride, sodium and potassium in grapevines. Aust. J. Agric. Res. 1977, 28, 879–889. [Google Scholar] [CrossRef]
- Stevens, R.M.; Harvey, G.; Partington, D.L. Irrigation of grapevines with saline water at different growth stages: Effects on leaf, wood and juice composition. Aust. J. Grape Wine Res. 2011, 17, 239–248. [Google Scholar] [CrossRef]
- Walker, R.R.; Blackmore, D.H.; Clingeleffer, P.R.; Emanuelli, D. Rootstock type determines tolerance of Chardonnay and Shiraz to long-term saline irrigation. Aust. J. Grape Wine Res. 2014, 20, 496–506. [Google Scholar] [CrossRef]
- Hassena, A.B.; Zouari, M.; Trabelsi, L.; Khabou, W.; Zouari, N. Physiological improvements of young olive tree (Olea europaea L. cv. Chetoui) under short term irrigation with treated wastewater. Agric. Water Manag. 2018, 207, 53–58. [Google Scholar] [CrossRef]
- Martin, L.; Vila, H.; Bottini, R.; Berli, F. Rootstocks increase grapevine tolerance to NaCl through ion compartmentalization and exclusion. Acta Physiol. Plant. 2020, 42, 145. [Google Scholar] [CrossRef]
- Aydemir, B.Ç.; Özmen, C.Y.; Kibar, U.; Mutaf, F.; Büyük, P.B.; Bakır, M.; Ergül, A. Salt stress induces endoplasmic reticulum stress-responsive genes in a grapevine rootstock. PLoS ONE 2020, 15, e0236424. [Google Scholar]
- Li, F.; Zhao, X.; Yang, X.; He, X. Influence of NaCl stress on ABA synthesis and hardness of Vitis vinifera L.‘Cabernet Sauvignon’ berry in veraison. In Proceedings of the AIP Conference Proceedings, Proceedings of the 2nd International Conference on Frontiers of Biological Sciences and Engineering (FBSE 2019), Jinan, China, 19–20 October 2019; AIP Publishing LLC: Melville, NY, USA, 2020; Volume 2208, p. 020002. [Google Scholar]
- Zhao, P.; Yang, X.; Han, N.; He, X. Influence of salt-alkali stress on quality formation of Vitis vinifera L.‘Cabernet Sauvignon’ of wine grape. In Proceedings of the AIP Conference Proceedings, Proceedings of the 2nd International Conference on Frontiers of Biological Sciences and Engineering (FBSE 2019), Jinan, China, 19–20 October 2019; AIP Publishing LLC: Melville, NY, USA, 2020; Volume 2208, p. 020006. [Google Scholar]
- Walker, R.R.; Read, P.E.; Blackmore, D.H. Rootstock and salinity effects on rates of berry maturation, ion accumulation and colour development in Shiraz grapes. Aust. J. Grape Wine Res. 2000, 6, 227–239. [Google Scholar] [CrossRef]
- Teakle, N.L.; Tyerman, S.D. Mechanisms of Cl- transport contributing to salt tolerance. Plant Cell Environ. 2010, 33, 566–589. [Google Scholar] [CrossRef]
- Degaris, K.A.; Walker, R.R.; Loveys, B.R.; Tyerman, S.D. Comparative effects of deficit and partial root-zone drying irrigation techniques using moderately saline water on ion partitioning in Shiraz and Grenache grapevines. Aust. J. Grape Wine Res. 2016, 22, 296–306. [Google Scholar] [CrossRef]
- Upreti, K.K.; Murti, G.S.R. Response of grape rootstocks to salinity: Changes in root growth, polyamines and abscisic acid. Biol. Plant. 2010, 54, 730–734. [Google Scholar] [CrossRef]
- Paranychianakis, N.V.; Angelakis, A.N. The effect of water stress and rootstock on the development of leaf injuries in grapevines irrigated with saline effluent. Agric. Water Manag. 2008, 95, 375–382. [Google Scholar] [CrossRef]
- Vincent, D.; Ergül, A.; Bohlman, M.C.; Tattersall, E.A.R.; Tillett, R.L.; Wheatley, M.D.; Woolsey, R.; Quilici, D.R.; Joets, J.; Schlauch, K.; et al. Proteomic analysis reveals differences between Vitis vinifera L. cv. Chardonnay and cv. Cabernet Sauvignon and their responses to water deficit and salinity. J. Exp. Bot. 2007, 58, 1873–1892. [Google Scholar] [CrossRef] [Green Version]
- Pye, M.F.; Dye, S.M.; Resende, R.S.; MacDonald, J.D.; Bostock, R.M. Abscisic acid as a dominant signal in tomato during salt stress predisposition to phytophthora root and crown rot. Front. Plant Sci. 2018, 9, 525. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marusig, D.; Tombesi, S. Abscisic Acid Mediates Drought and Salt Stress Responses in Vitis vinifera—A Review. Int. J. Mol. Sci. 2020, 21, 8648. https://doi.org/10.3390/ijms21228648
Marusig D, Tombesi S. Abscisic Acid Mediates Drought and Salt Stress Responses in Vitis vinifera—A Review. International Journal of Molecular Sciences. 2020; 21(22):8648. https://doi.org/10.3390/ijms21228648
Chicago/Turabian StyleMarusig, Daniel, and Sergio Tombesi. 2020. "Abscisic Acid Mediates Drought and Salt Stress Responses in Vitis vinifera—A Review" International Journal of Molecular Sciences 21, no. 22: 8648. https://doi.org/10.3390/ijms21228648
APA StyleMarusig, D., & Tombesi, S. (2020). Abscisic Acid Mediates Drought and Salt Stress Responses in Vitis vinifera—A Review. International Journal of Molecular Sciences, 21(22), 8648. https://doi.org/10.3390/ijms21228648





