Isolation of Antimicrobial Genes from Oryza rufipogon Griff by Using a Bacillus subtilis Expression System with Potential Antimicrobial Activities
Abstract
:1. Introduction
2. Results
2.1. Screening of Antimicrobial Genes from O. Rufipogon cDNA Libraries by the B. subtilis System
2.2. Antimicrobial Activity of Resistance Genes against Pathogenic and Non-Pathogenic Bacteria
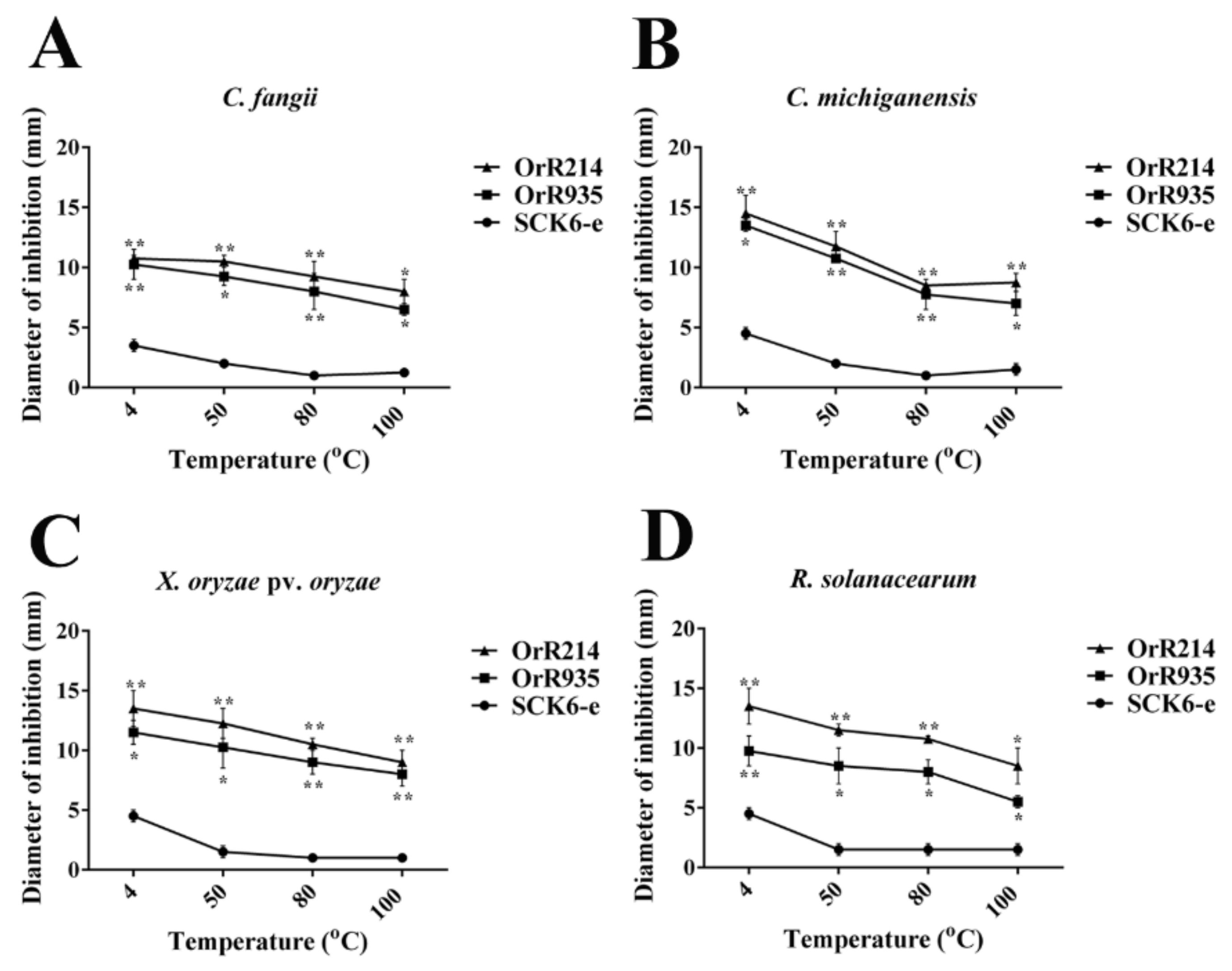
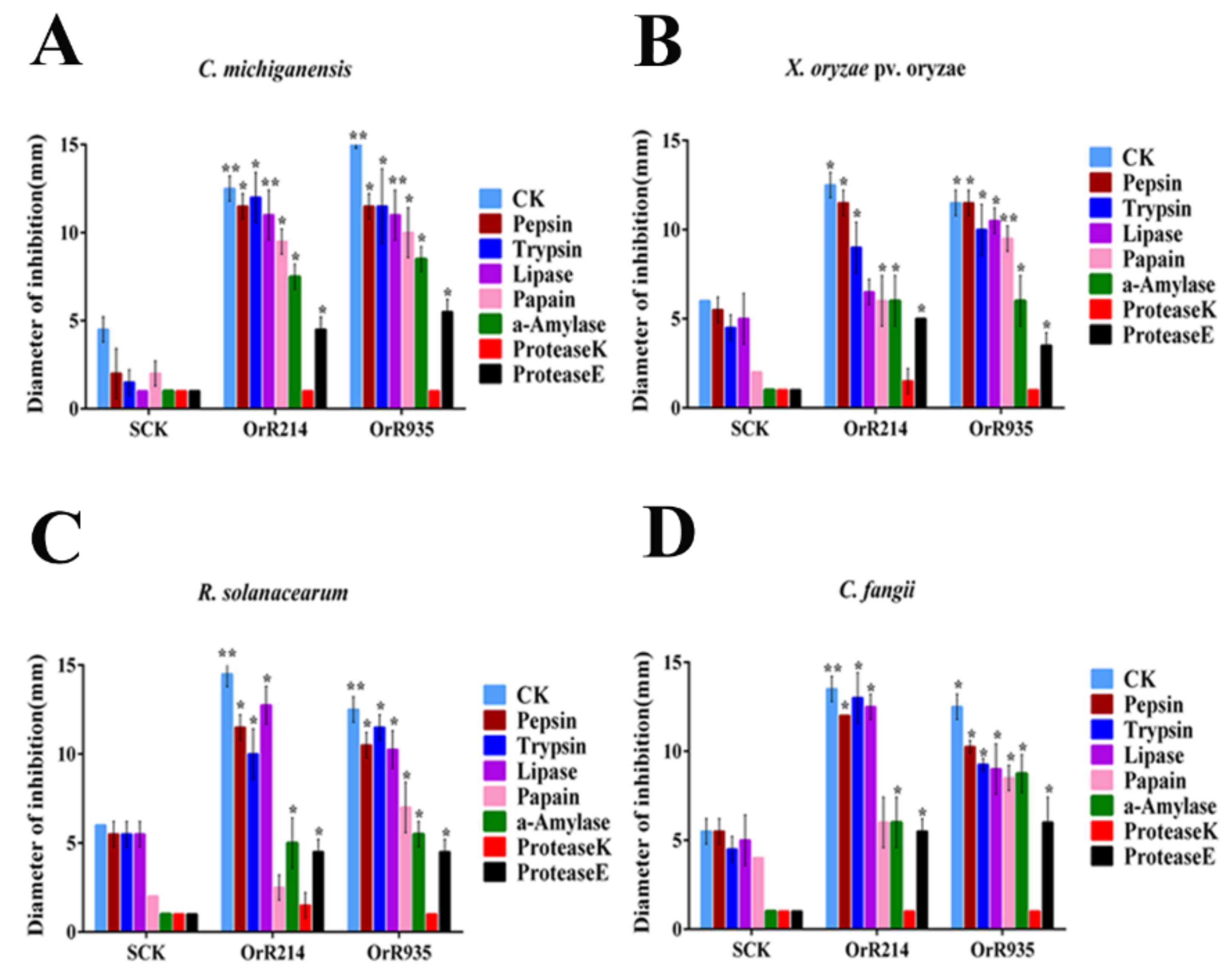
2.3. Stabilities of Antimicrobial Peptides Influenced by Temperature and Enzymes
2.4. SDS-PAGE Analysis Revealed the Peptide Expression and Sizes of OrR214 and OrR935 and Minimum Inhibitory Concentration (MIC) Assay
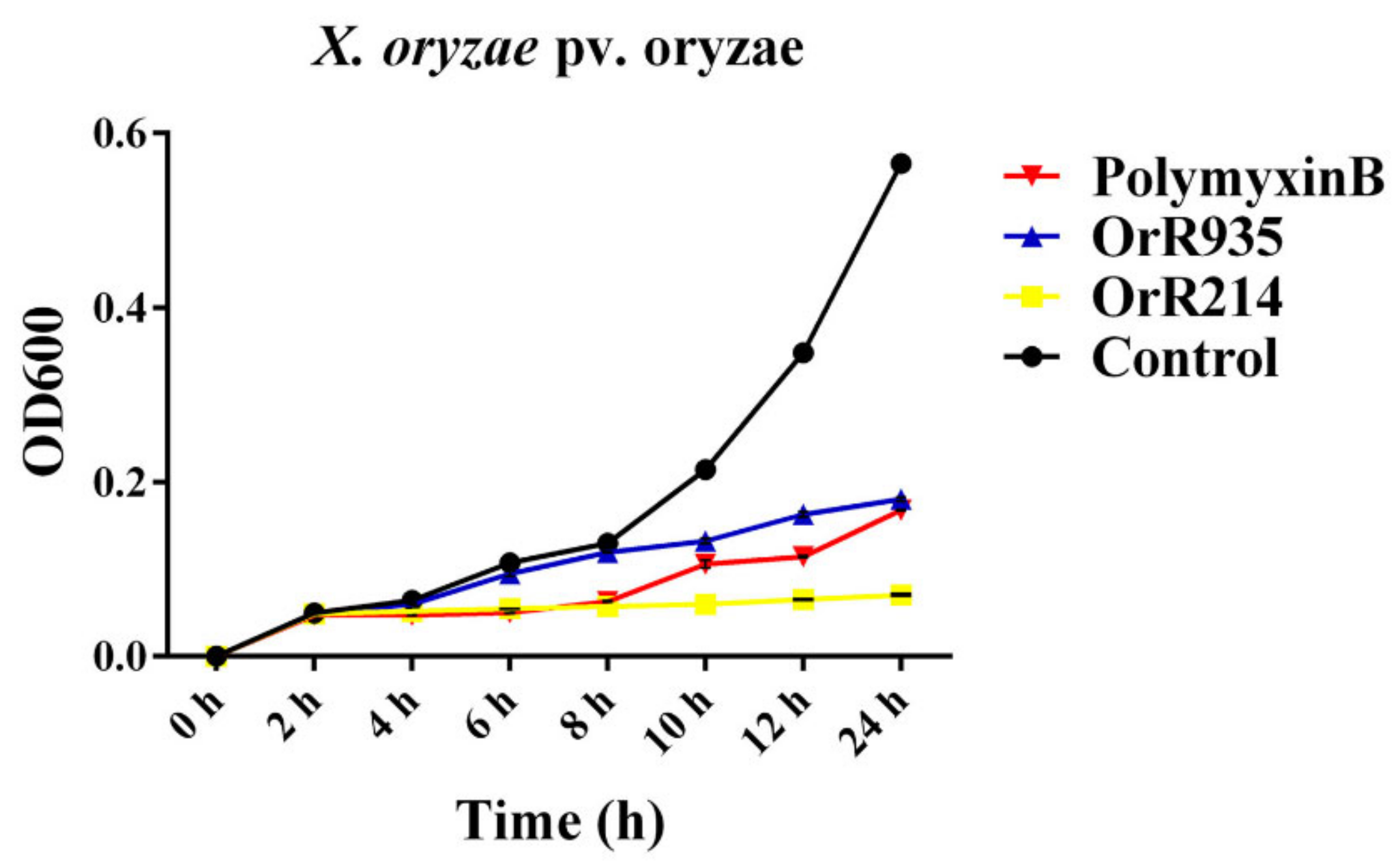
2.5. Time-Kill Curve Analysis
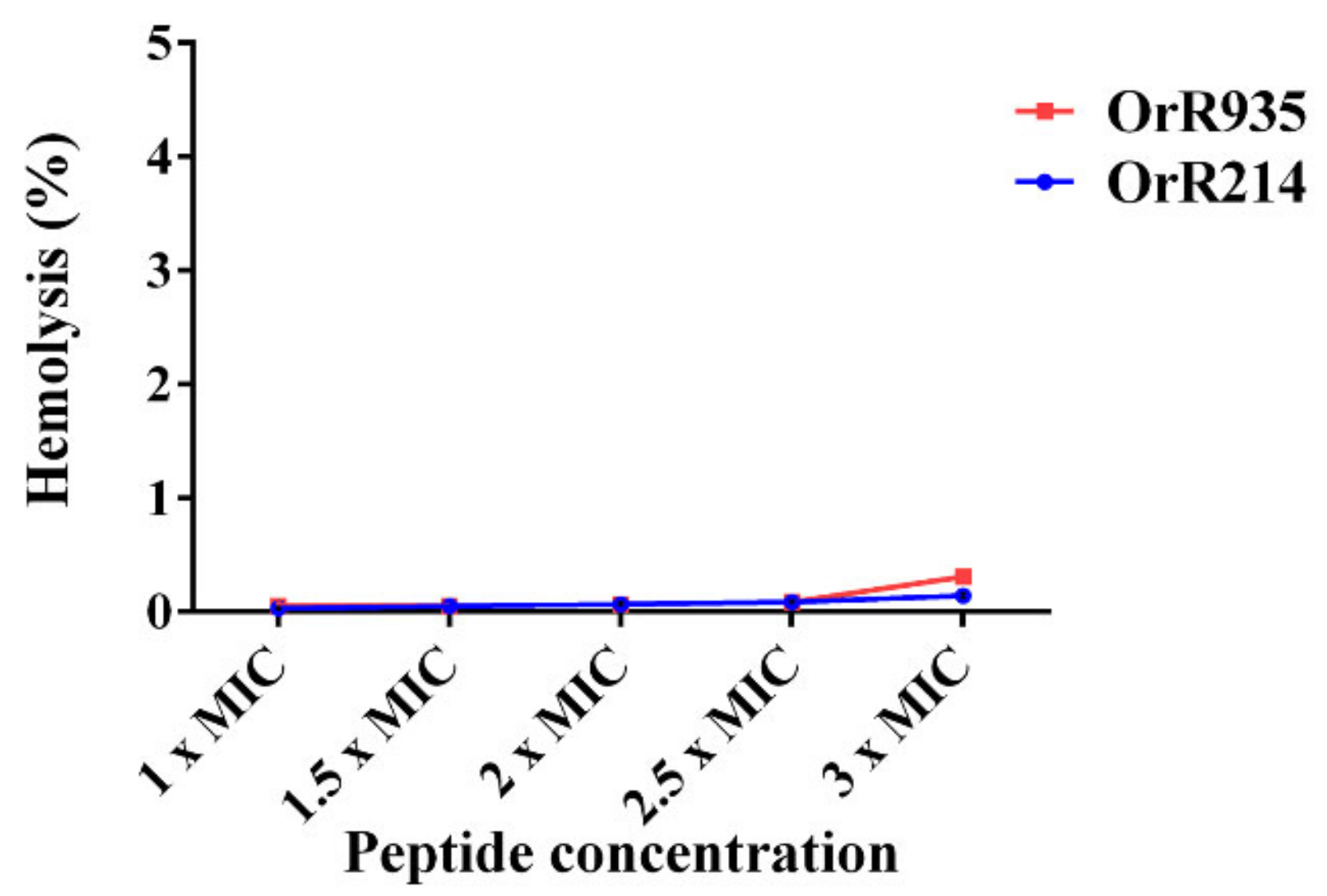
2.6. Assay of Hemolytic Activity of Antimicrobial Peptides Against Mammalian Cells
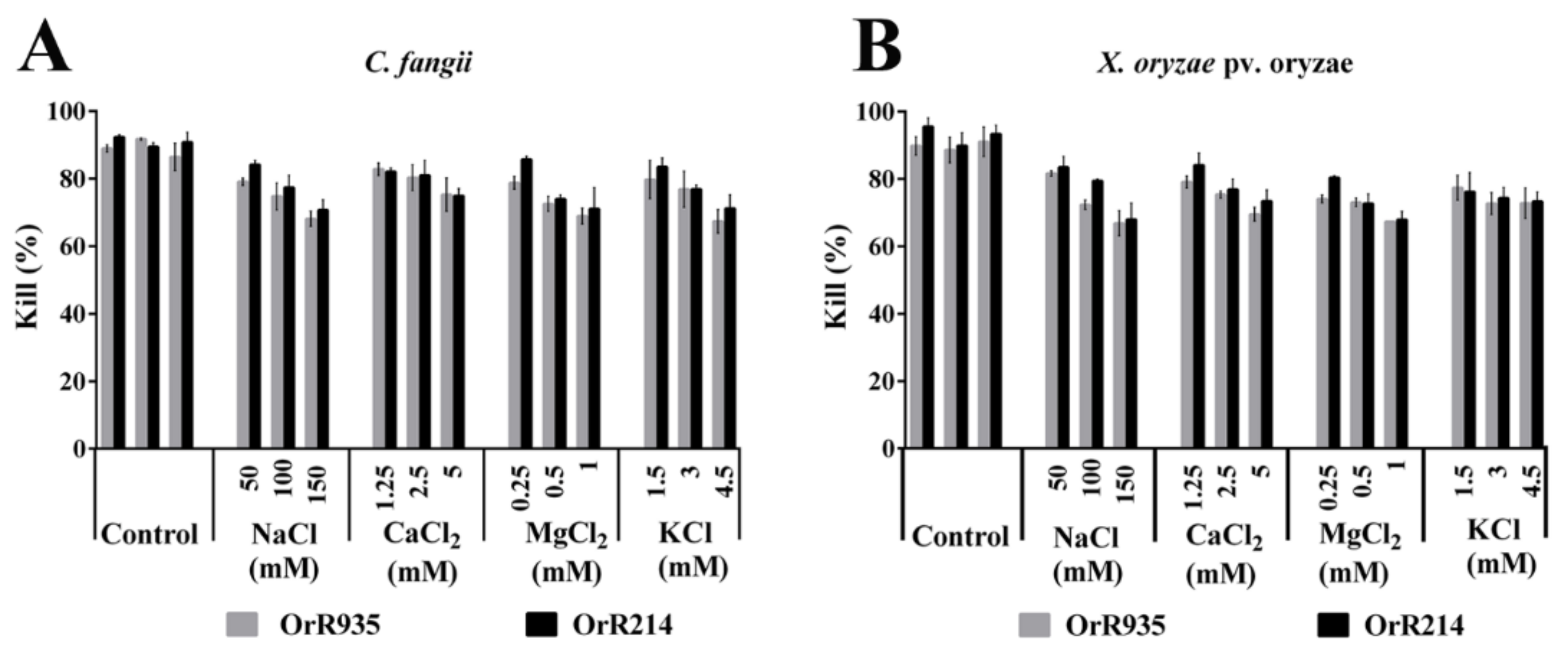
2.7. Salt Sensitivity Assay of the Antimicrobial Peptides OrR214 and OrR935
2.8. Antimicrobial Peptides OrR214 and OrR935 Induce Reactive Oxygen Species (ROS) Production
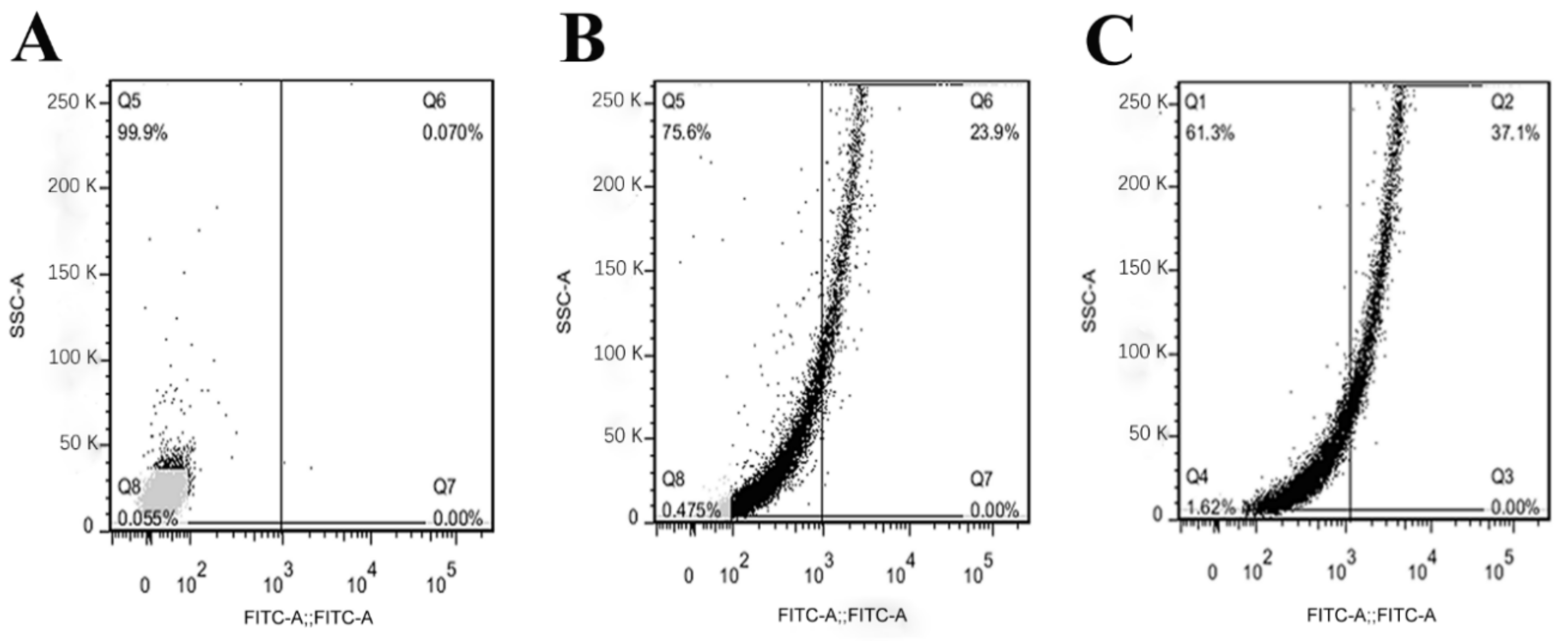
2.9. Effects of OrR214 and OrR935 Peptides on Cell Membrane Permeability
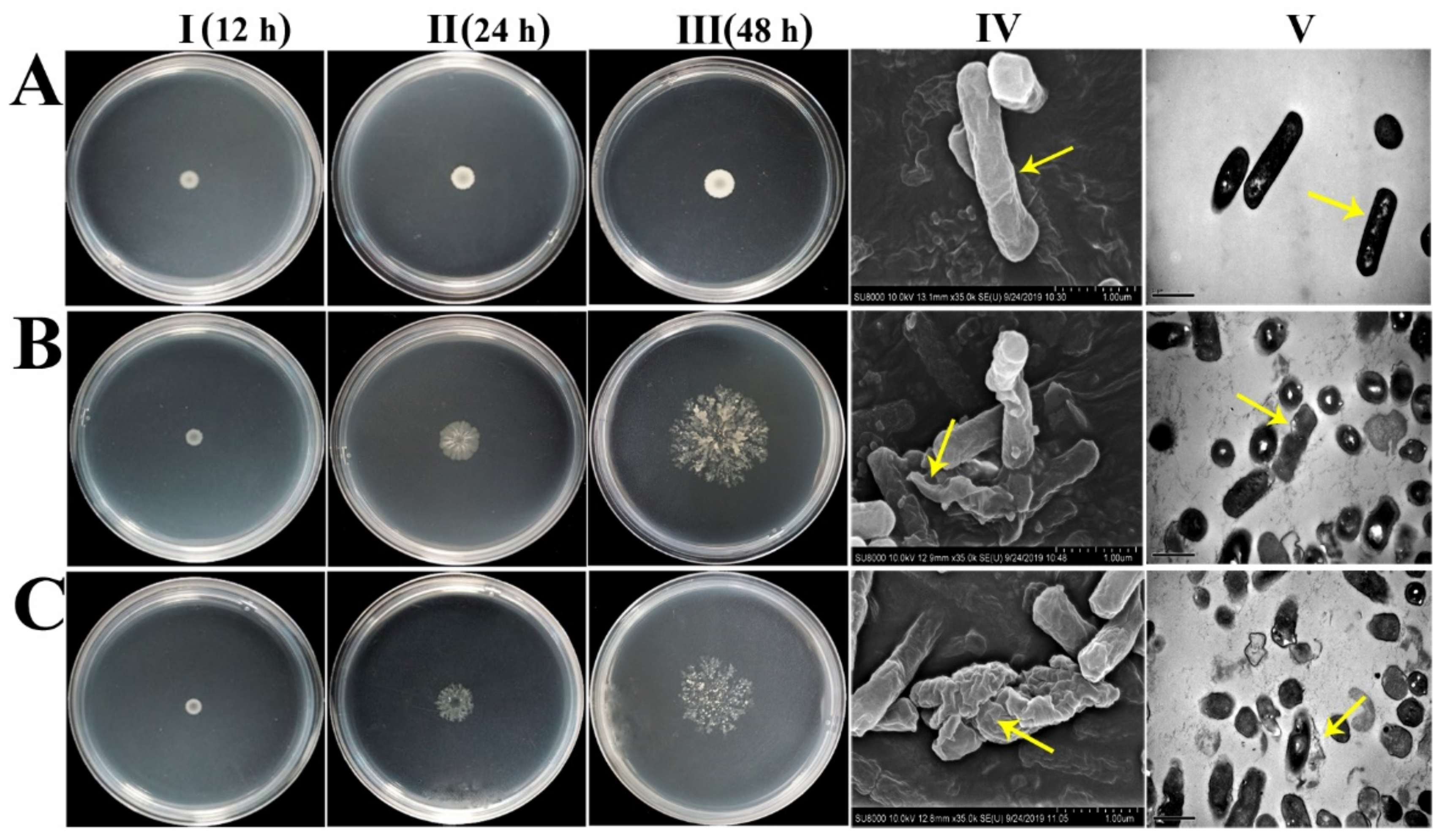
2.10. Antimicrobial Mechanisms of Peptides OrR214 and OrR935
3. Discussion
4. Materials and Methods
4.1. Maintenance of Plant and Pathogen Cultures
4.2. Construction of the cDNA Library and Quality Assessment
4.3. Candidate Resistance Gene Screening and Confirmation from cDNA Libraries
4.4. Expression of the Antimicrobial Peptide Against Pathogenic Bacteria and Thermal Stability
4.5. His-Tag Fusion Peptide Purification
4.6. Minimum Inhibitory Concentration (MIC) and Growth Time-Kill Curve Analyses
4.7. Salt Dependence Test
4.8. Hemolytic Assay
4.9. Reactive Oxygen Species (ROS) Determination Test
4.10. Detection of Cell Membrane Permeability
4.11. Electron Microscopy Analysis
4.12. Pathogenicity Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res. 2017, 36, 22–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Li, J.; Zhang, L.; Chen, X.; Yu, Y.-D.; Huang, H. Post-graphene 2D materials-based antimicrobial agents: Focus on fabrication strategies and biosafety assessments. J. Mater. Sci. 2020, 55, 7226–7246. [Google Scholar] [CrossRef]
- Bin Zaman, S.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A Review on Antibiotic Resistance: Alarm Bells are Ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Xu, H.; Wang, F. Research Advances of Antimicrobial Peptides and Applications in Food Industry and Agriculture. Curr. Protein Pept. Sci. 2010, 11, 264–273. [Google Scholar] [CrossRef]
- Islam, S.; Mahmud, S.; Sultana, R.; Dong, W. Identification and in silico molecular modelling study of newly isolated Bacillus subtilis SI-18 strain against S9 protein of Rhizoctonia solani. Arab. J. Chem. 2020. [Google Scholar] [CrossRef]
- Lin, P.; Wong, J.H.; Ng, T.B. A defensin with highly potent antipathogenic activities from the seeds of purple pole bean. Biosci. Rep. 2009, 30, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Fu, T.; Islam, S.; Ali, M.; Wu, J.; Dong, W. Two antimicrobial genes from Aegilops tauschii Cosson identified by the Bacillus subtilis expression system. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Jung, Y.-J.; Lee, S.-Y.; Moon, Y.-S.; Kang, K.-K. Enhanced resistance to bacterial and fungal pathogens by overexpression of a human cathelicidin antimicrobial peptide (hCAP18/LL-37) in Chinese cabbage. Plant Biotechnol. Rep. 2012, 6, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Qutb, A.M.; Wei, F.; Dong, W. Prediction and Characterization of Cationic Arginine-Rich Plant Antimicrobial Peptide SM-985 From Teosinte (Zea mays ssp. mexicana). Front. Microbiol. 2020, 11, 1353. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, L.; Wang, J.; Ma, Z.; Xu, W.; Li, J.; Shan, A. Characterization of antimicrobial activity and mechanisms of low amphipathic peptides with different α-helical propensity. Acta Biomater. 2015, 18, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Ganewatta, M.S.; Tang, C. Controlling macromolecular structures towards effective antimicrobial polymers. Polymer 2015, 63, A1–A29. [Google Scholar] [CrossRef]
- Liu, S.; Wang, J.; Feng, Y.; Ye, Q.; Wen, L.; Xu, G.; Zou, J. Effects of compound antimicrobial peptides on the growth performance, antioxidant and immune responses and disease resistance of grass carp (Ctenopharyngodon idellus). Fish Shellfish. Immunol. 2020, 107, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.F.N.; Marques, L.S.; Oliveira, J.T.; Lima, P.G.; Dias, L.P.; Neto, N.A.; Lopes, F.E.; Sousa, J.S.; Silva, A.F.; Caneiro, R.F.; et al. Synthetic antimicrobial peptides: From choice of the best sequences to action mechanisms. Biochimie 2020, 175, 132–145. [Google Scholar] [CrossRef]
- Hancock, R.E.; Haney, E.F.; Gill, E.E. The immunology of host defense peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef]
- Mishra, B.; Wang, X.; Lushnikova, T.; Zhang, Y.; Golla, R.M.; Narayana, J.L.; Wang, C.; McGuire, T.R.; Wang, G. Antibacterial, antifungal, anticancer activities and structural bioinformatics analysis of six naturally occurring temporins. Peptides 2018, 106, 9–20. [Google Scholar] [CrossRef]
- Li, C.; Zhu, J.; Wang, Y.; Chen, Y.; Song, L.; Zheng, W.; Li, J.; Yu, R. Antibacterial Activity of AI-Hemocidin 2, a Novel N-Terminal Peptide of Hemoglobin Purified from Arca inflata. Mar. Drugs 2017, 15, 205. [Google Scholar] [CrossRef]
- Lin, M.-C.; Lin, S.-B.; Lee, S.-C.; Lin, C.-C.; Hui, C.-F.; Chen, J.-Y. Antimicrobial peptide of an anti-lipopolysaccharide factor modulates of the inflammatory response in RAW264.7 cells. Peptides 2010, 31, 1262–1272. [Google Scholar] [CrossRef]
- Shenkarev, Z.; Balandin, S.V.; Trunov, K.I.; Paramonov, A.S.; Sukhanov, S.V.; Barsukov, L.I.; Arseniev, A.S.; Ovchinnikova, T.V. Molecular Mechanism of Action of β-Hairpin Antimicrobial Peptide Arenicin: Oligomeric Structure in Dodecylphosphocholine Micelles and Pore Formation in Planar Lipid Bilayers. Biochemistry 2011, 50, 6255–6265. [Google Scholar] [CrossRef]
- Jindal, H.M.; Zandi, K.; Ong, K.C.; Velayuthan, R.D.; Rasid, S.M.; Samudi Raju, C.; Sekaran, S.D. Mechanisms of action and in vivo antibacterial efficacy assessment of five novel hybrid peptides derived from Indolicidin and Ranalexin against Streptococcus pneumoniae. PeerJ 2017, 5, e3887. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Yan, J.; Liu, X.; Zhang, J.; Chen, R.; Zhang, B.; Dang, W.; Zhang, W.; Kai, M.; Song, J.; et al. Novel cytotoxity exhibition mode of polybia-CP, a novel antimicrobial peptide from the venom of the social wasp Polybia paulista. Toxicology 2011, 288, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Maróti, G.; Kereszt, A.; Kondorosi, E.; Mergaert, P. Natural roles of antimicrobial peptides in microbes, plants and animals. Res. Microbiol. 2011, 162, 363–374. [Google Scholar] [CrossRef]
- Oh, R.; Lee, M.J.; Kim, Y.-O.; Nam, B.-H.; Kong, H.J.; Kim, J.-W.; Park, J.-Y.; Seo, J.-K.; Kim, D.-G. Purification and characterization of an antimicrobial peptide mytichitin-chitin binding domain from the hard-shelled mussel, Mytilus coruscus. Fish Shellfish Immunol. 2018, 83, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Oh, R.; Lee, M.J.; Kim, Y.-O.; Nam, B.-H.; Kong, H.J.; Kim, J.-W.; Park, J.-Y.; Seo, J.-K.; Kim, D.-G. Myticusin-beta, antimicrobial peptide from the marine bivalve, Mytilus coruscus. Fish Shellfish Immunol. 2020, 99, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Ladram, A. Antimicrobial peptides from frog skin biodiversity and therapeutic promises. Front. Biosci. 2016, 21, 1341–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mygind, P.H.; Fischer, R.L.; Schnorr, K.M.; Hansen, M.T.; Sönksen, C.P.; Ludvigsen, S.; Raventós, D.; Buskov, S.; Christensen, B.; De Maria, L.; et al. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nat. Cell Biol. 2005, 437, 975–980. [Google Scholar] [CrossRef]
- Kong, X.; Yang, M.; Abbas, H.M.K.; Wu, J.; Li, M.; Dong, W. Antimicrobial genes from Allium sativum and Pinellia ternata revealed by a Bacillus subtilis expression system. Sci. Rep. 2018, 8, 14514. [Google Scholar] [CrossRef]
- Fjell, C.D.; Jenssen, H.; Cheung, W.A.; Hancock, R.E.W.; Cherkasov, A. Optimization of Antibacterial Peptides by Genetic Algorithms and Cheminformatics. Chem. Biol. Drug Des. 2010, 77, 48–56. [Google Scholar] [CrossRef]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef] [Green Version]
- Ling, J.; Liu, Z.; Tong, T.; Fan, H.; Zhang, D.; Chen, H.; Guo, A. Construction and Eukaryotic Expression of Recombinant Plasmid Encoding Fusion Protein of Goat Complement C3d and Foot-and-Mouth Disease Virus VP1. Chin. J. Biotechnol. 2008, 24, 209–213. [Google Scholar] [CrossRef]
- Groisillier, A.; Hervé, C.; Jeudy, A.; Rebuffet, E.; Pluchon, P.F.; Chevolot, Y.; Flament, D.; Geslin, C.; Morgado, I.; Power, D.M.; et al. MARINE-EXPRESS: Taking advantage of high throughput cloning and expression strategies for the post-genomic analysis of marine organisms. Microb. Cell Factories 2010, 9, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khow, O.; Suntrarachun, S. Strategies for production of active eukaryotic proteins in bacterial expression system. Asian Pac. J. Trop. Biomed. 2012, 2, 159–162. [Google Scholar] [CrossRef] [Green Version]
- Wenzel, M.; Müller, A.; Siemann-Herzberg, M.; Altenbuchner, J. Self-Inducible Bacillus subtilis Expression System for Reliable and Inexpensive Protein Production by High-Cell-Density Fermentation. Appl. Environ. Microbiol. 2011, 77, 6419–6425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Feng, Q.; Lu, H.; Li, Y.; Wang, A.; Tian, Q.; Zhan, Q.; Lu, Y.; Zhang, L.; Huang, T.; et al. Pan-genome analysis highlights the extent of genomic variation in cultivated and wild rice. Nat. Genet. 2018, 50, 278–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Z.; Li, B.; Chen, J.; Lu, B.-R. Genetic diversity and conservation of common wild rice (Oryza rufipogon) in China. Plant Species Biol. 2005, 20, 83–92. [Google Scholar] [CrossRef]
- Tian, F.; Li, D.J.; Fu, Q.; Zhu, Z.F.; Fu, Y.C.; Wang, X.K.; Sun, C. Construction of introgression lines carrying wild rice (Oryza rufipogon Griff.) segments in cultivated rice (Oryza sativa L.) background and characterization of introgressed segments associated with yield-related traits. Theor. Appl. Genet. 2006, 112, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, P.; Cui, F.; Zhang, F.; Luo, X.; Xie, J. Transcriptome Analysis of Salt Stress Responsiveness in the Seedlings of Dongxiang Wild Rice (Oryza rufipogon Griff.). PLoS ONE 2016, 11, e0146242. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Ghouri, F.; Yu, H.; Li, X.; Yu, S.; Shahid, M.Q.; Liu, X. Genome wide re-sequencing of newly developed Rice Lines from common wild rice (Oryza rufipogon Griff.) for the identification of NBS-LRR genes. PLoS ONE 2017, 12, e0180662. [Google Scholar] [CrossRef] [Green Version]
- Zhai, C.; Dong, Z.; He, X.; Zeng, X.; Wang, L.; Lin, F.; Yuan, B.; Pan, Q. The isolation and characterization of Pik, a rice blast resistance gene which emerged after rice domestication. New Phytol. 2010, 189, 321–334. [Google Scholar] [CrossRef]
- Das, A.; Soubam, D.; Singh, P.K.; Thakur, S.; Sharma, T.R. A novel blast resistance gene, Pi54rh cloned from wild species of rice, Oryza rhizomatis confers broad spectrum resistance to Magnaporthe oryzae. Funct. Integr. Genom. 2012, 12, 215–228. [Google Scholar] [CrossRef]
- Hong-Jing; Xiang-Hua; Jing-Hua; Xiao; Shi-Ping; Wang. Ortholog Alleles at Xa3/Xa26 Locus Confer Conserved Race-Specific Resistance against Xanthomonas oryzae in Rice. Mol. Plant 2012, 5, 281–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, Q.; Xu, X.; Shang, J.; Jiang, G.; Pang, Z.; Zhou, Z.; Wang, J.; Liu, Y.; Li, T.; Li, X.; et al. Functional Analysis of Pid3-A4, an Ortholog of Rice Blast Resistance Gene Pid3 Revealed by Allele Mining in Common Wild Rice. Phytopathology 2013, 103, 594–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Abbas, H.M.K.; Li, J.; Yuan, Y.; Liu, Y.; Wang, G.; Dong, W. Cell Membrane-Interrupting Antimicrobial Peptides from Isatis indigotica Fortune Isolated by a Bacillus subtilis Expression System. Biomolecules 2019, 10, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maria-Neto, S.; De Almeida, K.C.; Macedo, M.L.R.; Franco, O.L. Understanding bacterial resistance to antimicrobial peptides: From the surface to deep inside. Biochim. Biophys. Acta Biomembr. 2015, 1848, 3078–3088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, A.T.Y.; Gellatly, S.L.; Hancock, R.E. Multifunctional cationic host defence peptides and their clinical applications. Cell. Mol. Life Sci. 2011, 68, 2161–2176. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.M.; Lim, B.L. Co-expression of a prophage system and a plasmid system in Bacillus subtilis. Protein Expr. Purif. 2003, 32, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Sun, J.; Zhou, M.; Zhou, J.; Lao, X.; Zheng, S.; Xu, H. DRAMP: A comprehensive data repository of antimicrobial peptides. Sci. Rep. 2016, 6, 24482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazam, P.K.; Goyal, R.; Ramakrishnan, V. Peptide based antimicrobials: Design strategies and therapeutic potential. Prog. Biophys. Mol. Biol. 2019, 142, 10–22. [Google Scholar] [CrossRef]
- Wimley, W.C. Describing the Mechanism of Antimicrobial Peptide Action with the Interfacial Activity Model. ACS Chem. Biol. 2010, 5, 905–917. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [Green Version]
- Zucca, M.; Scutera, S.; Savoia, D. New Antimicrobial Frontiers. Mini Rev. Med. Chem. 2011, 11, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Huang, J.; Chen, Y. Alpha-helical cationic antimicrobial peptides: Relationships of structure and function. Protein Cell 2010, 1, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Shan, A.; Wang, J.; Shang, L.; Akhtar, M.U.; Wang, Z.; Shi, B.; Feng, X.; Shan, A. Short, symmetric-helical peptides have narrow-spectrum activity with low resistance potential and high selectivity. Biomater. Sci. 2019, 7, 2394–2409. [Google Scholar] [CrossRef]
- Mai, J.; Tian, X.-L.; Gallant, J.W.; Merkley, N.; Biswas, Z.; Syvitski, R.; Douglas, S.E.; Ling, J.; Li, Y.-H. A Novel Target-Specific, Salt-Resistant Antimicrobial Peptide against the Cariogenic Pathogen Streptococcus mutans. Antimicrob. Agents Chemother. 2011, 55, 5205–5213. [Google Scholar] [CrossRef] [Green Version]
- Salvatore, F.; Galdiero, S.; Cantisani, M.; Di Noto, R.; Vitiello, M.; Galdiero, M.; Naclerio, G.; Cassiman, J.-J.; Pedone, C.; Castaldo, G.; et al. Novel Synthetic, Salt-Resistant Analogs of Human Beta-Defensins 1 and 3 Endowed with Enhanced Antimicrobial Activity. Antimicrob. Agents Chemother. 2010, 54, 2312–2322. [Google Scholar] [CrossRef] [Green Version]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A Common Mechanism of Cellular Death Induced by Bactericidal Antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef] [Green Version]
- Mahl, C.D.; Behling, C.S.; Hackenhaar, F.S.; de Carvalho e Silva, M.N.; Putti, J.; Salomon, T.B.; Alves, S.H.; Fuentefria, A.; Benfato, M.S. Induction of ROS generation by fluconazole in Candida glabrata: Activation of antioxidant enzymes and oxidative DNA damage. Diagn. Microbiol. Infect. Dis. 2015, 82, 203–208. [Google Scholar] [CrossRef]
- Gautam, A.; Chaudhary, K.; Singh, S.; Joshi, A.; Anand, P.; Tuknait, A.; Mathur, D.; Varshney, G.C.; Raghava, G. Hemolytik: A database of experimentally determined hemolytic and non-hemolytic peptides. Nucleic Acids Res. 2013, 42, D444–D449. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-T.; Neerman, M.F.; Parrish, A.R.; Simanek, E.E. Cytotoxicity, Hemolysis, and Acute in Vivo Toxicity of Dendrimers Based on Melamine, Candidate Vehicles for Drug Delivery. J. Am. Chem. Soc. 2004, 126, 10044–10048. [Google Scholar] [CrossRef]
- Ling, P.; Wang, M.; Chen, X.; Garland-Campbell, K. Construction and characterization of a full-length cDNA library for the wheat stripe rust pathogen (Puccinia striiformis f. sp. tritici). BMC Genom. 2007, 8, 145. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.F.; Gai, J.Y.; Zhu, Y.L.; Chen, G.; Wei, G.P.; Wang, C.; Liu, Q.Q. Construction and Characterization of Full-Length cDNA Library and Expressed Sequence Tags Analysis in Developing Seeds of Vegetable Soybean. Hortic. Environ. Biotechnol. 2009, 50, 51–56. [Google Scholar]
- Saporito, P.; Mojsoska, B.; Olesen, A.L.; Jenssen, H. Antibacterial mechanisms of GN-2 derived peptides and peptoids against Escherichia coli. Biopolymers 2019, 110, e23275. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antimicrobial mechanism of clove oil on Listeria monocytogenes. Food Control. 2018, 94, 140–146. [Google Scholar] [CrossRef]
- Guangming, S.; Yang, Y.; Yuan, Q.; Shi, G.; Wu, L.; Lou, Z.; Huo, R.; Wu, H.; Borriss, R.; Gao, X. Bacillomycin D Produced by Bacillus amyloliquefaciens Is Involved in the Antagonistic Interaction with the Plant-Pathogenic Fungus Fusarium graminearum. Appl. Environ. Microbiol. 2017, 83, e01075–e01117. [Google Scholar] [CrossRef] [Green Version]
- Bie, X.; Wei, D.; Yan, P.; Zhu, X.; Shan, A.; Bi, Z. Characterization of cell selectivity, physiological stability and endotoxin neutralization capabilities of α-helix-based peptide amphiphiles. Biomaterials 2015, 52, 517–530. [Google Scholar] [CrossRef]
- Zhu, X.; Dong, N.; Wang, Z.; Ma, Z.; Zhang, L.; Ma, Q.; Shan, A. Design of imperfectly amphipathic α-helical antimicrobial peptides with enhanced cell selectivity. Acta Biomater. 2014, 10, 244–257. [Google Scholar] [CrossRef]
- Maurya, I.K.; Pathak, S.; Sharma, M.; Sanwal, H.; Chaudhary, P.; Tupe, S.G.; Deshpande, M.; Chauhan, V.S.; Prasad, R. Antifungal activity of novel synthetic peptides by accumulation of reactive oxygen species (ROS) and disruption of cell wall against Candida albicans. Peptides 2011, 32, 1732–1740. [Google Scholar] [CrossRef]
- Dong, N.; Ma, Q.; Shan, A.; Lv, Y.; Hu, W.; Gu, Y.; Li, Y. Strand Length-Dependent Antimicrobial Activity and Membrane-Active Mechanism of Arginine- and Valine-Rich β-Hairpin-Like Antimicrobial Peptides. Antimicrob. Agents Chemother. 2012, 56, 2994–3003. [Google Scholar] [CrossRef] [Green Version]
- Mares, D. Electron microscopy of Microsporum cookei after in vitro treatment with protoanemonin: A combined SEM and TEM study. Mycopathology 1989, 108, 37–46. [Google Scholar] [CrossRef]
- Ke, Y.; Hui, S.; Yuan, M. Xanthomonas oryzae pv. oryzae Inoculation and Growth Rate on Rice by Leaf Clipping Method. Bio Protoc. 2017, 7, 2568. [Google Scholar] [CrossRef]



| Strain | MIC (µM) | ||
|---|---|---|---|
| Polymyxin B | OrR214 | OrR935 | |
| C. fangi | 25.0 | 10.7 | 37.7 |
| C. michiganensis | 12.5 | 10.5 | 34.6 |
| X. oryzae pv. oryzae | 5.0 | 10.1 | 44.0 |
| R. solanacearum | 7.5 | 8.1 | 33 |
| X. oryzae pv. oryzicola | 10.0 | 7.7 | 31.4 |
| B. subtilis (168) | 15.0 | 8.3 | 36.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Islam, S.; Guo, P.; Hu, X.; Dong, W. Isolation of Antimicrobial Genes from Oryza rufipogon Griff by Using a Bacillus subtilis Expression System with Potential Antimicrobial Activities. Int. J. Mol. Sci. 2020, 21, 8722. https://doi.org/10.3390/ijms21228722
Li J, Islam S, Guo P, Hu X, Dong W. Isolation of Antimicrobial Genes from Oryza rufipogon Griff by Using a Bacillus subtilis Expression System with Potential Antimicrobial Activities. International Journal of Molecular Sciences. 2020; 21(22):8722. https://doi.org/10.3390/ijms21228722
Chicago/Turabian StyleLi, Jiale, Samiul Islam, Pengfei Guo, Xiaoqing Hu, and Wubei Dong. 2020. "Isolation of Antimicrobial Genes from Oryza rufipogon Griff by Using a Bacillus subtilis Expression System with Potential Antimicrobial Activities" International Journal of Molecular Sciences 21, no. 22: 8722. https://doi.org/10.3390/ijms21228722
APA StyleLi, J., Islam, S., Guo, P., Hu, X., & Dong, W. (2020). Isolation of Antimicrobial Genes from Oryza rufipogon Griff by Using a Bacillus subtilis Expression System with Potential Antimicrobial Activities. International Journal of Molecular Sciences, 21(22), 8722. https://doi.org/10.3390/ijms21228722






