Abstract
Tumours were recently revealed to undergo a phylostratic and phenotypic shift to unicellularity. As well, aggressive tumours are characterized by an increased proportion of polyploid cells. In order to investigate a possible shared causation of these two features, we performed a comparative phylostratigraphic analysis of ploidy-related genes, obtained from transcriptomic data for polyploid and diploid human and mouse tissues using pairwise cross-species transcriptome comparison and principal component analysis. Our results indicate that polyploidy shifts the evolutionary age balance of the expressed genes from the late metazoan phylostrata towards the upregulation of unicellular and early metazoan phylostrata. The up-regulation of unicellular metabolic and drug-resistance pathways and the downregulation of pathways related to circadian clock were identified. This evolutionary shift was associated with the enrichment of ploidy with bivalent genes (p < 10−16). The protein interactome of activated bivalent genes revealed the increase of the connectivity of unicellulars and (early) multicellulars, while circadian regulators were depressed. The mutual polyploidy-c-MYC-bivalent genes-associated protein network was organized by gene-hubs engaged in both embryonic development and metastatic cancer including driver (proto)-oncogenes of viral origin. Our data suggest that, in cancer, the atavistic shift goes hand-in-hand with polyploidy and is driven by epigenetic mechanisms impinging on development-related bivalent genes.
1. Introduction
Whole-genome duplications (WGD) and recurrent polyploidization, providing a source for gene divergence and adaptability to environmental changes, are central in the evolution of biodiversity [1,2,3]. Polyploid cells are also present in the tissues of vertebrates: in humans and other mammalians, cells with multiplied genomes appear during normal organogenesis of definitive and provisional organs (placenta, heart, brain, liver, skin, blood) [4,5,6,7,8,9,10]. Polyploidy also arises in response to stress, wounding, and in cancer [11,12,13,14,15,16,17,18].
The relationship between polyploidy and stemness, both found as typical features of aggressive tumours [19,20,21,22], may be associated with the re-activation of evolutionarily ancient programs. The observations show that malignant cells often acquire the phenotypes and reproductive behaviour of unicellular organisms through transient polyploidy “life-cycle”, which is reciprocally linked with a cell cycle [23,24,25,26,27]. This behaviour is characteristic for cancer cells resistant to therapeutic treatments [28].
The atavistic theory of oncogenesis suggests that cancer is a reversal from a multicellular to a unicellular state [7,29,30,31,32,33]. The two giant clusters were revealed in the human coexpression gene network (also based on bulk tissue analysis): a widely expressed cluster enriched in genes of unicellular origin enriching the cancer tissues and the other giant cluster of multicellular genes [34,35]. Phylostratigraphic tracking of the genes involved in cancer [36] suggests their link to the emergence of multicellularity in metazoan, many cancer genes have a viral origin and even the typical somatic mutations of the cancer genes can be traced as ancestral [37].
In turn, the studies of Trigos and colleagues [38,39] also showed that the genes of unicellular origin are overexpressed in human cancers as compared to their normal counterpart tissues, whereas the genes appearing at multicellular stages of evolution were downregulated. Moreover, in tumours, the interaction between the unicllularity and multicellularity gene networks is weakened [38,40].
The upregulation of the unicellular giant cluster in cancer cells was shown in the single-cells transcriptomes of various cancer types and in invasive as compared with non-invasive cancer [35,41]. These two clusters can be defined as “echoing” a proper dynamical attractor given, strictly speaking, they are not mutually exclusive (both in cancer and normal cell genes from both clusters are activated). The prevalence of ancient unicellular state of cancer cells with respect to normal tissue corresponds to a partial “going back” to the more ancient unicellular pattern preceding the rise of multicellularity. This implies we do not expect that cancer cells become ’identical to unicellular organism’ but that lose some phenotypic characters essential for the existence of an organized tissue like contact inhibition, the crucial role played by shape changes in cancer development is consistent with this view [42,43].
The epigenetic mechanisms favouring transition to unicellularity and cancerogenesis and, thus, the proposed evolution reversal of human cancer cells remain unclear. Since oncogenesis is frequently associated with polyploidy [2,26,44,45,46,47], we suggested the involvement of polyploidy in this process and addressed here the phylostratigraphy and protein networks of the genes differentially expressed in polyploid versus diploid tissues.
We choose this approach, because mammalian homologous tissues differ by cell ploidy levels [1,2,5,8,12]. Some species have predominantly polyploid heart and diploid liver (pig and primates), whereas others possess mainly polyploid liver and predominantly diploid heart (rodents) [1,5,8]. Here, we rely on this fact to generate a balanced factorial design with a criss-cross distribution of ploidy/tissue across human and mouse that allows to separate ploidy effect from both tissue and species influences (see Methods).
To promote understanding of the epigenetic nature of ploidy-associated gene regulation, we also investigated how polyploidy influences the expression of bivalent genes. Bivalent genes are characterized by epigenetic ambiguity, bearing in their promoters or enhancers two opposite epigenetic modifications of the histone H3, the repressing H3K27me3 and the activating H3K4me3, poised for transcription but capable for its rapid activation. These poised genes were established in embryonic stem cells [48,49] and are the main players in the early and post-implantation development and cell fate change. Changes of bivalent chromatin coincide with the increased expression of developmental genes in cancer and are also likely involved there in the epigenetic changes [50,51,52,53,54,55]. On a more general ground the notion of cancer as ‘development gone awry’ supports the focus on such genes [56].
The obtained results indicate that polyploidy activates unicellular metabolic pathways and ancient programs of development related to carcinogenesis. This evolutionary retour is accompanied by the activation of bivalent genes and deregulation of circadian rhythms. Altogether, these results provide evidence that polyploidy is an important driver of epigenetic changes linked to cancer that may reorganize gene regulatory networks.
2. Results
2.1. Polyploidy Causes Transition of Gene Phylostratic Balance towards Evolutionary Old Unicellular Phylostrata
To find out whether polyploidy affects the expression of genes of various evolutionary ages, we first applied pairwise cross-species transcriptome comparison to polyploid vs. diploid organs in human and mouse (human heart vs. mouse heart and mouse liver vs. human liver). Then we evaluated the effects of polyploidy at various thresholds of the expression difference. This evaluation gave 584 upregulated and 711 downregulated genes at two-fold difference and 1028 upregulated and 887 downregulated genes at a 1.3-fold difference (Tables S1 and S2).
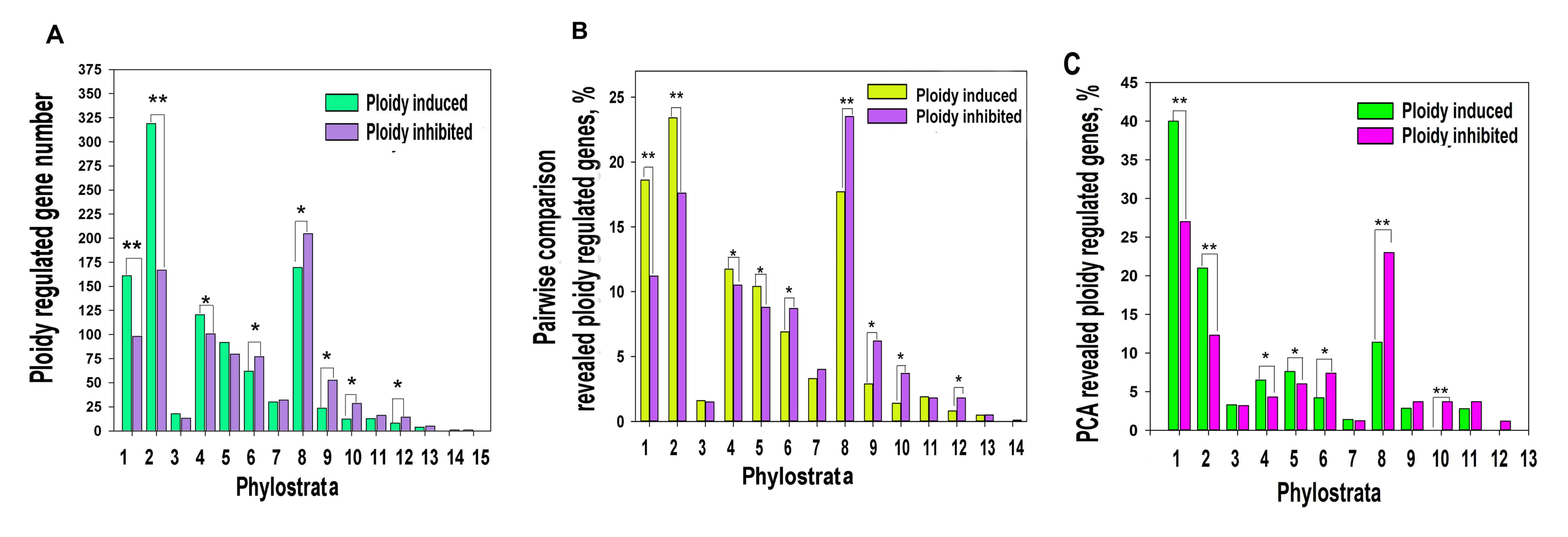
Phylostratic distribution of ploidy-associated genes was analysed by grouping genes according to their age of evolutionary origin taken from [38]. Our data indicated that polyploidy is associated with the upregulation of ancient genes originating in unicellular ancestors (1-3 phylostrata) and the downregulation of genes starting with phylostratum 6 (Bilateria) and onwards, while the early multicellularity strata 4–5 (Metazoa and Eumetazoa) did not reveal a clear difference (Figure 1A,B).
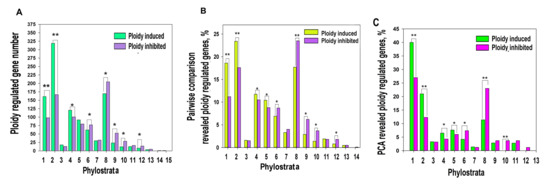
Figure 1.
Ploidy associated gene distribution across evolutionary phylostrata. Phylostratigraphy of ploidy associated genes revealed by pairwise cross-species (criss-cross) comparison (A,B) and by PCA (C). This figure illustrates that polyploidy shifts the gene age balance of expressed genes from metazoan phylostrata (6–16, Bilateria and later) towards unicellular phylostrata (1–3) via transitional early metazoan phylostrata (4–5) * p < 0.05 for the difference, ** p < 0.01 for the difference. Gene expression difference above two-fold. The phylostrata are as follows: 1—cellular organisms, 2—Eukaryota, 3—Opisthokonta, 4—Metazoa, 5—Eumetazoa, 6—Bilateria, 7—Chordata, 8—Euteleostomi, 9—Amniota, 10—Mammalia, 11—Ttheria, 12—Eutheria, 13—Euarchontoglires, 14—Catarrhini, 15—Homininae.
To verify this result with a well-known traditional approach, we applied principal component analysis (PCA). PCA identified 103 upregulated and 96 downregulated genes having above two standard deviations. Of these genes, 78 and 87 genes demonstrated a more than two-fold expression difference (Tables S1 and S2). With excluding the tissue-specific component, the phylostratic distribution of PCA-revealed genes also indicated the activation by polyploidy of evolutionarily conserved programs (genes from phylostrata 1–3) and the suppression of young programs maintaining multicellularity (phylostrata 6–13, Figure 1C). The phylostrata 4–5 were up-regulated.
2.2. Polyploidy Activates Recapitulation of Evolutionary Developmental Programs Associated with Carcinogenesis
To investigate the functional consequences of ploidy-associated phylostrata rearrangement at the gene module level, we performed gene module enrichment analysis for genes with more than two-fold expression difference between polyploid vs. diploid organs. The results are presented in Table S3. For unicellular phylostrata (1–3) the main effects of polyploidy were the induction of modules related to drug ABC pump and drug metabolism (hsa02010;GO:0017144), protein and ribosome synthesis (hsa01230;hsa03010), oxidation-reduction (GO:0055114) and energy metabolism (both aerobic respiration and carbohydrate metabolism (GO:0019752; GO:0008152; GO:0044262; hsa00051). This picture is seen from the higher significance of enrichment and the larger gene number for upregulated genes than for downregulated ones. For the up-regulated by polyploidy early metazoan phylostrata (4–5) our data reveal the upregulation of modules related to embryonic development (GO:0009790), stem cell commitment (GO:0045165), pluripotency (hsa04550) and Kegg pathways of carcinogenesis (hsa05200; hsa05205). The phylostrata 6–8 were down-regulated by polyploidy. They are involved in the development of the multicellular organism complexity. The downregulated genes of the 6–8 strata were mostly enriched in biological processes related to immunity (GO:0002682), inflammation (GO:0050727) and communication (GO:0010646) that are the specific biological features of multi-cellularity. Consistently, multicellular phylostrata (10–15, i.e., Mammalia and later) were not enriched for gene modules depending on polyploidy.
The results of functional enrichment of PCA-ploidy-revealed genes for old phylostrata were consistent with the results of pair-wise cross-species comparison (Table S4). Thus, the carcinogenesis pathways favoured by polyploidy appear in the early metazoan (strata 4–5) in one pack with embryogenesis and cell fate change (stemness commitment), associated with asexual reproduction.
Similar changes in the general phylostratigraphic landscape shifted towards unicellularity found here for polyploidy were previously shown for tumorigenesis as such [38]. The authors also paid attention to the enrichment of the intermediate between unicellularity and multicellularity phylostrata with cancer driver genes [40]. In comparison with our data, this similarity suggests that polyploidy may favour the epigenetic evolutionary shift of normal cells to cancer. The interactome of c-MYC proto-oncogene provides a useful link to verify this suggestion because c-MYC in an important regulator of both ploidy and cancer.
2.3. c-MYC Induction Drives Polyploidy-Associated Transcriptomic Changes towards Unicellularity
c-MYC is a regulator of the normal cell cycle. However, when over-expressed it disjoins replication from cell division and favours polyploidy [57,58]. Moreover, while the overexpressed non-mutant (often amplified) c-MYC is a powerful reprogramming factor and oncogene, its downregulation in transgenic mice causes tumour regression [59,60,61,62].
The pleiotropic c-MYC is capable to drive the opposite processes, proliferation versus apoptosis and is acting as a master activator of the bivalent genes involved in the epigenetic regulation of development [60,63,64,65].
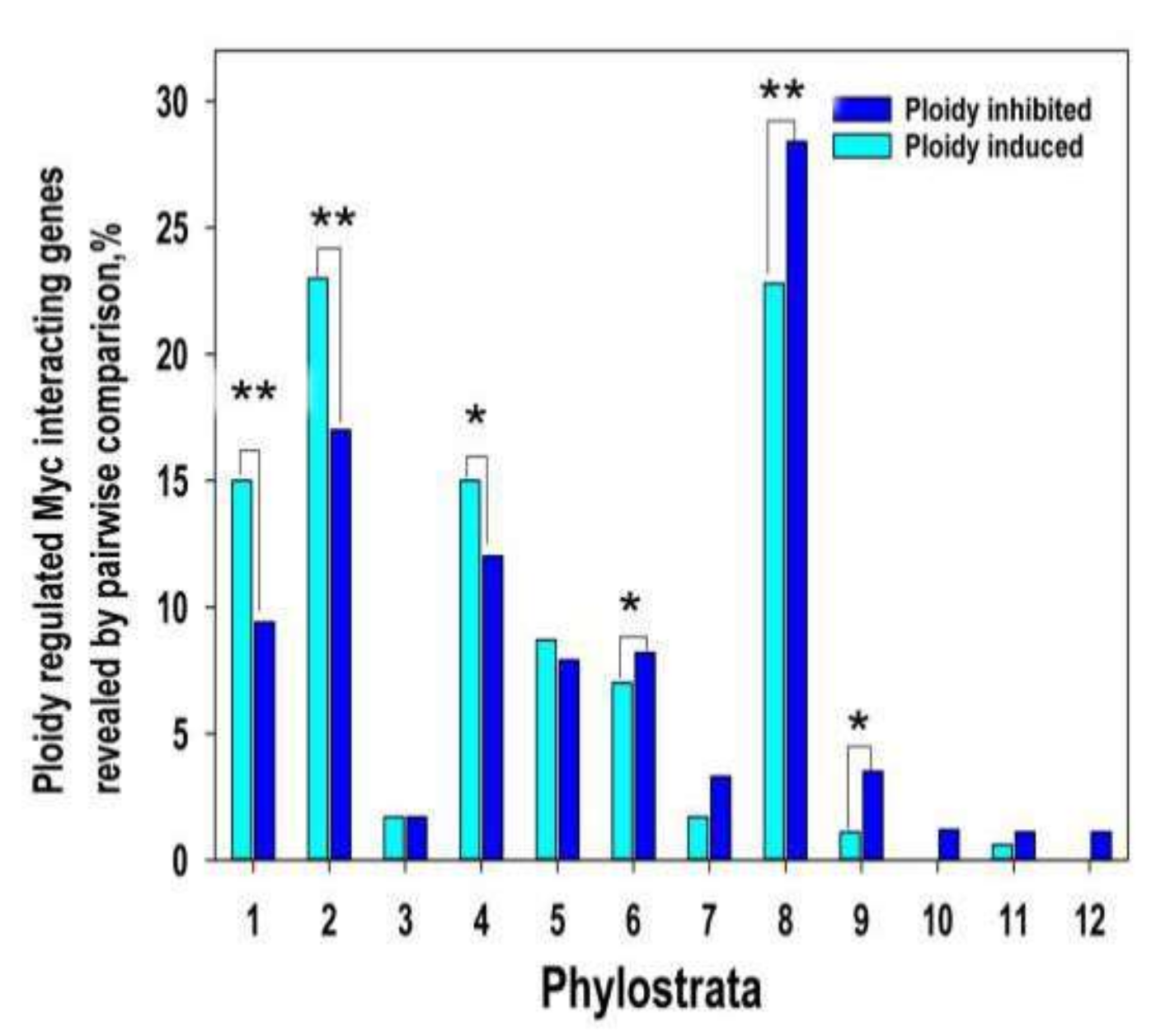
Here we evaluated the phylostratic distribution of ploidy-dependent c-MYC -interacting genes at two-fold expression difference (Tables S5 and S6) and explored its link through polyploidy with bivalent genes (Figure 2). The data on ploidy -Myc-ploidy-related gene phylostratigraphic changes repeat the ploidy-dependent relationships (compare with Figure 1)
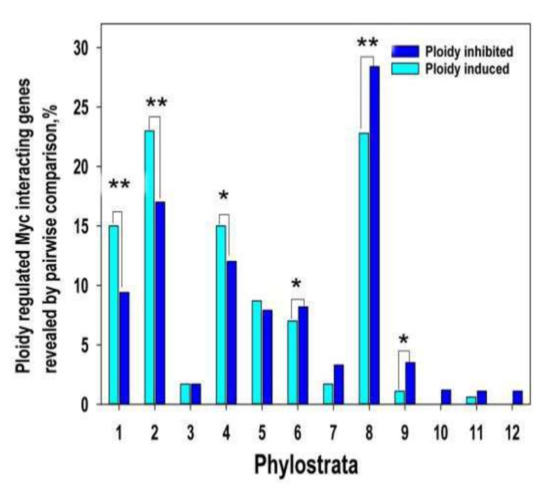
Figure 2.
Ploidy associated c-MYC interacting gene distribution across evolutionary phylostrata. This figure illustrates that c-MYC -interacting genes repeat pattern of all ploidy related gene age distribution. Gene age is shifted from young multicellular phylostrata (6–16) towards unicellular phylostrata (1–3 phylostrata) via transitional metazoan phylostrata (4–5). * p < 0.05 for the difference, ** p < 0.01. Gene expression difference is above two-fold.
It is clear that the non-mutant c-MYC should elaborate these effects in the epigenetic modus. Therefore, we paid attention to the fact that c-MYC controls gene transcription by activating bivalent genes.
2.4. The Phylostratigraphic Polyploidy-Associated Effect of c-MYC Is Associated with the Regulation of Bivalent Genes
The genome-wide studies indicate that the overexpressed c-MYC demethylates the H3K27me3 repressive domain of bivalent genes and thereby switches these genes to the active state [63,64], it also activates Polymerase II paused in bivalent genes [53]. To find out whether overexpressed Myc also increases expression of ploidy-related bivalent genes, we first identified these genes among ploidy-regulated c-MYC interacting genes with expression difference above two-fold. Genes of interest were identified using the list of bivalent genes in human embryonic stem cells [54]. Our data identified 60 bivalent genes of 161 ploidy-upregulated Myc interactants and 27 bivalent genes among 101 ploidy-downregulated c-MYC interactants (Figure 3A,B, Tables S7 and S8).
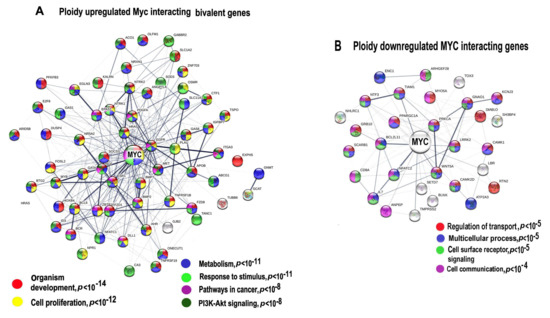
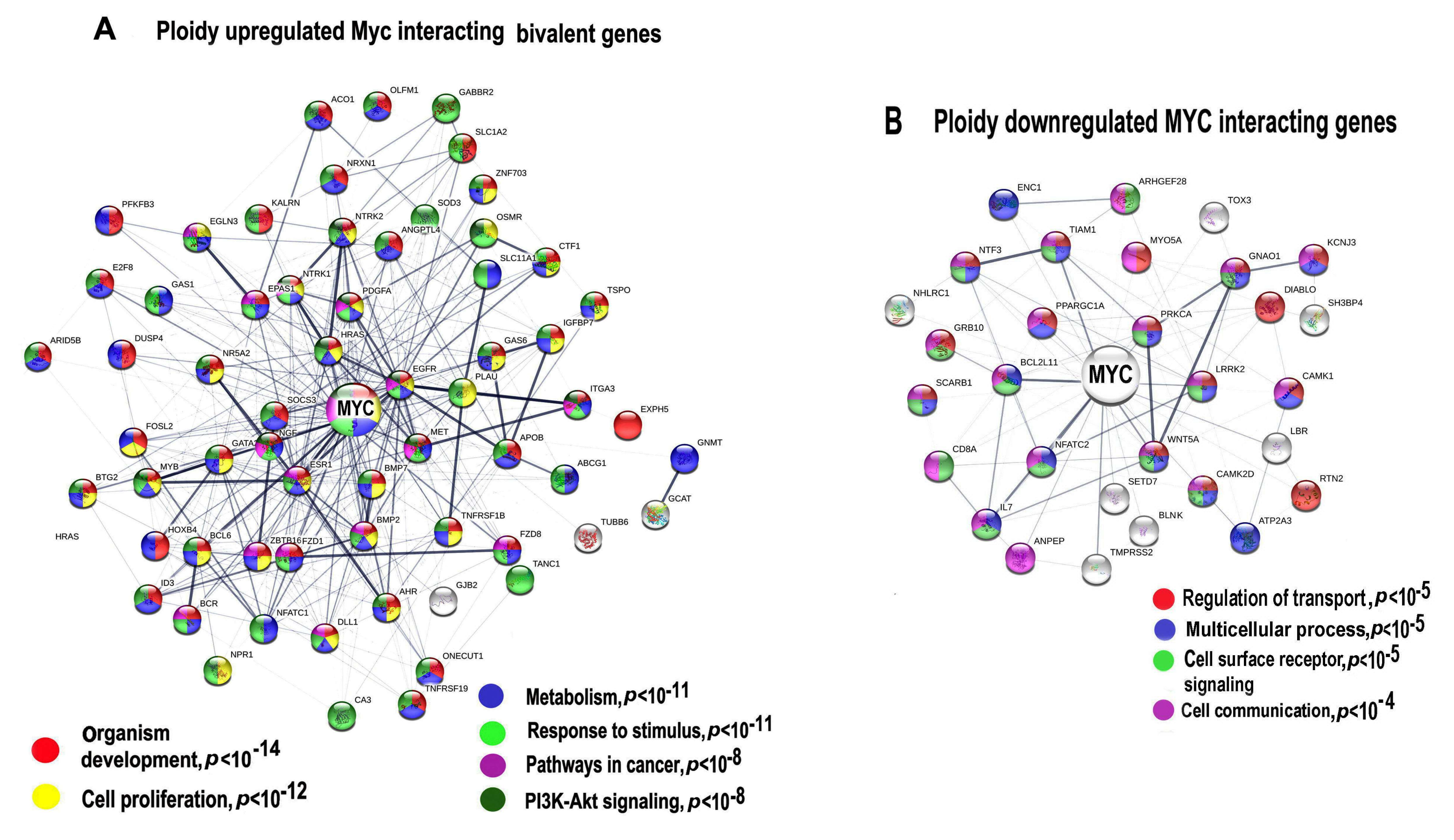
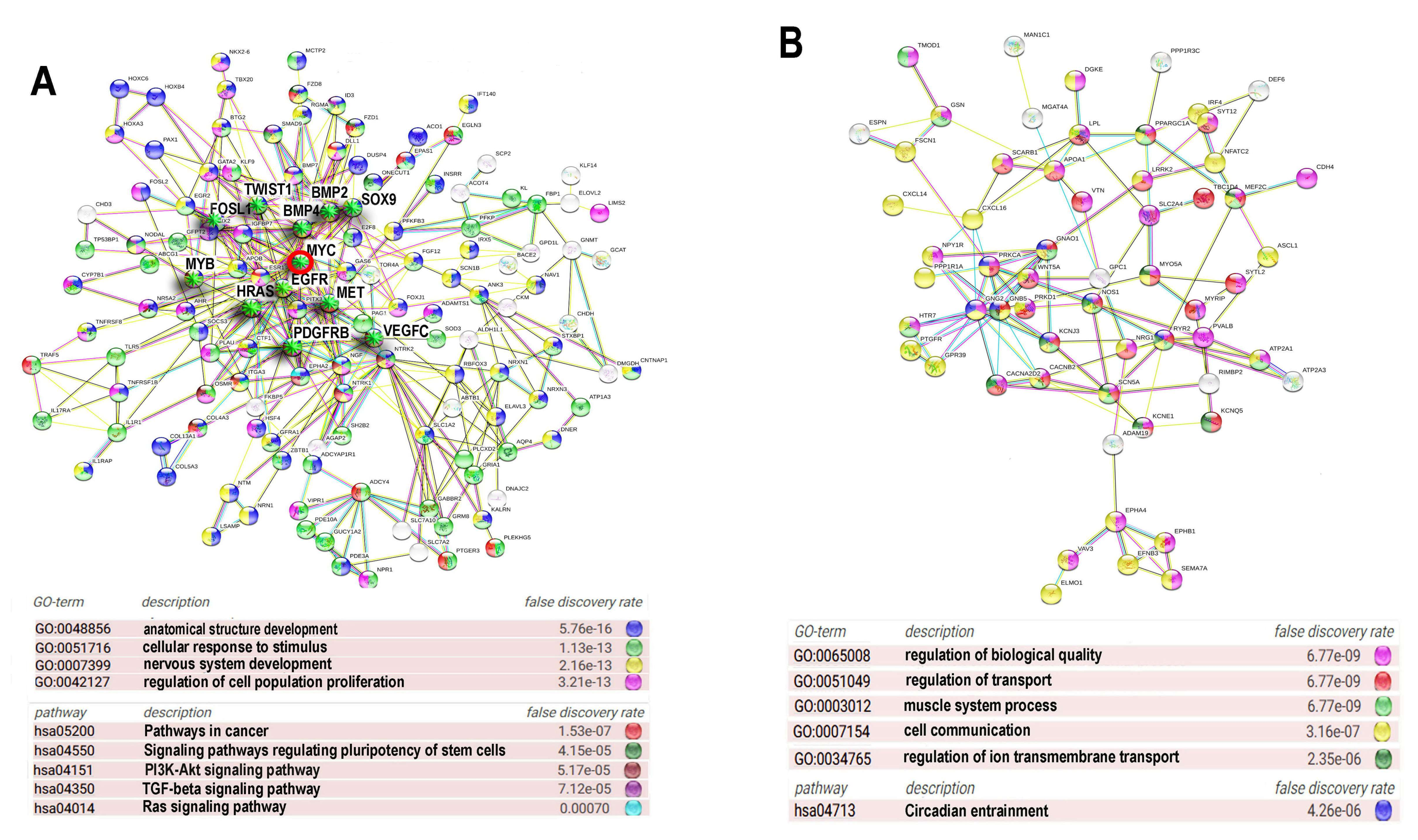
Figure 3.
Protein interaction networks (PPIs) for ploidy associated c-MYC -interacting bivalent genes. PPI for upregulated (A) and downregulated (B) genes. Gene expression difference is above two-fold. The networks are constructed with server String. Gene pathway enrichment was found using the same server.
Protein interaction network presented in Figure 3A indicates the Myc interacting and ploidy upregulated bivalent genes. Gene module functional enrichment analysis indicated that these genes are enriched in pathways of development, proliferation, stress response, and pathways in cancer with high significance. Figure 3B that presents c-MYC interacting ploidy downregulated genes shows that the cell surface-mediated functions (transport, reception, communication) are downregulated.
It is important to note that the network for the upregulated bivalent genes shows higher connectivity (13.5 connection per a node) compared to the downregulated genes (only 5.13 connections per a node), which implies that c-MYC and polyploidy- induced activity of bivalent genes considerably increases epigenetic plasticity of protein interaction network. It should be noted that the overexpressed functional pack again included a tandem of development and carcinogenesis modules, which we have found in the ploidy-related gene phylostratigraphic analysis. Therefore, we decided to extend the investigation of bivalent genes in relation to ploidy and gene phylostrata through the entire transcriptome.
2.5. Ploidy-Associated Genes Are Enriched in Bivalent Genes of the Entire Genome, Prevailing among Unicellular and Eumetazoan Phylostrata.
We compared the genes with above two-fold expression difference dependent on polyploidy (vs. diploidy) with bivalent genes in human embryonic stem cells [54]. Among the 584 up and 711 downregulated by ploidy genes (Tables S1 and S2) we found 267 (45.7%) and 222 (31.6%) of up-and down-regulated bivalent genes, respectively (Tables S9 and S10). The results of the binomial test indicate that bivalent genes enrich ploidy-upregulated genes compared to the entire transcriptome (3024 vs. 14093) with high significance (p < 10−16 binomial test). For the downregulated genes the significance was p < 10−8 (for 21.3 vs. 31.2%), respectively. These results indicate that polyploidy mostly induced bivalent genes.
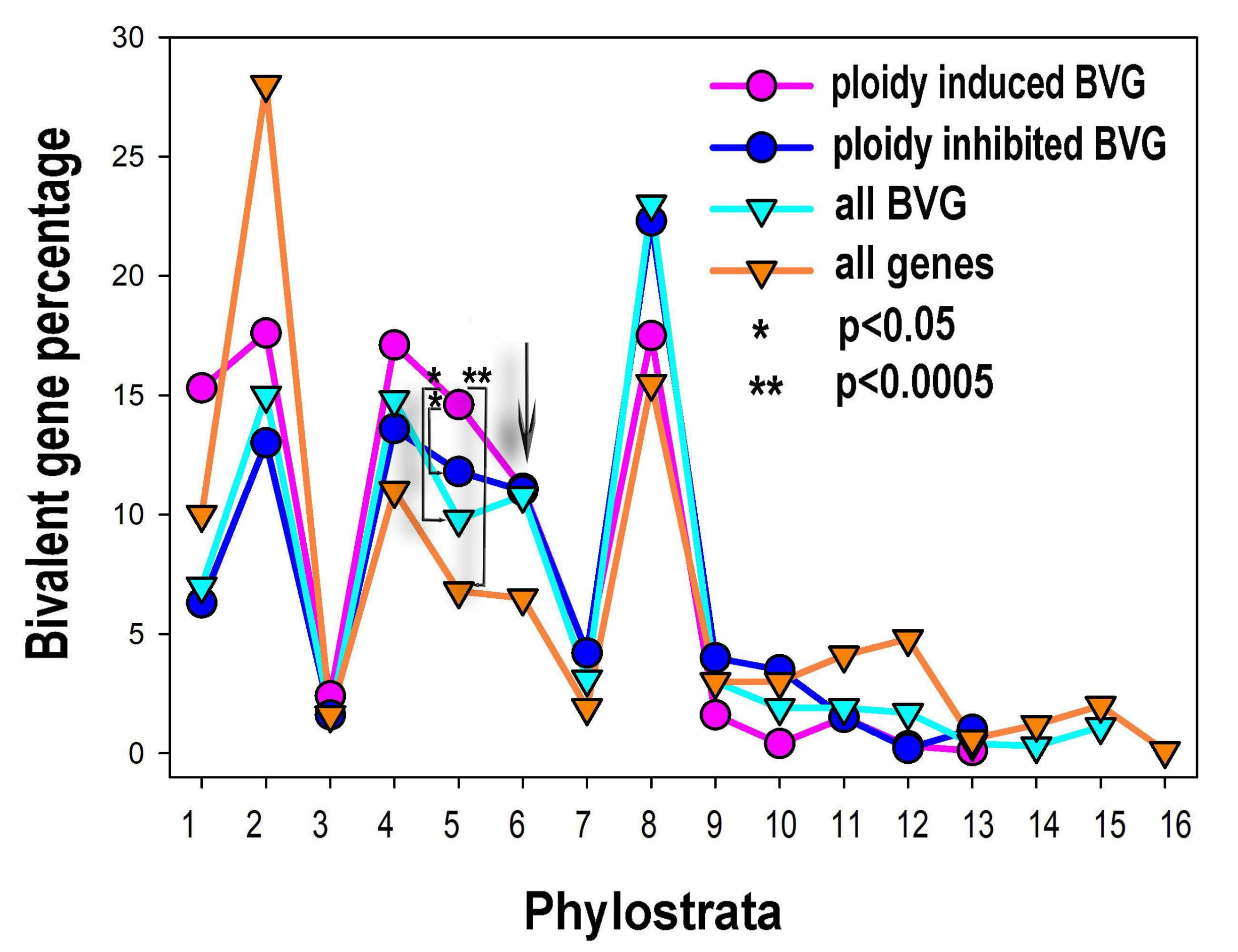
The percentage of phylostratigraphic distribution of ploidy associated bivalent and common genes is shown in Figure 4. The bivalent genes, comprising ~21% of the human genome, originated in evolution in all phylostrata but in the larger proportion with development of multicellularity, from phylostratum 4 (Metazoa) to 8 (Euteleostomi). The distribution of the expression of the ploidy upregulated bivalent genes by phylostrata is different from that for all bivalent genes. It is clearly seen that these genes repeat the general effect of all ploidy-regulated genes and c-MYC-ploidy regulated genes. Among the ploidy upregulated bivalent genes prevail the proportions of phylostrata 1 (Prokaryota) to 5 (Eumetazoa). Proportions of phylostrata 6 and 7 (Bilateria and Chordata) are ambivalent. Proportions of phylostrata 8 (Euteleostomi) to 10 (Mammalia) show clear decrease (Figure 4). The ploidy downregulated bivalent genes generally repeat the pattern for all bivalent genes.
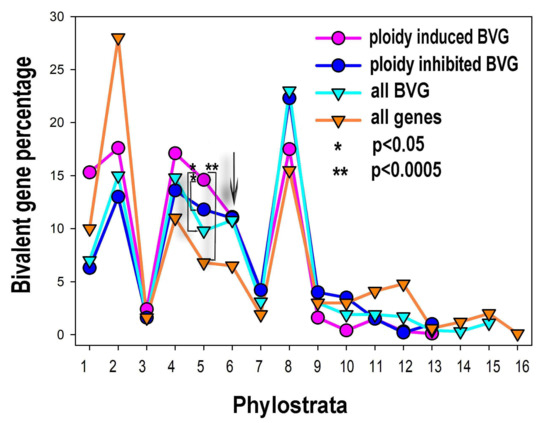
Figure 4.
The percentual proportions of gene origins and distribution of bivalent genes (BVG) in the phylostratic tree of life (strata 1–16) and the effect of polyploidy on it. The upregulation of bi-valent genes by polyploidy includes strata 1, 2 (unicellularians), stratum 4 (metazoa) and, prominently, stratum 5 (eumetazoa—the appearance of embryo, germ layer, and gastrulation). The arrowed cross-point starting down-regulation of bivalent genes by polyploidy in late metazoa falls upon stratum 6 (bilateria).
2.6. Protein Interaction Networks for Ploidy-Associated Bivalent Genes Are Involved in the Upregulation of the Developmental and Carcinogenesis Genes and the Downregulation of the Networks Related to Differentiation Biological Quality and Circadian Clock
We constructed protein interaction networks (PPIs) for ploidy up- and downregulated bivalent genes marked with H3K27me3 and H3K4me3 in human ESC using the String server. We also included c-MYC that is not a bivalent gene, but it is of particular interest because this important oncogene is an inducer of bivalent genes [64]. The networks were constructed for genes with above two-fold expression difference. Then we extracted the whole connected component from the up- and downregulated networks and clustered them using the same server. For the upregulated genes the network contains 165 bivalent of 267 ploidy upregulated bivalent genes (62%). For the ploidy downregulated bivalent genes it contains 63 genes of 222 (24%). As in the case of c-MYC, the striking difference in gene numbers of the connected components of up- and downregulated networks (163 vs. 63) by bivalent genes in the whole transcriptome, despite approximately the same numbers of up- and downregulated genes, shows that the upregulated by ploidy bivalent genes create essentially more functional connections and master regulators compared to the downregulated genes. Below we provide a functional description of the up and downregulated PPIs (Figure 5A,B). It is clearly seen that the upregulated network contains more hub regulators than the downregulated network (29 vs. 13).
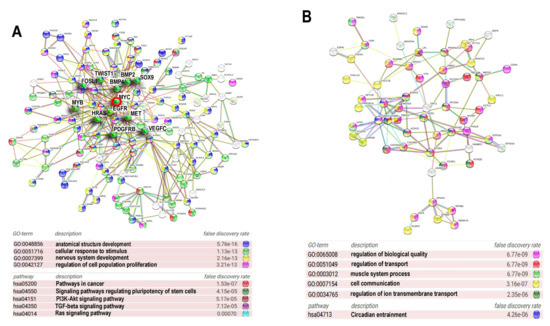
Figure 5.
Protein interaction networks for ploidy associated bivalent genes. The most connected components of protein interaction networks of upregulated (A) and downregulated (B) bivalent genes constructed using STRING server. Gene expression difference is above two-fold. Gene module analysis and node degree evaluation was done using the same server. The titles of GO biological processes are given with for false discovery rate. The important drivers of carcinogenesis are marked with green asterisks. Myc oncogene is marked with a red circle.
The PPI for the ploidy upregulated bivalent genes (Figure 5A) is enriched in the GO biological processes related to the development of anatomical structures (GO:0048856) and the nervous system (GO:007399), proliferation (GO:0042127), response to stress and stimulus (GO:0051716), and in KEGG pathways involved in the regulation of pluripotency (hsa04550), pathway of carcinogenesis (hsa05200) and several pro-carcinogenic and development related pathways, including PI3K-AKT (hsa04151) TGFB (hsa04350) and RAS (hsa04014) signalling pathways (Figure 5A). It is important to note that this PIN unifies proteins involved both in development and metastatic cancer that comprise dense functional nucleus of the induced network and are the hub proteins with high degree of connectivity originating from 1–6 phylostrata genes (Table 1). The PPI for the downregulated genes is enriched for GO biological processes participating in transport (GO:0051049, GO:0034765), cell communication (GO:0007154), regulation of biological quality (GO:0065008), muscle system processes (GO:003012) and circadian entrainment (hsa04713). Thus, the functional picture provided by PPI for the upregulated sgenes indicates that ploidy-related bivalent genes are associated with the induction of embryonic developmental programs including drivers of carcinogenesis (Figure 5A, Table 1). PPI for the downregulated genes illustrates the depression of circadian clocks.

Table 1.
Characteristics of the central hubs of Myc-related network of proteins encoded by bivalent genes upregulated by polyploidy (presented in Figure 5A).
2.7. The Relation of Cancer Driver Genes to Polyploidy, Bivalency and Their Phylostratigraphic Origin
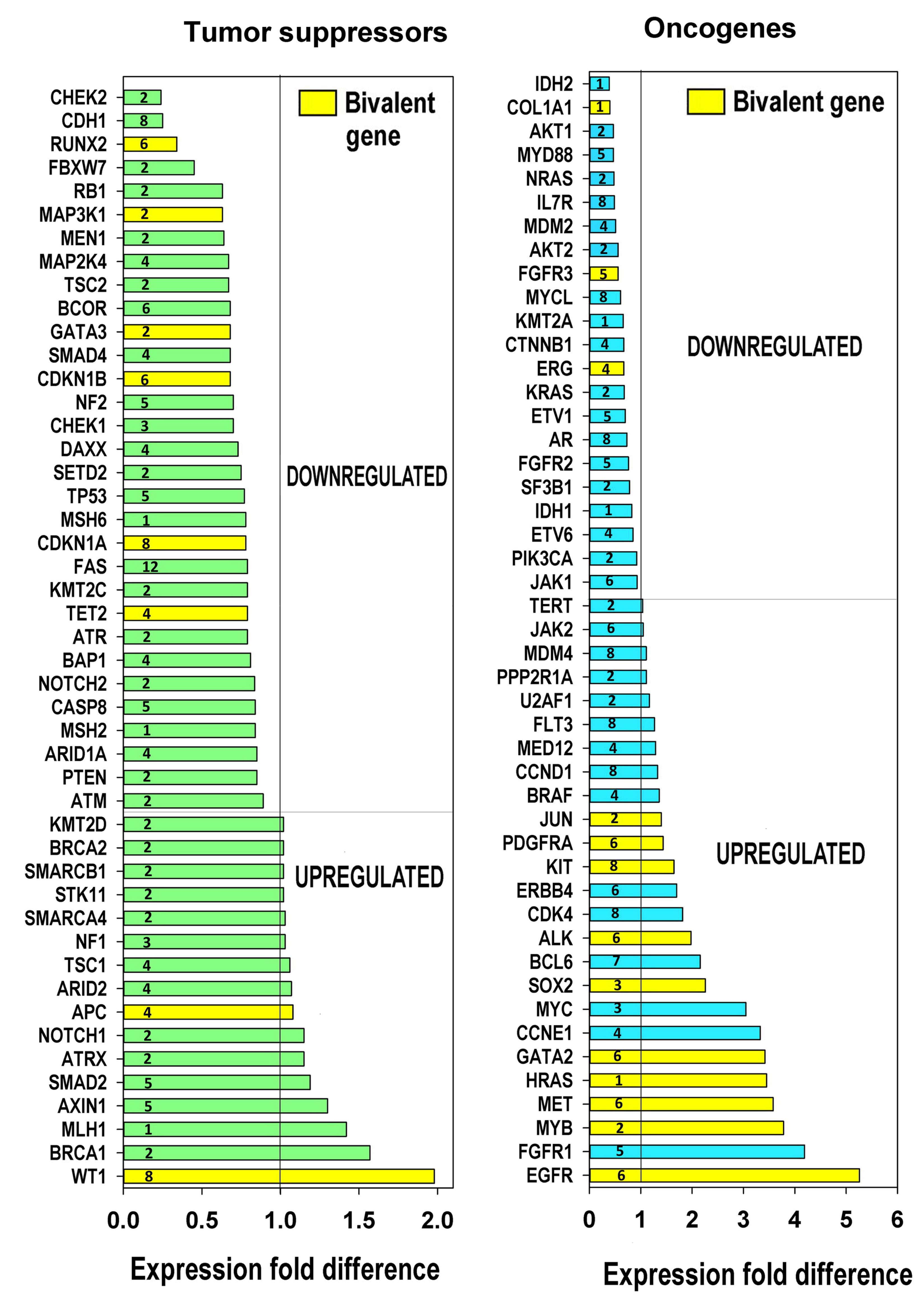
To obtain more evidence concerning features of carcinogenesis, we evaluated the expression of tumour suppressors and oncogenes in relation to polyploidy based on the list from [78]. The obtained data presented in Figure 6, together with phylostratic origin of genes, indicates that polyploidy is associated with preferential downregulation of tumour suppressors and strong upregulation of many oncogenes. It is important to note that the upregulated oncogenes were more strongly enriched with bivalent genes than the downregulated oncogenes and tumour suppressors (p < 0.02 for all comparisons, binomial test). The notably down-regulated by polyploidy tumour suppressors belong to DNA damage response: ATR, (2nd phylostratum), ATR (2nd), CHEK2 (2nd), CHEK1 (3rd), TP53 (5th)—however, its protein inhibitor MDM2 (seen in the column for oncogenes) is also inhibited; apoptosis executor Fas, prominent tumour suppressor PTEN (2nd) and the E-cadherin-associated gene CDH1 (8th phylostratum). Thus, cell communication is suppressed by polyploidy, while cells become more tolerant to DNA damage and inhibit apoptosis.
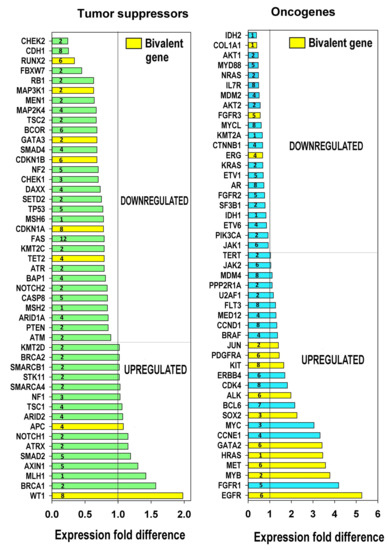
Figure 6.
Ploidy associated tumor suppressors and oncogenes. This figure illustrates the downregulation of tumour suppressors, the upregulation of oncogenes and the enrichment of the induced oncogenes with bivalent genes p < 0.05, binomial test. The phylostrata numbers of gene evolutionary origins are indicated.
Among the prominent oncogenes upregulated by polyploidy, we find a master transcription factor (TF) of early stress response Jun (2nd phylostratum), KIT-tyrosine-protein-kinase (6th); among 3–5-fold upregulated oncogenes, we see GATA 2 TF (6th) responsible for embryonic development, c-MYC TF (3rd, viral origin); MYB TF (2nd, viral origin); MET Tyrosin-protein-kinase (6th)— often a target for avian leukemia retrovirus [79] HA-RAS G-protein (1st phylostratum, viral origin)—the most common oncogene in at least 30% of all tumours and, finally, its upstream regulator EGFR (6th); both are bivalent (Table S9).
Clearly, in this analysis, we have used the transcriptome data from polyploid tissues, where the activated protooncogenes were not mutated. In addition to their relation to bivalency and embryonic development, we see that the main tumour suppressor TP53 and strong oncogene EGFR belong to the intermediate phylostrata 5–6, while many important driver oncogenes of this list activated by polyploidy are from the earliest evolutionary phylostrata. Furthermore, the most often involved in carcinogenesis prominent complementary pair of oncogenes c-MYC and HRAS (phylostrata 1–3) are of the most ancient origin.
3. Discussion
Despite extensive development of therapies, metastatic cancer disease remains incurable, resulting in high morbidity and mortality [80]. The association of aggressive incurable cancer with polyploid giant cells has been shown [28]. The extraordinary resistance to extinction therapies suggests that cancers recapitulate the phylogenetic endurance acquired in the long evolution of the lifeforms on Earth [81]. The cancer genome sequencing projects compromised, to some extent, a somatic mutation theory [82,83]. In recent times, the epigenetic aspects of the whole genome regulation in cancer stepped forward and are now intensively explored. One of these aspects is associated with typical for cancers whole genome duplications, or polyploidy (and aneuploidy inevitably linked to it), as a feature characteristically accompanying tumour growth and aggravating with tumour aggression [18]. The genetic sequences of polyploidy and aneuploidy in the microevolution of cancer were in the focus of studies for rather a long time [84,85]. However, the epigenetic aspects of aneuploidy became also attractive [86,87]. Considering a possible atavistic effect of cancer polyploidy, the phylostratigraphic analysis considering the evolutionary origin of genes and the change of their expression balance may be helpful.
Therefore, an interesting epigenetic approach may represent the study of the phylostratigraphic effect of polyploidy of not mutant normal mammalian tissues. In this paper we have investigated these issues by bioinformatical means using transcriptome data of polyploid versus diploid cells of normal human heart and mouse liver.
We have revealed that polyploidy changes the phylostratigraphic balance of the mammalian cellular network in favour of the enhanced expression of the evolutionary most ancient gene phylostrata—prokaryotic, early eukaryotic and early metazoan (1–5 phylostrata) up to bilateria, after which the trend is changed to the opposite. Although found here in normal polyploid tissues, this shift is surprisingly similar to the changes revealed for tumours when compared with normal counterparts [34,35,36,38]. With this revelation we arrive to the immediate conclusion: this epigenetic shift in tumours to the unicellular and early multicellular forms of life is associated or at least highly favoured by polyploidization.
The question arises—how it is regulated (or rather disregulated)? One of the mechanisms found in our study—it is by use of so-called bivalent genes, the epigenetically modified by histone H3 repressive and activating modifications of the same gene promoter or enhancer, namely by their prevailing activation. The particularity of bivalent genes comprising ~21% of human genes and employed for cell fate change in development is that they are poised (paused) but can be rapidly activated. That means that they favour cell fate change by the rules of non-equilibrium thermodynamics, in particular by critical state transition [88].
It is known now that the chromatin conformation at the supranucleosome and higher levels of the genome architecture is much a subject of the biophysical processes which assemble the active and inactive chromatin in two separate self-organizing high-order domains [89,90,91,92,93]. Self-organization is an important driving force of evolution [94]. On one hand, polyploidy is well known as driving gene diversification and speciation in evolution [1], which is a slow process. On the other hand, its epigenetic effect causing rapid reorganization of the transcriptional regulatory network after genome duplication was shown [95,96].
It can be reasoned that multiplication in polyploid cells of the identical chromosome alleles with the identical epigenetic status of bivalent genes should not just linearly increase their epigenetic effect proportionally to gene dosage but potentiate it by the thermodynamics of structural self-organization (phase transition). The role of the closely-juxtaposed DNA fibres’ electrostatic forces yielding fine-tuned structure-specific recognition and pairing [97,98] should also contribute in the chromatin self-organization by polyploidy. A similar thought on the potentiating structural effect of additional DNA by the chromatin modifiers and transcription factors has been proposed earlier [99,100].
The Kegg “pathways in carcinogenesis”, along with the genes known as being mutants in cancers [101], also include many developmental genes. Here we revealed that this developmental component is largely introduced through the polyploidy-dependent enrichment of the expression networks with the activated bivalent genes upregulated by c-MYC master hub (stratum 3). While in general gene evolution the proportion of bivalent genes increases from phylostrata 3 to 8, the polyploidy is mostly affected by enrichment with expression of bivalency in the phylostrata of Prokaryote and Eumetazoa (1—5). Very likely, that it is achieved by Myc up-regulating both polyploidy and bivalency. Thus, the carcinogenic H-RAS-c-MYC feed-back loop (strata 1–3) linked to developmental genes of early metazoans (strata 4–5) enriched with developmental pathways becomes involved, while stratum 6 (Bilateria) becomes a cross-point of the ambivalence (with repulsion from vertebrates).
c-MYC–mtH-RAS complementary pair has been distinguished in the early cancer chemical carcinogenesis research in a two-hit theory of cancer with Myc determining immortality and cancer initiation and H-RAS mutant in the codon 12 or 61 accomplishing cancer promotion [71,102]. Afterwards, ample studies have extended this theory by demonstrating that a potent oncogenicity of Myc can be further enhanced by collaborations with not only RAS mutant but also many extracellular growth stimuli that activate RAS, such as epidermal growth factor and its receptor (EGFR) or transforming growth factors [103,104]. The found phylostratic effect of ploidy-associated c-MYC collaborating with bivalent genes, e.g., both H-RAS (stratum 1) and EGFR (stratum 6) may explain its critical role in cancer initiation when overexpressed, and cancer regression when locked [59,60,62]. This phylostratic effect induced by the oncogenic potency of c-MYC linked to induced polyploidy, metabolic stemness, and bivalency likely creates the evolutionary “cancer attractor” postulated by Kauffman [94] as situated close to the summit of metaphoric Waddington Hill of the development potential [105,106]. Moreover, the upregulated c-MYC cooperates with mutant ras through programming inflammation and suppressing the tumour immunity [61], and c-MYC acts by cell selection promoting proliferation versus apoptosis depending on the supply of growth factors [60]. Likely, this trigger can occur through asymmetric cell division of epigenetically diverged bi-nuclear cells [107,108]. In fact, the main actors of this complex “story” including the complementary c-MYC and RAS pair can be found in Table 1 describing tumour and stromal components (like angiogenesis) of the polyploidy-dependent “cancer attractor”.
Interestingly, cnidaria (Hydra), an organism where the basic processes of the multicellularity were established represents phylogenetically the oldest organisms, where the tumours were revealed [109].
The early eukaryotic gene cluster in human genome is more stable than the cluster of complex metazoans (starting from vertebrates and peaking in acquired number of new genes at stratum 8) [38,40,41]. The unicellular gene network is responsible for basic cellular functions and also for resistance to extinction elaborated through series of earth catastrophes in billion years—we highlighted here that the genes responsible for DNA damage response, drug resistance, early stress response, and the proto-oncogenes of the viral origin, are from the 1–3 phylostrata. These likely act through feedback loops as the driving belts epigenetically attracting the cells from the basin of early metazoans into a unicellularity network.
On the other hand, the basic evolutionary processes of the early embryonal development of mammals are conserved from their origin in Eumetazoa, stratum 5 [110]. Therefore, aggressive cancer associated with polyploidy and multinuclearity often bears the features of the early embryo [111,112], including such inalienable components of morphogenesis as cell motility and angiogenesis. Moreover, through polyploidy, the TP53 cancer mutants can acquire the phenotypical features of more ancient unicellular eukaryotes (“amoeboidisation”) [24,27,33,108,113,114], in line with the atavistic theory of cancer [29,30,33,115,116], confirmed by gene phylostratigraphic analysis [38] and here.
The early metazoans are tolerant to polyploidy because use it reversibly in their life-cycles [117]; it is sufficient to mention Amoeba vulgaris and Entamoeba histolytica [33,118,119] and amoebal slime molds [120]. When associated with polyploidy, as we found, the bivalent genes increase the connectivity and basin of attraction and become embedded in the network with the oncogene hubs of the viral origin, such as c-MYC, H-RAS, MYB—originated and having strong established interactions in the older strata (1–2–3). Moreover, polyploidy permits DNA replication but stops cell division and induces suppression of the bivalent genes regulating circadian rhythms, which entrain many cellular processes, including cell cycles [121]. The cell-autonomous circadian timers established already in cyanobacteria are composed of a transcription–translation-based auto-regulatory feedback loops [122]. Apparently, this detrainment is an important factor causing along with epigenetic bibivalency a metastable dynamic state, which can also favour a reshape and glide from the multi-cellular to unicellular gene expression network.
However, de-polyploidisation, in turn, can probably cause the back-shift of the transcription network: the two cycles, polyploidy (life-cycle-like) and diploidy (cell cycle) are reciprocally linked in tumours [23,87]; the recovery diploid fraction reciprocally downregulates the expression of amoebal genes in polyploidized human breast cancer treated with anticancer drugs; in addition, the amoebal giant cells can play a “nursing” function for the reproductive cell line [27]. Without any doubt, the constitutively activating mutations of oncogenes increase, push and likely stabilize the epigenetic shift to unicellularity. Such prominent tumour suppressors as TP53 and HYPPO/YAP pathway, which also serve as guardians of the barrier to polyploidy [123], normally prevent the carcinogenic epigenetic shift to unicellularity caused by overcoming this barrier.
4. Methods
4.1. Data Sources and Comparative Criss-Cross Analysis
In this study we applied the method of reciprocal pair-wise cross-species transcriptome comparison that we used previously [12,57,124]. We choose this approach, because mammalian homologous tissues differ by cell ploidy levels [1,2,5,8,12]. Certain species have predominantly polyploid heart and diploid liver (pig and primates), whereas others possess mainly polyploid liver and predominantly diploid heart (rodents) [1,5,8]. Humans have highly polyploid hearts where cardiomyocytes contain nuclei with 4–16 genomes, whereas mouse hearts, on the contrary, consist of cardiomyocytes with mostly diploid nuclei [12]. At the same time, human hepatocytes are mostly diploid, whereas mouse hepatocytes contain nuclei with 4–8 genomes [5,12]. This inverse pattern of polyploidization enables identifying pure ploidy specific effects since it removes species-specific and tissue-specific noise [12]. In this study we applied pairwise cross species transcriptome comparison and principal component analysis (PCA) to comparisons of polyploid vs. diploid tissues: human heart vs. mouse heart and mouse liver vs. human liver. In the present work there are three main advancements over previous studies. Firstly, here we made the functional analysis of all ploidy-associated genes based on the whole-transcriptome data. Previously, the analysis was limited to the c-MYC interactome only [58,124]. Secondly, we studied the gene evolutionary origin (phylostratic distribution of all polyploidy-associated genes), which is important for cancer research in the context of the main contradiction arising between the unicellular and multicellular level features in tissue organization [34,38,41]. Thirdly, we addressed possible epigenetic mechanisms underlying ploidy-related changes, focusing on the bivalent gene expression and interactome. The work builds upon the idea of reciprocal cross-species comparison. The cross-species approach is informative because evolutionary distance enhances the contrast allowing the polyploidy-specific signature to appear [125].
The signal-to-noise filtration, where signal corresponds to polyploidy and noise to species- and tissue-specific signatures, is especially important for investigation of polyploidy because polyploidy preserves gene-dosage balance and thus may exert only weak and idiosyncratic effects on gene expression [126]. We performed the reciprocal pair-wise cross-species comparison using the transcriptomic data for polyploid vs. diploid organs. Specifically, we compared human heart (polyploid) vs. mouse heart (diploid) and mouse liver (polyploid) vs. human liver (diploid). The data were taken from the database obtained using RNA-seq [127].
The processed data were taken from the Supplement at the journal site [127]. We matched human-mouse orthologous genes and normalized the data in pairwise comparisons (human-mouse heart, human-mouse liver) using “quantile” normalization implemented in the “limma” package [128], as was done previously [124,129]. Finally, there were 13,913 orthologous gene pairs for which we found stratigraphic data in Trigos et al. [38]. The transcriptomic data were uniformly obtained for all tissues and species [127]. This database was developed specially for interspecies comparisons (in particular, the samples were taken from the same parts of organs).
We detected the genes whose expression was changed in the same direction with regard to ploidy both in the heart and liver. In these comparisons the genes should have higher (or lower) expression in both polyploid tissues (human heart and mouse liver) compared with corresponding diploid tissues (mouse heart and human liver). Since we compared two different tissues in opposite directions in different species (human vs. mouse in the case of heart, and mouse vs. human in the case of liver), the tissue-specific and species-specific effects were presumably removed.
The one-to-one human-mouse orthologous genes were obtained from the NCBI database [130]. The expression levels of orthologous genes were analysed using the “limma” package specially developed for revealing differentially expressed genes in whole-transcriptome analyses [128]. A comparison of different software packages showed that limma is the method of choice for goals similar to those pursued in our work [131]. The data were normalized with “quantile” normalization implemented in “limma”. The differential gene expression (with statistical significance) was determined using the “voom” limma procedure. Then, we selected the genes with different expression contrasts between polyploid and diploid tissues as indicated in Results.
4.2. Principal Component Analysis
To determine whether the results of cross-species comparison can be confirmed by another approach, we applied principal component analysis (PCA) to the raw data matrix having samples as variables and genes as statistical units. The idea is to confirm the gene-by-gene a priori approach with a data-driven strategy, letting a ploidy-specific principal component to emerge from the data. The principal components are orthogonal to each other by construction; the data-driven emergence of a “ploidy” component distinct from tissue and “species” components is equivalent to an unsupervised normalization for tissue and species effects [132]. The genes endowed with extreme scores on such a “ploidy” component are the “image in light” of tissue and species independent ploidy effect on transcription pattern.
For this propose we used the same transcriptomic data for human and mouse heart and liver. This approach enabled us to evaluate the impact of shared variation (driven by housekeeping genes), species-specific, tissue-specific and ploidy-specific variables as separated mutually independent components [133].
As a result, we obtained two lists of genes demonstrating statistically significant ploidy-associated variation (above two standard deviations) for human and mouse heart and liver. Then these gene lists were subject to gene module enrichment analysis and the modules that are regulated by ploidy were identified. PCA is a purely geometrical non probabilistic procedure [132]. This means that each gene is simply projected into a rotated space (in this case having the same dimension of the original one, thus, with no loss of information) spanned by mutually orthogonal axes (components) extracted in decreasing order of explained variance. This unsupervised purely geometrical approach gave rise to a loading pattern mirroring the batch, tissue, species, ploidy a priori classification so demonstrating the tenability of the existence of “ploidy specific” genes. At this point the identification of genes having a score > |2| as markers of the ploidy component corresponds to the usual 95% confidence intervals (component scores are equivalent to z scores). The actual p-values for phylostratigraphic analysis were based on relative enrichment of genes pertaining to different stratigraphic level based on the entire gene ontology. The samples having same tissue and species origin are practically coincident [see 132] for the presence of a common gene expression ideal profile for same tissue independent samples). RNA-seq analysis was based on the shared gene products across different samples; these shared genes were the pivots for coupling the relative vectors.
The species-specific and tissue-specific effects were automatically removed by PCA [124]. As a matter of fact the principal component analysis applied in an unsupervised way to the gene expression profiles, generated a four-component solution correspondent to batch effect (PC1, 45.6% of variance explained), tissue-effect (PC2, 30.5% of variance); species-specific effect (PC3, 15% of variance) and ploidy effect (PC4, 9% of variance). We concentrated on genes having relevant score (higher than |2| corresponding to two standard deviations from mean) on PC4. Principal components are each other independent by construction, therefore batch, tissue and species confounding are eliminated by the spectral decomposition of the dataset.
4.3. Analysis of Gene Modules
To determine which biological modules were over-represented among the ploidy-associated genes, we applied a double control. We tested the genes from all three datasets with higher and lower expression in polyploid vs. diploid tissues for enrichment of Gene Ontology (GO) categories and molecular pathways with regard to all human-mouse orthologous genes. The enriched GO categories and molecular pathways were found using the hypergeometric distribution of probability (implemented in R package) as previously [134,135]. GO categories were taken from GO database [136]. For each GO category, we collected all its subcategories using Gene Ontology acyclic directed graphs, and a gene was regarded as belonging to a given category if it was mapped to any of its subcategories. As a source of molecular pathways, the NCBI BioSystems was used, which is a most complete compendium of molecular pathways from different databases [130]. Redundancy of this compendium was removed by uniting entries with identical gene sets. The adjustment for multiple comparisons was done according to the method by [137]. This procedure gives a q-value, which can be considered a p-value corrected for multiple tests.
4.4. Protein-Protein Interaction Network
The protein-protein interaction networks (PPI) were constructed and visualized using the STRING server [138]. The analysis of network connectivity and the identification of causal regulators in modular and network organization were done with the same server.
4.5. Phylostratigraphic Analysis of Ploidy Associated Genes
The attribution of genes to phylostrata was taken from [38]. The phylostrata are as follows: 1—cellular organisms, 2—Eukaryota, 3—Opisthokonta, 4—Metazoa, 5—Eumetazoa, 6—Bilateria, 7—Chordata, 8—Euteleostomi, 9—Amniota, 10—Mammalia, 11—Theria, 12—Eutheria, 13—Euarchontoglires, 14—Catarrhini, 15—Homininae, 16—Homo sapiens.
4.6. Identification of Ploidy Associated Bivalent Gene
To find out how polyploidy affects bivalent gene expression landscape, we evaluated the expression of ploidy-associated genes that are known to be marked by bivalent chromatin in stem cells. The list of these genes was taken from [54].
5. Conclusions
The obtained results indicate that polyploidy activates the unicellular pathways of resistance to extinction fusing with ancient programs of ontogenesis related to carcinogenesis which originated in the early metazoa while suppressing the expression of genes from the late metazoa. This evolutionary retour is favoured by the activation of bivalent genes and deregulation of circadian rhythms. The data highlight the paramount role of polyploidy in the atavistic origin of cancer, the reason for the incurability of metastatic cancer, and indicate to targeting polyploidy for overcoming resistance to therapies.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/21/22/8759/s1.
Author Contributions
Conceptualization: J.E., O.V.A., A.E.V.; methodology: O.V.A., A.E.V., A.G.; investigation: O.V.A., A.E.V., A.G., N.M.V.; validation: O.V.A., A.E.V., A.G.; formal analysis: O.V.A., A.E.V., A.G.; data curation: O.V.A., A.E.V., A.G.; visualization: O.V.A., N.M.V.; literature analysis: J.E., O.V.A., A.E.V., A.G., N.M.V.; writing—original draft preparation: J.E., O.V.A., A.E.V., A.G.; writing—review and editing: J.E., O.V.A., A.E.V., A.G., N.M.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly supported by a grant from the European Regional Development Fund (ERDF) project No. 1.1.1.1/18/A/099 for J.E and the Natural Sciences PhD Student Scholarship from the University of Latvia Foundation to N.M.V. Additionally, the work was partially supported by the Institute of Cytology Director’s Fund for OVA and AEV.
Acknowledgments
The authors are very grateful to Alexey N Tomilin for helpful comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Comai, L. The advantages and disadvantages of being polyploid. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Fox, D.T.; Soltis, D.E.; Soltis, P.S.; Ashman, T.-L.; Van de Peer, Y. Polyploidy: A Biological Force from Cells to Ecosystems. Trends Cell Biol. 2020, 30, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Avdeyev, P.; Alexeev, N.; Rong, Y.; Alekseyev, M.A. A unified ILP framework for core ancestral genome reconstruction problems. Bioinformatics 2020, 36, 2993–3003. [Google Scholar] [CrossRef] [PubMed]
- Anatskaya, O.V.; Vinogradov, A.E. Myocyte ploidy in heart chambers of birds with different locomotor activity. J. Exp. Zool. 2002, 293, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Anatskaya, O.V.; Vinogradov, A.E. Heart and liver as developmental bottlenecks of mammal design: Evidence from cell polyploidization. Biol. J. Linn. Soc. 2004, 83, 175–186. [Google Scholar] [CrossRef]
- Sher, N.; Von Stetina, J.R.; Bell, G.W.; Matsuura, S.; Ravid, K.; Orr-Weaver, T.L. Fundamental differences in endoreplication in mammals and Drosophila revealed by analysis of endocycling and endomitotic cells. Proc. Natl. Acad. Sci. USA 2013, 110, 9368–9373. [Google Scholar] [CrossRef]
- Orr-Weaver, T.L. When bigger is better: The role of polyploidy in organogenesis. Trends Genet. 2015, 31, 307–315. [Google Scholar] [CrossRef]
- Lazzeri, E.; Angelotti, M.L.; Conte, C.; Anders, H.-J.; Romagnani, P. Surviving Acute Organ Failure: Cell Polyploidization and Progenitor Proliferation. Trends Mol. Med. 2019, 25, 366–381. [Google Scholar] [CrossRef]
- Derks, W.; Bergmann, O. Polyploidy in Cardiomyocytes: Roadblock to Heart Regeneration? Circ. Res. 2020, 126, 552–565. [Google Scholar] [CrossRef]
- Gan, P.; Patterson, M.; Sucov, H.M. Cardiomyocyte Polyploidy and Implications for Heart Regeneration. Annu. Rev. Physiol. 2020, 82, 45–61. [Google Scholar] [CrossRef]
- Anatskaya, O.V.; Vinogradov, A.E.; Kudryavtsev, B.N. Cardiomyocyte ploidy levels in birds with different growth rates. J. Exp. Zool. 2001, 289, 48–58. [Google Scholar] [CrossRef]
- Anatskaya, O.V.; Vinogradov, A.E. Genome multiplication as adaptation to tissue survival: Evidence from gene expression in mammalian heart and liver. Genomics 2007, 89, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.S.; Ghiraldini, F.G.; Veronezi, G.M.B.; Mello, M.L.S. Polyploidy and nuclear phenotype characteristics of cardiomyocytes from diabetic adult and normoglycemic aged mice. Acta Histochem. 2018, 120, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Bensley, J.G.; Stacy, V.K.; De Matteo, R.; Harding, R.; Black, M.J. Cardiac remodelling as a result of pre-term birth: Implications for future cardiovascular disease. Eur. Heart J. 2010, 31, 2058–2066. [Google Scholar] [CrossRef]
- Gjelsvik, K.J.; Besen-McNally, R.; Losick, V.P. Solving the Polyploid Mystery in Health and Disease. Trends Genet. 2019, 35, 6–14. [Google Scholar] [CrossRef]
- Donne, R.; Saroul-Aïnama, M.; Cordier, P.; Celton-Morizur, S.; Desdouets, C. Polyploidy in liver development, homeostasis and disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 391–405. [Google Scholar] [CrossRef]
- Storchova, Z.; Pellman, D. From polyploidy to aneuploidy, genome instability and cancer. Nat. Rev. Mol. Cell Biol. 2004, 5, 45–54. [Google Scholar] [CrossRef]
- Coward, J.; Harding, A. Size Does Matter: Why Polyploid Tumor Cells are Critical Drug Targets in the War on Cancer. Front. Oncol. 2014, 4, 123. [Google Scholar] [CrossRef]
- Ben-Porath, I.; Thomson, M.W.; Carey, V.J.; Ge, R.; Bell, G.W.; Regev, A.; Weinberg, R.A. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat. Genet. 2008, 40, 499–507. [Google Scholar] [CrossRef]
- Salmina, K.; Jankevics, E.; Huna, A.; Perminov, D.; Radovica, I.; Klymenko, T.; Ivanov, A.; Jascenko, E.; Scherthan, H.; Cragg, M.; et al. Up-regulation of the embryonic self-renewal network through reversible polyploidy in irradiated p53-mutant tumour cells. Exp. Cell Res. 2010, 316, 2099–2112. [Google Scholar] [CrossRef]
- Nguyen, H.G.; Ravid, K. Polyploidy: Mechanisms and Cancer Promotion in Hematopoietic and Other Cells. In Polyploidization and Cancer; Poon, R.Y.C., Ed.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2010; Volume 676, pp. 105–122. ISBN 978-1-4419-6198-3. [Google Scholar]
- Lagadec, C.; Vlashi, E.; Della Donna, L.; Dekmezian, C.; Pajonk, F. Radiation-Induced Reprogramming of Breast Cancer Cells: Radiation-Induced Cancer Stem Cells. Stem Cells 2012, 30, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Erenpreisa, J.; Cragg, M.S. Cancer: A matter of life cycle? Cell Biol. Int. 2007, 31, 1507–1510. [Google Scholar] [CrossRef] [PubMed]
- Erenpreisa, J.; Cragg, M.S. Life-Cycle Features of Tumour Cells. In Evolutionary Biology from Concept to Application; Pontarotti, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 61–71. ISBN 978-3-540-78992-5. [Google Scholar]
- Mirzayans, R.; Andrais, B.; Scott, A.; Wang, Y.; Kumar, P.; Murray, D. Multinucleated Giant Cancer Cells Produced in Response to Ionizing Radiation Retain Viability and Replicate Their Genome. Int. J. Mol. Sci. 2017, 18, 360. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. The dualistic origin of human tumors. Semin. Cancer Biol. 2018, 53, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Salmina, K.; Bojko, A.; Inashkina, I.; Staniak, K.; Dudkowska, M.; Podlesniy, P.; Rumnieks, F.; Vainshelbaum, N.M.; Pjanova, D.; Sikora, E.; et al. “Mitotic Slippage” and Extranuclear DNA in Cancer Chemoresistance: A Focus on Telomeres. Int. J. Mol. Sci. 2020, 21, 2779. [Google Scholar] [CrossRef] [PubMed]
- Mirzayans, R.; Andrais, B.; Murray, D. Roles of Polyploid/Multinucleated Giant Cancer Cells in Metastasis and Disease Relapse Following Anticancer Treatment. Cancers 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.D. Cancer: Beyond Speciation. In Advances in Cancer Research; Elsevier: Amsterdam, The Netherlands, 2011; Volume 112, pp. 283–350. ISBN 978-0-12-387688-1. [Google Scholar]
- Davies, P.C.W.; Lineweaver, C.H. Cancer tumors as Metazoa 1.0: Tapping genes of ancient ancestors. Phys. Biol. 2011, 8, 015001. [Google Scholar] [CrossRef] [PubMed]
- Davies, P. Exposing cancer’s deep evolutionary roots. Phys. Cancer Phys. World 2013, 26, 37–40. [Google Scholar] [CrossRef]
- Aktipis, C.A.; Boddy, A.M.; Jansen, G.; Hibner, U.; Hochberg, M.E.; Maley, C.C.; Wilkinson, G.S. Cancer across the tree of life: Cooperation and cheating in multicellularity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370. [Google Scholar] [CrossRef]
- Niculescu, V.F. Developmental and Non Developmental Polyploidy in Xenic and Axenic Cultured Stem Cell Lines of Entamoeba invadens and E. histolytica. Insights Stem Cells 2016, 2, 1–9. [Google Scholar]
- Vinogradov, A.E. Human transcriptome nexuses: Basic-eukaryotic and metazoan. Genomics 2010, 95, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, A.E.; Anatskaya, O.V. Evolutionary framework of the human interactome: Unicellular and multicellular giant clusters. Biosystems 2019, 181, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Domazet-Lošo, T.; Tautz, D. Phylostratigraphic tracking of cancer genes suggests a link to the emergence of multicellularity in metazoa. BMC Biol. 2010, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zou, Z.; Yang, J. Tracing Evolutionary Ages of Cancer-Driving Sites by Cancer Somatic Mutations. bioRxiv 2020. [Google Scholar] [CrossRef]
- Trigos, A.S.; Pearson, R.B.; Papenfuss, A.T.; Goode, D.L. Altered interactions between unicellular and multicellular genes drive hallmarks of transformation in a diverse range of solid tumors. Proc. Natl. Acad. Sci. USA 2017, 114, 6406–6411. [Google Scholar] [CrossRef] [PubMed]
- Bussey, K.J.; Cisneros, L.H.; Lineweaver, C.H.; Davies, P.C.W. Ancestral gene regulatory networks drive cancer. Proc. Natl. Acad. Sci. USA 2017, 114, 6160–6162. [Google Scholar] [CrossRef] [PubMed]
- Trigos, A.S.; Pearson, R.B.; Papenfuss, A.T.; Goode, D.L. How the evolution of multicellularity set the stage for cancer. Br. J. Cancer 2018, 118, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, A.E.; Anatskaya, O.V. Cell-cycle dependence of transcriptome gene modules: Comparison of regression lines. FEBS J. 2020, 287, 4427–4439. [Google Scholar] [CrossRef]
- Bizzarri, M.; Giuliani, A.; Cucina, A.; D’Anselmi, F.; Soto, A.M.; Sonnenschein, C. Fractal analysis in a systems biology approach to cancer. Semin. Cancer Biol. 2011, 21, 175–182. [Google Scholar] [CrossRef]
- Bizzarri, M.; Giuliani, A.; Minini, M.; Monti, N.; Cucina, A. Constraints Shape Cell Function and Morphology by Canalizing the Developmental Path along the Waddington’s Landscape. Bioessays 2020, 42, e1900108. [Google Scholar] [CrossRef]
- Erenpreisa, J.; Cragg, M.S. Three steps to the immortality of cancer cells: Senescence, polyploidy and self-renewal. Cancer Cell Int. 2013, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Van de Peer, Y.; Mizrachi, E.; Marchal, K. The evolutionary significance of polyploidy. Nat. Rev. Genet. 2017, 18, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Moein, S.; Adibi, R.; da Silva Meirelles, L.; Nardi, N.B.; Gheisari, Y. Cancer regeneration: Polyploid cells are the key drivers of tumor progression. Biochim. Biophys. Acta (BBA) Rev. Cancer 2020, 188408. [Google Scholar] [CrossRef] [PubMed]
- Mirzayans, R.; Murray, D. Intratumor Heterogeneity and Therapy Resistance: Contributions of Dormancy, Apoptosis Reversal (Anastasis) and Cell Fusion to Disease Recurrence. Int. J. Mol. Sci. 2020, 21, 1308. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, B.E.; Mikkelsen, T.S.; Xie, X.; Kamal, M.; Huebert, D.J.; Cuff, J.; Fry, B.; Meissner, A.; Wernig, M.; Plath, K.; et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 2006, 125, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, T.S.; Ku, M.; Jaffe, D.B.; Issac, B.; Lieberman, E.; Giannoukos, G.; Alvarez, P.; Brockman, W.; Kim, T.-K.; Koche, R.P.; et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 2007, 448, 553–560. [Google Scholar] [CrossRef]
- Widschwendter, M.; Fiegl, H.; Egle, D.; Mueller-Holzner, E.; Spizzo, G.; Marth, C.; Weisenberger, D.J.; Campan, M.; Young, J.; Jacobs, I.; et al. Epigenetic stem cell signature in cancer. Nat. Genet. 2007, 39, 157–158. [Google Scholar] [CrossRef]
- Balch, C.; Nephew, K.P.; Huang, T.H.-M.; Bapat, S.A. Epigenetic “bivalently marked” process of cancer stem cell-driven tumorigenesis. Bioessays 2007, 29, 842–845. [Google Scholar] [CrossRef]
- Blanco, E.; González-Ramírez, M.; Alcaine-Colet, A.; Aranda, S.; Di Croce, L. The Bivalent Genome: Characterization, Structure, and Regulation. Trends Genet. 2020, 36, 118–131. [Google Scholar] [CrossRef]
- Bernhart, S.H.; Kretzmer, H.; Holdt, L.M.; Jühling, F.; Ammerpohl, O.; Bergmann, A.K.; Northoff, B.H.; Doose, G.; Siebert, R.; Stadler, P.F.; et al. Changes of bivalent chromatin coincide with increased expression of developmental genes in cancer. Sci. Rep. 2016, 6, 37393. [Google Scholar] [CrossRef]
- Court, F.; Arnaud, P. An annotated list of bivalent chromatin regions in human ES cells: A new tool for cancer epigenetic research. Oncotarget 2017, 8, 4110–4124. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.I.; Kang, H.; De, S.; Kassis, J.A. Dynamic Competition of Polycomb and Trithorax in Transcriptional Programming. Annu. Rev. Biochem. 2020, 89, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, C.; Soto, A.M. Cancer Metastases: So Close and So Far. JNCI J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dang, C.V. Myc Overexpression Uncouples DNA Replication from Mitosis. Mol. Cell. Biol. 1999, 19, 5339–5351. [Google Scholar] [CrossRef] [PubMed]
- Grendler, J.; Lowgren, S.; Mills, M.; Losick, V.P. Wound-induced polyploidization is driven by Myc and supports tissue repair in the presence of DNA damage. Development 2019, 146, dev173005. [Google Scholar] [CrossRef]
- Soucek, L.; Whitfield, J.; Martins, C.P.; Finch, A.J.; Murphy, D.J.; Sodir, N.M.; Karnezis, A.N.; Swigart, L.B.; Nasi, S.; Evan, G.I. Modelling Myc inhibition as a cancer therapy. Nature 2008, 455, 679–683. [Google Scholar] [CrossRef]
- Evan, G. Taking a Back Door to Target Myc. Science 2012, 335, 293–294. [Google Scholar] [CrossRef]
- Kortlever, R.M.; Sodir, N.M.; Wilson, C.H.; Burkhart, D.L.; Pellegrinet, L.; Brown Swigart, L.; Littlewood, T.D.; Evan, G.I. Myc Cooperates with Ras by Programming Inflammation and Immune Suppression. Cell 2017, 171, 1301–1315. [Google Scholar] [CrossRef]
- Sui, Y.; Gu, R.; Janknecht, R. Crucial Functions of the JMJD1/KDM3 Epigenetic Regulators in Cancer. Mol. Cancer Res. 2020. [Google Scholar] [CrossRef]
- Neri, F.; Zippo, A.; Krepelova, A.; Cherubini, A.; Rocchigiani, M.; Oliviero, S. Myc Regulates the Transcription of the PRC2 Gene to Control the Expression of Developmental Genes in Embryonic Stem Cells. Mol. Cell. Biol. 2012, 32, 840–851. [Google Scholar] [CrossRef]
- Ullius, A.; Luscher-Firzlaff, J.; Costa, I.G.; Walsemann, G.; Forst, A.H.; Gusmao, E.G.; Kapelle, K.; Kleine, H.; Kremmer, E.; Vervoorts, J.; et al. The interaction of MYC with the trithorax protein ASH2L promotes gene transcription by regulating H3K27 modification. Nucleic Acids Res. 2014, 42, 6901–6920. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, A.E.; Shilina, M.A.; Anatskaya, O.V.; Alekseenko, L.L.; Fridlyanskaya, I.I.; Krasnenko, A.; Kim, A.; Korostin, D.; Ilynsky, V.; Elmuratov, A.; et al. Molecular Genetic Analysis of Human Endometrial Mesenchymal Stem Cells That Survived Sublethal Heat Shock. Stem Cells Int 2017, 17, 2362630. [Google Scholar] [CrossRef]
- Kallioniemi, A. Bone morphogenetic protein 4—A fascinating regulator of cancer cell behavior. Cancer Genet. 2012, 205, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Bach, D.-H.; Park, H.J.; Lee, S.K. The Dual Role of Bone Morphogenetic Proteins in Cancer. Mol. Ther. Oncolytics 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, H.; Qing, G. Targeting oncogenic Myc as a strategy for cancer treatment. Signal Transduct. Target. Ther. 2018, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Tovar, E.A.; Graveel, C.R. MET in human cancer: Germline and somatic mutations. Ann. Transl. Med. 2017, 5, 205. [Google Scholar] [CrossRef]
- Mitra, P. Transcription regulation of MYB: A potential and novel therapeutic target in cancer. Ann. Transl. Med. 2018, 6, 443. [Google Scholar] [CrossRef]
- Erenpreiss, J.O. Current Concepts of Malignant Growth; Zinâtne Publ.: Riga, Latvia, 1993. [Google Scholar]
- Moore, A.R.; Rosenberg, S.C.; McCormick, F.; Malek, S. RAS-targeted therapies: Is the undruggable drugged? Nat. Rev. Drug Discov. 2020, 19, 533–552. [Google Scholar] [CrossRef]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef]
- Hosaka, K.; Yang, Y.; Seki, T.; Du, Q.; Jing, X.; He, X.; Wu, J.; Zhang, Y.; Morikawa, H.; Nakamura, M.; et al. Therapeutic paradigm of dual targeting VEGF and PDGF for effectively treating FGF-2 off-target tumors. Nat. Commun. 2020, 11, 3704. [Google Scholar] [CrossRef]
- Khurana, N.; Sikka, S.C. Interplay between SOX9, Wnt/β-Catenin and Androgen Receptor Signaling in Castration-Resistant Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 2066. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Xu, Y.; He, T.; Qin, C.; Xu, J. Normal and disease-related biological functions of Twist1 and underlying molecular mechanisms. Cell Res. 2012, 22, 90–106. [Google Scholar] [CrossRef] [PubMed]
- Petrizzo, A.; Caruso, F.P.; Tagliamonte, M.; Tornesello, M.L.; Ceccarelli, M.; Costa, V.; Aprile, M.; Esposito, R.; Ciliberto, G.; Buonaguro, F.M.; et al. Identification and Validation of HCC-specific Gene Transcriptional Signature for Tumor Antigen Discovery. Sci. Rep. 2016, 6, 29258. [Google Scholar] [CrossRef] [PubMed]
- Davoli, T.; Xu, A.W.; Mengwasser, K.E.; Sack, L.M.; Yoon, J.C.; Park, P.J.; Elledge, S.J. Cumulative Haploinsufficiency and Triplosensitivity Drive Aneuploidy Patterns and Shape the Cancer Genome. Cell 2013, 155, 948–962. [Google Scholar] [CrossRef]
- Justice, J.; Malhotra, S.; Ruano, M.; Li, Y.; Zavala, G.; Lee, N.; Morgan, R.; Beemon, K. The MET Gene Is a Common Integration Target in Avian Leukosis Virus Subgroup J-Induced Chicken Hemangiomas. J. Virol. 2015, 89, 4712–4719. [Google Scholar] [CrossRef] [PubMed]
- Pienta, K.J.; Hammarlund, E.U.; Axelrod, R.; Amend, S.R.; Brown, J.S. Convergent Evolution, Evolving Evolvability, and the Origins of Lethal Cancer. Mol. Cancer Res. 2020, 18, 801–810. [Google Scholar] [CrossRef]
- Walther, V.; Hiley, C.T.; Shibata, D.; Swanton, C.; Turner, P.E.; Maley, C.C. Can oncology recapitulate paleontology? Lessons from species extinctions. Nat. Rev. Clin. Oncol. 2015, 12, 273–285. [Google Scholar] [CrossRef]
- Versteeg, R. Tumours outside the mutation box. Nature 2014, 506, 438–439. [Google Scholar] [CrossRef]
- Gatenby, R.A. Is the Genetic Paradigm of Cancer Complete? Radiology 2017, 284, 1–3. [Google Scholar] [CrossRef]
- Ganem, N.J.; Storchova, Z.; Pellman, D. Tetraploidy, aneuploidy and cancer. Curr. Opin. Genet. Dev. 2007, 17, 157–162. [Google Scholar] [CrossRef]
- López, S.; Lim, E.L.; Horswell, S.; Haase, K.; Huebner, A.; Dietzen, M.; Mourikis, T.P.; Watkins, T.B.K.; Rowan, A.; Dewhurst, S.M.; et al. Interplay between whole-genome doubling and the accumulation of deleterious alterations in cancer evolution. Nat. Genet. 2020, 52, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Beach, R.R.; Ricci-Tam, C.; Brennan, C.M.; Moomau, C.A.; Hsu, P.-H.; Hua, B.; Silberman, R.E.; Springer, M.; Amon, A. Aneuploidy Causes Non-genetic Individuality. Cell 2017, 169, 229–242.e21. [Google Scholar] [CrossRef] [PubMed]
- Salmina, K.; Huna, A.; Kalejs, M.; Pjanova, D.; Scherthan, H.; Cragg, M.S.; Erenpreisa, J. The Cancer Aneuploidy Paradox: In the Light of Evolution. Genes 2019, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Mojtahedi, M.; Skupin, A.; Zhou, J.; Castaño, I.G.; Leong-Quong, R.Y.Y.; Chang, H.; Trachana, K.; Giuliani, A.; Huang, S. Cell Fate Decision as High-Dimensional Critical State Transition. PLoS Biol. 2016, 14, e2000640. [Google Scholar] [CrossRef]
- Cavalli, G. Chromosome kissing. Curr. Opin. Genet. Dev. 2007, 17, 443–450. [Google Scholar] [CrossRef]
- Lieberman-Aiden, E.; van Berkum, N.L.; Williams, L.; Imakaev, M.; Ragoczy, T.; Telling, A.; Amit, I.; Lajoie, B.R.; Sabo, P.J.; Dorschner, M.O.; et al. Comprehensive Mapping of Long-Range Interactions Reveals Folding Principles of the Human Genome. Science 2009, 326, 289–293. [Google Scholar] [CrossRef]
- Solovei, I.; Thanisch, K.; Feodorova, Y. How to rule the nucleus: Divide et impera. Curr. Opin. Cell Biol. 2016, 40, 47–59. [Google Scholar] [CrossRef]
- Bakhmet, E.I.; Nazarov, I.B.; Gazizova, A.R.; Vorobyeva, N.E.; Kuzmin, A.A.; Gordeev, M.N.; Sinenko, S.A.; Aksenov, N.D.; Artamonova, T.O.; Khodorkovskii, M.A.; et al. hnRNP-K Targets Open Chromatin in Mouse Embryonic Stem Cells in Concert with Multiple Regulators: hnRNP-K Targets Open Chromatin in Mouse ESCs. Stem Cells 2019, 37, 1018–1029. [Google Scholar] [CrossRef]
- Nazarov, I.B.; Bakhmet, E.I.; Tomilin, A.N. KH-Domain Poly(C)-Binding Proteins as Versatile Regulators of Multiple Biological Processes. Biochem. Mosc. 2019, 84, 205–219. [Google Scholar] [CrossRef]
- Kauffman, S.A. The Origins of Order: Self-Organization and Selection in Evolution; Series on Directions in Condensed Matter Physics; World Scientific: Singapore, 1992; Volume 6, pp. 61–100. ISBN 978-9971-5-0537-0. [Google Scholar]
- Conant, G.C. Rapid reorganization of the transcriptional regulatory network after genome duplication in yeast. Proc. Biol. Sci. 2010, 277, 869–876. [Google Scholar] [CrossRef]
- Conant, G.C. The lasting after-effects of an ancient polyploidy on the genomes of teleosts. PLoS ONE 2020, 15, e0231356. [Google Scholar] [CrossRef] [PubMed]
- Cherstvy, A.G.; Teif, V.B. Structure-driven homology pairing of chromatin fibers: The role of electrostatics and protein-induced bridging. J. Biol. Phys. 2013, 39, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Jerković, I.; Szabo, Q.; Bantignies, F.; Cavalli, G. Higher-Order Chromosomal Structures Mediate Genome Function. J. Mol. Biol. 2020, 432, 676–681. [Google Scholar] [CrossRef]
- Vinogradov, A.E. Buffering: A Possible Passive-homeostasis Role for Redundant DNA. J. Theor. Biol. 1998, 193, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, A.E. Genome size and chromatin condensation in vertebrates. Chromosoma 2005, 113, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Sato, Y.; Furumichi, M.; Morishima, K.; Tanabe, M. New approach for understanding genome variations in KEGG. Nucleic Acids Res. 2019, 47, D590–D595. [Google Scholar] [CrossRef]
- Land, H.; Parada, L.F.; Weinberg, R.A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature 1983, 304, 596–602. [Google Scholar] [CrossRef]
- Knudson, A.G. Two genetic hits (more or less) to cancer. Nat. Rev. Cancer 2001, 1, 157–162. [Google Scholar] [CrossRef]
- Wang, C.; Lisanti, M.P.; Liao, D.J. Reviewing once more the c-myc and Ras collaboration: Converging at the cyclin D1-CDK4 complex and challenging basic concepts of cancer biology. Cell Cycle 2011, 10, 57–67. [Google Scholar] [CrossRef]
- Huang, S.; Ernberg, I.; Kauffman, S. Cancer attractors: A systems view of tumors from a gene network dynamics and developmental perspective. Semin. Cell Dev. Biol. 2009, 20, 869–876. [Google Scholar] [CrossRef]
- Huang, S.; Kauffman, S. How to escape the cancer attractor: Rationale and limitations of multi-target drugs. Semin. Cancer Biol. 2013, 23, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Erenpreisa, J.; Roach, H.I. Epigenetic selection as a possible component of transdifferentiation. Further study of the commitment of hypertrophic chondrocytes to become osteocytes. Mech. Ageing Dev. 1996, 87, 165–182. [Google Scholar] [CrossRef]
- Erenpreisa, J.; Salmiņa, K.; Belyayev, A.; Inashkina, I.; Cragg, M.S. Survival at the Brink. In Autophagy: Cancer, Other Pathologies, Inflammation, Immunity, Infection, and Aging; Elsevier: Amsterdam, The Netherlands, 2017; pp. 275–294. ISBN 978-0-12-812146-7. [Google Scholar]
- Domazet-Lošo, T.; Klimovich, A.; Anokhin, B.; Anton-Erxleben, F.; Hamm, M.J.; Lange, C.; Bosch, T.C.G. Naturally occurring tumours in the basal metazoan Hydra. Nat. Commun. 2014, 5, 4222. [Google Scholar] [CrossRef] [PubMed]
- Arthur, W. Evolution: A Developmental Approach; Wiley-Blackwell: Chichester, UK, 2011; ISBN 978-1-4051-8658-2. [Google Scholar]
- Erenpreisa, J.; Salmina, K.; Huna, A.; Jackson, T.R.; Vazquez-Martin, A.; Cragg, M.S. The “virgin birth”, polyploidy, and the origin of cancer. Oncoscience 2014, 2, 3–14. [Google Scholar] [CrossRef]
- Niu, N.; Mercado-Uribe, I.; Liu, J. Dedifferentiation into blastomere-like cancer stem cells via formation of polyploid giant cancer cells. Oncogene 2017, 36, 4887–4900. [Google Scholar] [CrossRef] [PubMed]
- Fais, S.; Overholtzer, M. Cell-in-cell phenomena, cannibalism, and autophagy: Is there a relationship? Cell Death Dis. 2018, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Goodkov, A.V.; Berdieva, M.A.; Podlipaeva, Y.I.; Demin, S.Y. The Chromatin Extrusion Phenomenon in Amoeba proteus Cell Cycle. J. Eukaryot. Microbiol. 2020, 67, 203–208. [Google Scholar] [CrossRef]
- Arguello, F. Atavistic Metamorphosis: A New and Logical Explanation for the Origin and Biological Nature of Cancer: With a Discussion on a Novel Approach to Treat Cancer. CreateSpace: Scotts Valley, CA, USA, 2011; ISBN 978-1-4609-6899-4. [Google Scholar]
- Lineweaver, C.H.; Davies, P.C.W.; Vincent, M.D. Targeting cancer’s weaknesses (not its strengths): Therapeutic strategies suggested by the atavistic model: Insights & Perspectives. BioEssays 2014, 36, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Raikov, I.B. Nuclear apparatus of Mesokaryotic Protozoa. In The Protozoan Nucleus, Morphology and Evolution; Cell Biology Monographs; Springer: New York, NY, USA, 1982; pp. 1–174. [Google Scholar]
- Demin, S.Y.; Berdieva, M.A.; Podlipaeva, Y.I.; Goodkov, A.V. Karyotypic instability of endoprophase and mitotic cells of Amoeba sp. strain Cont from the “proteus-type” group (Amoebozoa, Euamoebida, Amoebidae). Eur. J. Protistol. 2020, 74, 125691. [Google Scholar] [CrossRef]
- Niculescu, V.F. The reproductive life cycle of cancer: Hypotheses of cell of origin, TP53 drivers and stem cell conversions in the light of the atavistic cancer cell theory. Med Hypotheses 2019, 123, 19–23. [Google Scholar] [CrossRef]
- Nanjundiah, V. Cellular slime mold development as a paradigm for the transition from unicellular to multicellular life. In Multicellularity: Origins and Evolution; MIT Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Andreani, T.S.; Itoh, T.Q.; Yildirim, E.; Hwangbo, D.-S.; Allada, R. Genetics of Circadian Rhythms. Sleep Med. Clin. 2015, 10, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Camponeschi, I.; Damasco, A.; Uversky, V.N.; Giuliani, A.; Bianchi, M.M. Phenotypic suppression caused by resonance with light-dark cycles indicates the presence of a 24-hours oscillator in yeast and suggests a new role of intrinsically disordered protein regions as internal mediators. J. Biomol. Struct. Dyn. 2020. [Google Scholar] [CrossRef] [PubMed]
- Øvrebø, J.I.; Edgar, B.A. Polyploidy in tissue homeostasis and regeneration. Development 2018, 145. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Martin, A.; Anatskaya, O.V.; Giuliani, A.; Erenpreisa, J.; Huang, S.; Salmina, K.; Inashkina, I.; Huna, A.; Nikolsky, N.N.; Vinogradov, A.E. Somatic polyploidy is associated with the upregulation of c-MYC interacting genes and EMT-like signature. Oncotarget 2016, 7, 75235–75260. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, A.; Crawford, D.L. Variation within and among species in gene expression: Raw material for evolution: Review of gene expression variation. Mol. Ecol. 2006, 15, 1197–1211. [Google Scholar] [CrossRef]
- Otto, S.P. The Evolutionary Consequences of Polyploidy. Cell 2007, 131, 452–462. [Google Scholar] [CrossRef]
- Brawand, D.; Soumillon, M.; Necsulea, A.; Julien, P.; Csárdi, G.; Harrigan, P.; Weier, M.; Liechti, A.; Aximu-Petri, A.; Kircher, M.; et al. The evolution of gene expression levels in mammalian organs. Nature 2011, 478, 343–348. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Vinogradov, A.E.; Anatskaya, O.V. Systemic evolutionary changes in mammalian gene expression. Biosystems 2020, 198, 104256. [Google Scholar] [CrossRef]
- Sayers, E.W.; Beck, J.; Brister, J.R.; Bolton, E.E.; Canese, K.; Comeau, D.C.; Funk, K.; Ketter, A.; Kim, S.; Kimchi, A.; et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2020, 48, D9–D16. [Google Scholar] [CrossRef]
- Seyednasrollah, F.; Laiho, A.; Elo, L.L. Comparison of software packages for detecting differential expression in RNA-seq studies. Brief. Bioinform. 2015, 16, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A. The application of principal component analysis to drug discovery and biomedical data. Drug Discov. Today 2017, 22, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Roden, J.C.; King, B.W.; Trout, D.; Mortazavi, A.; Wold, B.J.; Hart, C.E. Mining gene expression data by interpreting principal components. BMC Bioinform. 2006, 7, 194. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, A.E. ’Genome design’ model and multicellular complexity: Golden middle. Nucleic Acids Res. 2006, 34, 5906–5914. [Google Scholar] [CrossRef]
- Vinogradov, A.E.; Anatskaya, O.V. DNA helix: The importance of being AT-rich. Mamm. Genome 2017, 28, 455–464. [Google Scholar] [CrossRef]
- The Gene Ontology Consortium. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019, 47, D330–D338. [Google Scholar] [CrossRef]
- Storey, J.D.; Tibshirani, R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA 2003, 100, 9440–9445. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).