Carvedilol, an Adrenergic Blocker, Suppresses Melanin Synthesis by Inhibiting the cAMP/CREB Signaling Pathway in Human Melanocytes and Ex Vivo Human Skin Culture
Abstract
1. Introduction
2. Results
2.1. Carvedilol Suppresses Melanogenesis
2.2. Carvedilol Inhibits the Expression of MITF and Its Target Genes and Decreases Phospho-CREB Levels in NHMs
2.3. Melanin Index and Immunohistochemical Staining in Ex Vivo Human Skin Culture
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Lines and Cell Culture
4.3. Antibodies and Western Blots
4.4. Melanin Content
4.5. Cellular Tyrosinase Activity
4.6. Immunohistochemical Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Gillbro, J.M.; Olsson, M.J. The melanogenesis and mechanisms of skin-lightening agents—Existing and new approaches. Int. J. Cosmet. Sci. 2011, 33, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Shin, H.J.; Noh, T.K.; Choi, K.H.; Chang, S.E. Treatment of Melasma and Post-Inflammatory Hyperpigmentation by a Picosecond 755-nm Alexandrite Laser in Asian Patients. Ann. Dermatol. 2017, 29, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Park, J.H.; Lee, D.Y.; Lee, J.H. Acquired Bilateral Dyspigmentation on Face and Neck: Clinically Appropriate Approaches. J. Korean Med. Sci. 2016, 31, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Na, J.I.; Lee, J.H.; Roh, M.R.; Ko, J.Y.; Chang, S.E. Postinflammatory hyperpigmentation associated with treatment of solar lentigines using a Q-Switched 532-nm Nd: YAG laser: A multicenter survey. J. Dermatolog. Treat. 2017, 28, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Araki, J.; Eto, H.; Doi, K.; Hirai, R.; Kuno, S.; Higashino, T.; Yoshimura, K. A prospective randomized controlled study of oral tranexamic acid for preventing postinflammatory hyperpigmentation after Q-switched ruby laser. Dermatol. Surg. 2011, 37, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.R.; Anderson, R.R. Ineffective treatment of refractory melasma and postinflammatory hyperpigmentation by Q-switched ruby laser. J. Dermatol. Surg. Oncol. 1994, 20, 592–597. [Google Scholar] [CrossRef]
- Gillbro, J.M.; Marles, L.K.; Hibberts, N.A.; Schallreuter, K.U. Autocrine catecholamine biosynthesis and the beta-adrenoceptor signal promote pigmentation in human epidermal melanocytes. J. Investig. Dermatol. 2004, 123, 346–353. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Korner, C.; Pittelkow, M.R.; Swanson, N.N.; Gardner, M.L. The induction of the alpha-1-adrenoceptor signal transduction system on human melanocytes. Exp. Dermatol. 1996, 5, 20–23. [Google Scholar] [CrossRef]
- Sivamani, R.K.; Shi, B.; Griffiths, E.; Vu, S.M.; Lev-Tov, H.A.; Dahle, S.; Chigbrow, M.; La, T.D.; Mashburn, C.; Peavy, T.R.; et al. Acute wounding alters the beta2-adrenergic signaling and catecholamine synthetic pathways in keratinocytes. J. Investig. Dermatol. 2014, 134, 2258–2266. [Google Scholar] [CrossRef]
- Emery, A.C. Catecholamine receptors: Prototypes for GPCR-based drug discovery. Adv. Pharmacol. 2013, 68, 335–356. [Google Scholar]
- Do Vale, G.T.; Ceron, C.S.; Gonzaga, N.A.; Simplicio, J.A.; Padovan, J.C. Three Generations of beta-blockers: History, Class Differences and Clinical Applicability. Curr. Hypertens. Rev. 2019, 15, 22–31. [Google Scholar] [PubMed]
- McTavish, D.; Campoli-Richards, D.; Sorkin, E.M. Carvedilol: A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs 1993, 45, 232–258. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Lee, J.Y. Pronounced facial flushing and persistent erythema of rosacea effectively treated by carvedilol, a nonselective beta-adrenergic blocker. J. Am. Acad. Dermatol. 2012, 67, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Pietschke, K.; Schaller, M. Long-term management of distinct facial flushing and persistent erythema of rosacea by treatment with carvedilol. J. Dermatolog. Treat. 2018, 29, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Yeung, S.; Thakkar, A.; Huang, K.M.; Liu, M.M.; Kanassatega, R.S.; Parsa, C.; Orlando, R.; Jackson, E.K.; Andresen, B.T.; et al. Prevention of skin carcinogenesis by the beta-blocker carvedilol. Cancer Prev. Res. 2015, 8, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liang, S.; Shahid, A.; Andresen, B.T.; Huang, Y. The beta-Blocker Carvedilol Prevented Ultraviolet-Mediated Damage of Murine Epidermal Cells and 3D Human Reconstructed Skin. Int. J. Mol. Sci. 2020, 21, 798. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.M.; Liang, S.; Yeung, S.; Oiyemhonlan, E.; Cleveland, K.H.; Parsa, C.; Orlando, R.; Meyskens, F.L., Jr.; Andresen, B.T.; Huang, Y. Topically Applied Carvedilol Attenuates Solar Ultraviolet Radiation Induced Skin Carcinogenesis. Cancer Prev. Res. 2017, 10, 598–606. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar]
- Cleveland, K.H.; Liang, S.; Chang, A.; Huang, K.M.; Chen, S.; Guo, L.; Huang, Y.; Andresen, B.T. Carvedilol inhibits EGF-mediated JB6 P+ colony formation through a mechanism independent of adrenoceptors. PLoS ONE 2019, 14, e0217038. [Google Scholar] [CrossRef]
- Dezong, G.; Zhongbing, M.; Qinye, F.; Zhigang, Y. Carvedilol suppresses migration and invasion of malignant breast cells by inactivating Src involving cAMP/PKA and PKCdelta signaling pathway. J. Cancer Res. Ther. 2014, 10, 998–1003. [Google Scholar]
- Rzepka, Z.; Buszman, E.; Beberok, A.; Wrzesniok, D. From tyrosine to melanin: Signaling pathways and factors regulating melanogenesis. Postepy Hig. Med. Dosw. Online 2016, 70, 695–708. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, J.Y.; Oh, S.W.; Kang, M.; Lee, S.E.; Jung, E.; Park, Y.S.; Lee, J. Globular adiponectin acts as a melanogenic signal in human epidermal melanocytes. Br. J. Dermatol. 2018, 179, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [PubMed]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Howe, J.; Costantino, R.; Slominski, A. On the putative mechanism of induction and regulation of melanogenesis by L-tyrosine. Acta Derm Venereol. 1991, 71, 150–152. [Google Scholar]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Jung, J.A.; Kim, B.J.; Kim, M.S.; You, H.J.; Yoon, E.S.; Dhong, E.S.; Park, S.H.; Kim, D.W. Protective Effect of Botulinum Toxin against Ultraviolet-Induced Skin Pigmentation. Plast. Reconstr. Surg. 2019, 144, 347–356. [Google Scholar] [CrossRef]
- Jeong, Y.M.; Oh, W.K.; Tran, T.L.; Kim, W.K.; Sung, S.H.; Bae, K.; Lee, S.; Sung, J.H. Aglycone of Rh4 inhibits melanin synthesis in B16 melanoma cells: Possible involvement of the protein kinase A pathway. Biosci. Biotechnol. Biochem. 2013, 77, 119–125. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, D.; Hong, A.R.; Kim, J.H.; Yoo, H.; Kim, J.; Kim, I.; Kang, S.W.; Chang, S.E.; Song, Y. Therapeutic Potential of Rottlerin for Skin Hyperpigmentary Disorders by Inhibiting the Transcriptional Activity of CREB-Regulated Transcription Coactivators. J. Investig. Dermatol. 2019, 139, 2359–2367.e2. [Google Scholar] [CrossRef]
- Das, A.; Ghosh, A.; Kumar, P. Chemical leukoderma due to hydroquinone: An unusual phenomenon. Indian J. Dermatol. Venereol. Leprol. 2019, 85, 567. [Google Scholar] [CrossRef]
- Jow, T.; Hantash, B.M. Hydroquinone-induced depigmentation: Case report and review of the literature. Dermatitis 2014, 25, e1–e5. [Google Scholar] [CrossRef]
- Kersey, P.; Stevenson, C.J. Vitiligo and occupational exposure to hydroquinone from servicing self-photographing machines. Contact Dermat. 1981, 7, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Kondo, M.; Sato, K.; Umeda, M.; Kawabata, K.; Takahashi, Y.; Suzuki, T.; Matsunaga, K.; Inoue, S. Rhododendrol, a depigmentation-inducing phenolic compound, exerts melanocyte cytotoxicity via a tyrosinase-dependent mechanism. Pigment Cell Melanoma Res. 2014, 27, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Sumikawa, Y.; Hida, T.; Kamiya, T.; Kase, K.; Ishii-Osai, Y.; Kato, J.; Kan, Y.; Kamiya, S.; Sato, Y.; et al. Clinical and epidemiological analysis in 149 cases of rhododendrol-induced leukoderma. J. Dermatol. 2017, 44, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.E. Chemical-Induced Vitiligo. Dermatol. Clin. 2017, 35, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Li-Sha, G.; Yi-He, C.; Na-Dan, Z.; Teng, Z.; Yue-Chun, L. Effects of carvedilol treatment on cardiac cAMP response element binding protein expression and phosphorylation in acute coxsackievirus B3-induced myocarditis. BMC Cardiovasc. Disord. 2013, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Steinberg, S.F. S49G and R389G polymorphisms of the beta(1)-adrenergic receptor influence signaling via the cAMP-PKA and ERK pathways. Physiol. Genom. 2013, 45, 1186–1192. [Google Scholar] [CrossRef]
- Lee, A.Y.; Noh, M. The regulation of epidermal melanogenesis via cAMP and/or PKC signaling pathways: Insights for the development of hypopigmenting agents. Arch. Pharm. Res. 2013, 36, 792–801. [Google Scholar] [CrossRef]
- Garcia-Borron, J.C.; Abdel-Malek, Z.; Jimenez-Cervantes, C. MC1R, the cAMP pathway, and the response to solar UV: Extending the horizon beyond pigmentation. Pigment Cell Melanoma Res. 2014, 27, 699–720. [Google Scholar] [CrossRef]
- Lin, C.B.; Babiarz, L.; Liebel, F.; Roydon Price, E.; Kizoulis, M.; Gendimenico, G.J.; Fisher, D.E.; Seiberg, M. Modulation of microphthalmia-associated transcription factor gene expression alters skin pigmentation. J. Investig. Dermatol. 2002, 119, 1330–1340. [Google Scholar] [CrossRef]
- Kim, J.H.; Hong, A.R.; Kim, Y.H.; Yoo, H.; Kang, S.W.; Chang, S.E.; Song, Y. JNK suppresses melanogenesis by interfering with CREB-regulated transcription coactivator 3-dependent MITF expression. Theranostics 2020, 10, 4017–4029. [Google Scholar] [CrossRef] [PubMed]
- Rios, M.; Habecker, B.; Sasaoka, T.; Eisenhofer, G.; Tian, H.; Landis, S.; Chikaraishi, D.; Roffler-Tarlov, S. Catecholamine synthesis is mediated by tyrosinase in the absence of tyrosine hydroxylase. J. Neurosci. 1999, 19, 3519–3526. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, M.; Marino, F.; Bombelli, R.; Ferrari, M.; Lecchini, S.; Frigo, G. Endogenous catecholamine synthesis, metabolism, storage and uptake in human neutrophils. Life Sci. 1999, 64, 975–981. [Google Scholar] [CrossRef]
- Marino, F.; Cosentino, M.; Bombelli, R.; Ferrari, M.; Lecchini, S.; Frigo, G. Endogenous catecholamine synthesis, metabolism storage, and uptake in human peripheral blood mononuclear cells. Exp. Hematol. 1999, 27, 489–495. [Google Scholar] [CrossRef]
- Sorriento, D.; Santulli, G.; Del Giudice, C.; Anastasio, A.; Trimarco, B.; Iaccarino, G. Endothelial cells are able to synthesize and release catecholamines both in vitro and in vivo. Hypertension 2012, 60, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Cucchi, M.L.; Frattini, P.; Santagostino, G.; Preda, S.; Orecchia, G. Catecholamines increase in the urine of non-segmental vitiligo especially during its active phase. Pigment Cell Res. 2003, 16, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Salzer, B.A.; Schallreuter, K.U. Investigation of the personality structure in patients with vitiligo and a possible association with impaired catecholamine metabolism. Dermatology 1995, 190, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A. Neuroendocrine activity of the melanocyte. Exp. Dermatol. 2009, 18, 760–763. [Google Scholar] [CrossRef]
- Gannu, R.; Vishnu, Y.V.; Kishan, V.; Rao, Y.M. In vitro permeation of carvedilol through porcine skin: Effect of vehicles and penetration enhancers. PDA J. Pharm. Sci. Technol. 2008, 62, 256–263. [Google Scholar]
- Kshirsagar, S.J.; Bhalekar, M.R.; Mohapatra, S.K. Development and evaluation of carvedilol-loaded transdermal drug delivery system: In-vitro and in-vivo characterization study. Drug Dev. Ind. Pharm. 2012, 38, 1530–1537. [Google Scholar] [CrossRef]
- Tanwar, Y.S.; Chauhan, C.S.; Sharma, A. Development and evaluation of carvedilol transdermal patches. Acta Pharm. 2007, 57, 151–159. [Google Scholar] [CrossRef] [PubMed]



 : inhibition,
: inhibition,  : decrease).
: decrease).
 : inhibition,
: inhibition,  : decrease).
: decrease).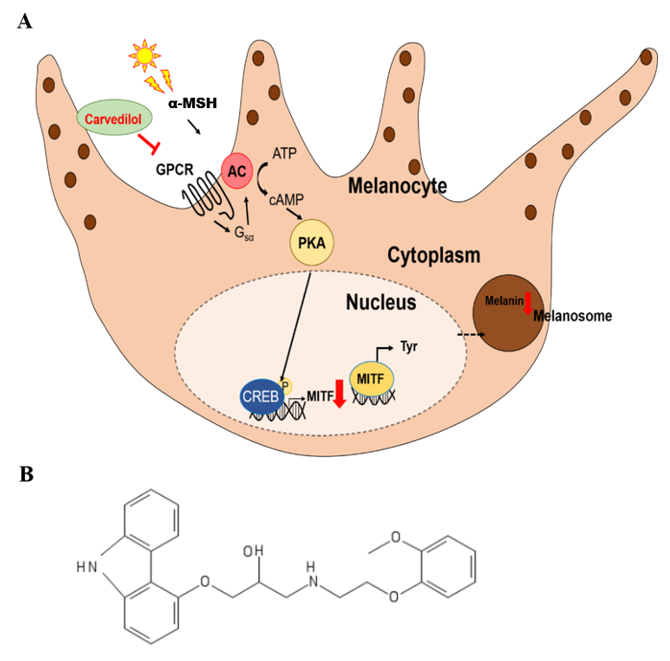
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, M.E.; Yoo, H.; Lee, H.-R.; Moon, I.J.; Lee, W.J.; Song, Y.; Chang, S.E. Carvedilol, an Adrenergic Blocker, Suppresses Melanin Synthesis by Inhibiting the cAMP/CREB Signaling Pathway in Human Melanocytes and Ex Vivo Human Skin Culture. Int. J. Mol. Sci. 2020, 21, 8796. https://doi.org/10.3390/ijms21228796
Choi ME, Yoo H, Lee H-R, Moon IJ, Lee WJ, Song Y, Chang SE. Carvedilol, an Adrenergic Blocker, Suppresses Melanin Synthesis by Inhibiting the cAMP/CREB Signaling Pathway in Human Melanocytes and Ex Vivo Human Skin Culture. International Journal of Molecular Sciences. 2020; 21(22):8796. https://doi.org/10.3390/ijms21228796
Chicago/Turabian StyleChoi, Myoung Eun, Hanju Yoo, Ha-Ri Lee, Ik Joon Moon, Woo Jin Lee, Youngsup Song, and Sung Eun Chang. 2020. "Carvedilol, an Adrenergic Blocker, Suppresses Melanin Synthesis by Inhibiting the cAMP/CREB Signaling Pathway in Human Melanocytes and Ex Vivo Human Skin Culture" International Journal of Molecular Sciences 21, no. 22: 8796. https://doi.org/10.3390/ijms21228796
APA StyleChoi, M. E., Yoo, H., Lee, H.-R., Moon, I. J., Lee, W. J., Song, Y., & Chang, S. E. (2020). Carvedilol, an Adrenergic Blocker, Suppresses Melanin Synthesis by Inhibiting the cAMP/CREB Signaling Pathway in Human Melanocytes and Ex Vivo Human Skin Culture. International Journal of Molecular Sciences, 21(22), 8796. https://doi.org/10.3390/ijms21228796




