The Molecular Network of YAP/Yorkie at the Cell Cortex and their Role in Ocular Morphogenesis
Abstract
1. Introduction
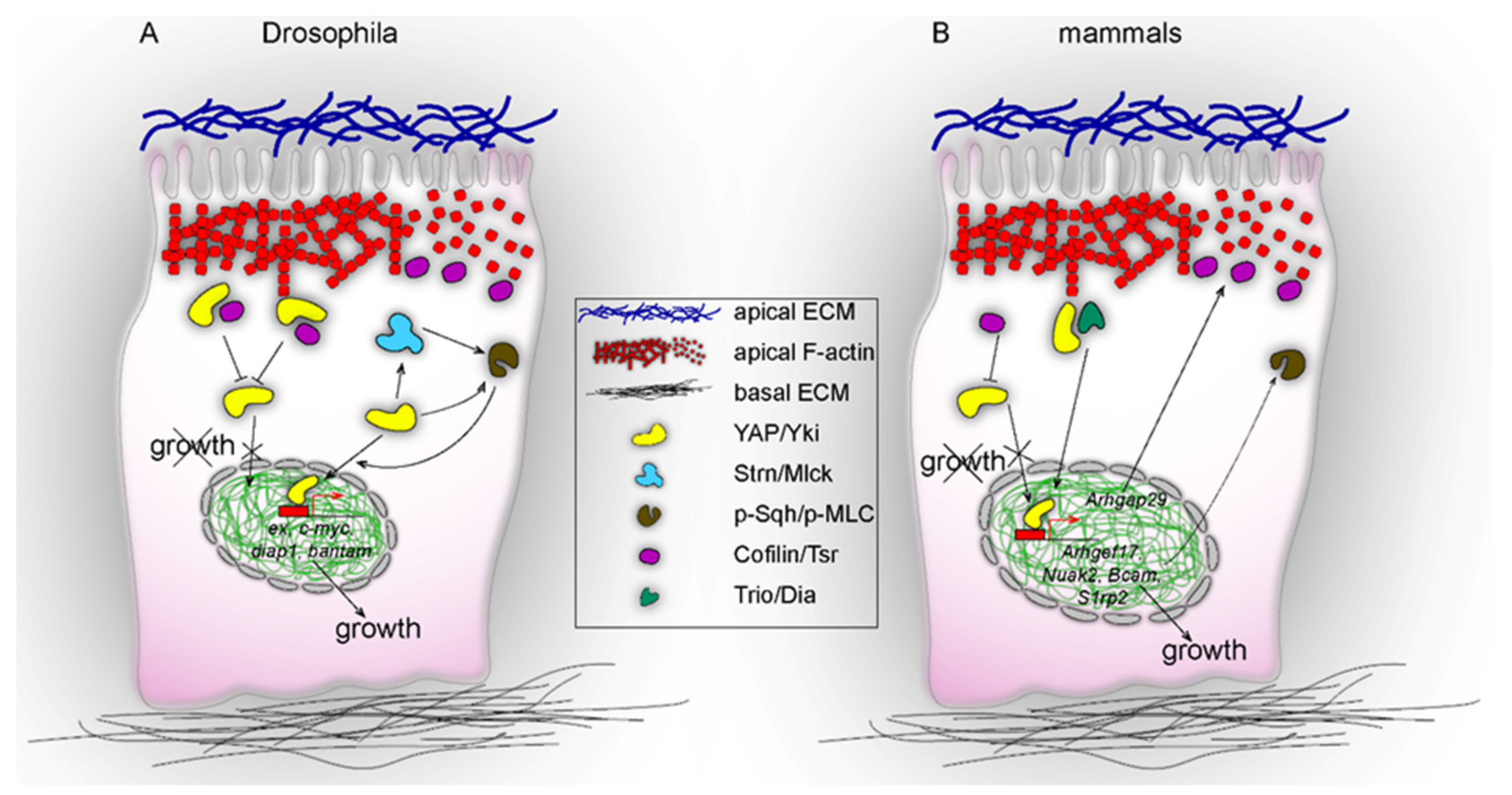
2. Apical Cell Cortex: A Site of YAP/Yki and Actin Cytoskeleton Interactions
3. YAP/Yki in Eye Development
4. YAP and Actin Cytoskeleton in Eye Development and Disease
5. Concluding Remarks
Funding
Conflicts of Interest
Abbreviations
| ARHGAP29 | Rho GTPase activating protein 29 |
| ARHGEF17 | Rho guanine nucleotide exchange factor 17 |
| Arp2/3 | Actin related protein 2/3 |
| CAPT | Capulet |
| ChIP | Chromatin immunoprecipitation |
| CPA | Capping protein alpha |
| CPB | Capping protein beta |
| Dia | Diaphanous |
| ERM | Ezrin-radixin-moesin |
| EYS | Eyes shut |
| HRMVEC | Human retinal microvascular endothelial cells |
| LATS1/2 | Large tumour-suppressor kinase 1/2 |
| LIMK | LIM Kinase |
| MLC | Myosin light chain |
| MLCK | Myosin light chain kinase |
| MST1/2 | Mammalian ste20-like kinase 1/2 |
| RhoGAP | Rho GTPase activating protein |
| ROCK | Rho-associated protein kinase |
| RPC | Retinal progenitor cell |
| RPE | Retinal pigmented epithelium |
| TBD | TEAD binding domain |
| TSR | Twinstar |
| YAP | Yes-associated protein |
| Yki | Yorkie |
References
- Chugh, P.; Paluch, E.K. The actin cortex at a glance. J. Cell Sci. 2018, 131, jcs186254. [Google Scholar] [CrossRef]
- Wagner, M.C.; Barylko, B.; E Albanesi, J. Tissue distribution and subcellular localization of mammalian myosin I. J. Cell Biol. 1992, 119, 163–170. [Google Scholar] [CrossRef]
- Mukhina, S.; Wang, Y.L.; Murata-Hori, M. Alpha-actinin is required for tightly regulated remodeling of the actin cortical network during cytokinesis. Dev. Cell 2007, 13, 554–565. [Google Scholar] [CrossRef]
- Reichl, E.M.; Ren, Y.; Morphew, M.K.; Delannoy, M.; Effler, J.C.; Girard, K.D.; Divi, S.; Iglesias, P.A.; Kuo, S.C.; Robinson, D.N. Interactions between Myosin and Actin Crosslinkers Control Cytokinesis Contractility Dynamics and Mechanics. Curr. Biol. 2008, 18, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Biro, M.; Romeo, Y.; Kroschwald, S.; Bovellan, M.; Boden, A.; Tcherkezian, J.; Roux, P.P.; Charras, G.; Paluch, E.K. Cell cortex composition and homeostasis resolved by integrating proteomics and quantitative imaging. Cytoskeleton 2013, 70, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Bretscher, A.; A Edwards, K.; Fehon, R.G. ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 2002, 3, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.G.; Moroishi, T.; Guan, K.-L. YAP and TAZ: a nexus for Hippo signaling and beyond. Trends Cell Biol. 2015, 25, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Moroishi, T.; Hansen, C.G.; Guan, K.-L. The emerging roles of YAP and TAZ in cancer. Nat. Rev. Cancer 2015, 15, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Moya, I.M.; Halder, G. Hippo–YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat. Rev. Mol. Cell Biol. 2019, 20, 211–226. [Google Scholar] [CrossRef]
- Fernandez, B.G.; Gaspar, P.; Bras-Pereira, C.; Jezowska, B.; Rebelo, S.R.; Janody, F. Actin-Capping Protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development 2011, 138, 2337–2346. [Google Scholar] [CrossRef]
- Sansores-Garcia, L.; Bossuyt, W.; Wada, K.-I.; Yonemura, S.; Tao, C.; Sasaki, H.; Halder, G. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 2011, 30, 2325–2335. [Google Scholar] [CrossRef] [PubMed]
- Aragona, M.; Panciera, T.; Manfrin, A.; Giulitti, S.; Michielin, F.; Elvassore, N.; Dupont, S.; Piccolo, S. A Mechanical Checkpoint Controls Multicellular Growth through YAP/TAZ Regulation by Actin-Processing Factors. Cell 2013, 154, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nat. Cell Biol. 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.; Morales-Navarrete, H.; Seifert, S.; Wilsch-Braeuninger, M.; Dahmen, U.; Tanaka, E.M.; Brusch, L.; Kalaidzidis, Y.; Zerial, M. Bile canaliculi remodeling activates YAP via the actin cytoskeleton during liver regeneration. Mol. Syst. Biol. 2020, 16, e8985. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Adler, P.N. Regulation of cell shape, wing hair initiation and the actin cytoskeleton by Trc/Fry and Wts/Mats complexes. Dev. Biol. 2010, 341, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Yao, E.; Zhang, K.; Jiang, X.; Croll, S.; Thompson-Peer, K.; Chuang, P.-T. YAP is essential for mechanical force production and epithelial cell proliferation during lung branching morphogenesis. eLife 2017, 6, e21130. [Google Scholar] [CrossRef] [PubMed]
- Lucas, E.P.; Khanal, I.; Gaspar, P.; Fletcher, G.C.; Polesello, C.; Tapon, N.; Thompson, B.J. The Hippo pathway polarizes the actin cytoskeleton during collective migration of Drosophila border cells. J. Cell Biol. 2013, 201, 875–885. [Google Scholar] [CrossRef]
- Skouloudaki, K.; Christodoulou, I.; Khalili, D.; Tsarouhas, V.; Samakovlis, C.; Tomancak, P.; Knust, E.; Papadopoulos, D.K. Yorkie controls tube length and apical barrier integrity during airway development. J. Cell Biol. 2019, 218, 2762–2781. [Google Scholar] [CrossRef]
- Xu, J.; Vanderzalm, P.J.; Ludwig, M.; Su, T.; Tokamov, S.A.; Fehon, R.G. Yorkie Functions at the Cell Cortex to Promote Myosin Activation in a Non-transcriptional Manner. Dev. Cell 2018, 46, 271–284.e5. [Google Scholar] [CrossRef]
- Densham, R.M.; O’Neill, E.; Munro, J.; Konig, I.; Anderson, K.; Kolch, W.; Olson, M.F. MST kinases monitor actin cytoskeletal integrity and signal via c-Jun N-terminal kinase stress-activated kinase to regulate p21Waf1/Cip1 stability. Mol. Cell Biol. 2009, 29, 6380–6390. [Google Scholar] [CrossRef]
- Hirota, T.; Morisaki, T.; Nishiyama, Y.; Marumoto, T.; Tada, K.; Hara, T.; Masuko, N.; Inagaki, M.; Hatakeyama, K.; Saya, H. Zyxin, a Regulator of Actin Filament Assembly, Targets the Mitotic Apparatus by Interacting with H-Warts/Lats1 Tumor Suppressor. J. Cell Biol. 2000, 149, 1073–1086. [Google Scholar] [CrossRef] [PubMed]
- Rauskolb, C.; Pan, G.; Reddy, B.V.V.G.; Oh, H.; Irvine, K.D. Zyxin Links Fat Signaling to the Hippo Pathway. PLoS Biol. 2011, 9, e1000624. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, K.; Hao, Y.; Li, D.-M.; Stewart, R.A.; Insogna, K.L.; Xu, T. LATS1 tumour suppressor affects cytokinesis by inhibiting LIMK1. Nat. Cell Biol. 2004, 6, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Rauskolb, C.; Sun, S.; Sun, G.; Pan, Y.; Irvine, K.D. Cytoskeletal Tension Inhibits Hippo Signaling through an Ajuba-Warts Complex. Cell 2014, 158, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Chen, J.; Lim, Y.B.; Finch-Edmondson, M.L.; Seshachalam, V.P.; Qin, L.; Jiang, T.; Low, B.C.; Singh, H.; Lim, C.T.; et al. YAP Regulates Actin Dynamics through ARHGAP29 and Promotes Metastasis. Cell Rep. 2017, 19, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wu, S.; Barrera, J.; Matthews, K.; Pan, D. The Hippo Signaling Pathway Coordinately Regulates Cell Proliferation and Apoptosis by Inactivating Yorkie, the Drosophila Homolog of YAP. Cell 2005, 122, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Hasegawa, H.; Hyodo, T.; Ito, S.; Asano, E.; Yuang, H.; Funasaka, K.; Shimokata, K.; Hasegawa, Y.; Hamaguchi, M.; et al. ARHGAP18, a GTPase-activating protein for RhoA, controls cell shape, spreading, and motility. Mol. Biol. Cell 2011, 22, 3840–3852. [Google Scholar] [CrossRef]
- Porazinski, S.; Wang, H.; Asaoka, Y.; Behrndt, M.; Miyamoto, T.; Morita, H.; Hata, S.; Sasaki, T.; Krens, S.F.G.; Osada, Y.; et al. YAP is essential for tissue tension to ensure vertebrate 3D body shape. Nat. Cell Biol. 2015, 521, 217–221. [Google Scholar] [CrossRef]
- Kim, J.; Park, D.-Y.; Bae, H.; Park, D.Y.; Kim, D.; Lee, C.-K.; Song, S.; Chung, T.-Y.; Lim, D.H.; Kubota, Y.; et al. Impaired angiopoietin/Tie2 signaling compromises Schlemm’s canal integrity and induces glaucoma. J. Clin. Investig. 2017, 127, 3877–3896. [Google Scholar] [CrossRef]
- Neto, F.; Klaus-Bergmann, A.; Ong, Y.T.; Alt, S.; Vion, A.C.; Szymborska, A.; Carvalho, J.R.; Hollfinger, I.; Bartels-Klein, E.; Franco, C.A.; et al. YAP and TAZ regulate adherens junction dynamics and endothelial cell distribution during vascular development. eLife 2018, 7, e31037. [Google Scholar] [CrossRef]
- Sakabe, M.; Fan, J.; Odaka, Y.; Liu, N.; Hassan, A.; Duan, X.; Stump, P.; Byerly, L.; Donaldson, M.; Hao, J.; et al. YAP/TAZ-CDC42 signaling regulates vascular tip cell migration. Proc. Natl. Acad. Sci. USA 2017, 114, 10918–10923. [Google Scholar] [CrossRef] [PubMed]
- Hamon, A.; Masson, C.; Bitard, J.; Gieser, L.; Roger, J.E.; Perron, M. Retinal Degeneration Triggers the Activation of YAP/TEAD in Reactive Müller Cells. Investig. Opthalmology Vis. Sci. 2017, 58, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, R.; Lee, J.H.J.; Shin, J.; Nickas, J.; Kim, S.; Cho, S.-H. Yap is essential for retinal progenitor cell cycle progression and RPE cell fate acquisition in the developing mouse eye. Dev. Biol. 2016, 419, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, Y.; Hata, S.; Namae, M.; Furutani-Seiki, M.; Nishina, H. The Hippo Pathway Controls a Switch between Retinal Progenitor Cell Proliferation and Photoreceptor Cell Differentiation in Zebrafish. PLoS ONE 2014, 9, e97365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Deo, M.; Thompson, R.C.; Uhler, M.D.; Turner, D.L. Negative regulation of Yap during neuronal differentiation. Dev. Biol. 2012, 361, 103–115. [Google Scholar] [CrossRef]
- Jukam, D.; Xie, B.; Rister, J.; Terrell, D.; Charlton-Perkins, M.; Pistillo, D.; Gebelein, B.; Desplan, C.; Cook, T.A. Opposite Feedbacks in the Hippo Pathway for Growth Control and Neural Fate. Science 2013, 342, 1238016. [Google Scholar] [CrossRef]
- Dong, J.; Feldmann, G.; Huang, J.; Wu, S.; Zhang, N.; Comerford, S.A.; Gayyed, M.F.; Anders, R.A.; Maitra, A.; Pan, D. Elucidation of a Universal Size-Control Mechanism in Drosophila and Mammals. Cell 2007, 130, 1120–1133. [Google Scholar] [CrossRef]
- Hong, W.; Guan, K.-L. The YAP and TAZ transcription co-activators: Key downstream effectors of the mammalian Hippo pathway. Semin. Cell Dev. Biol. 2012, 23, 785–793. [Google Scholar] [CrossRef]
- Skouloudaki, K.; Puetz, M.; Simons, M.; Courbard, J.-R.; Boehlke, C.; Hartleben, B.; Engel, C.; Moeller, M.J.; Englert, C.; Bollig, F.; et al. Scribble participates in Hippo signaling and is required for normal zebrafish pronephros development. Proc. Natl. Acad. Sci. USA 2009, 106, 8579–8584. [Google Scholar] [CrossRef]
- Zhao, B.; Ye, X.; Yu, J.; Li, L.; Li, W.; Li, S.; Lin, J.D.; Wang, C.-Y.; Chinnaiyan, A.M.; Lai, Z.-C.; et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008, 22, 1962–1971. [Google Scholar] [CrossRef]
- Song, J.Y.; Park, R.; Kim, J.Y.; Hughes, L.; Lu, L.; Kim, S.; Johnson, R.L.; Cho, S.-H. Dual function of Yap in the regulation of lens progenitor cells and cellular polarity. Dev. Biol. 2014, 386, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Miesfeld, J.B.; Gestri, G.; Clark, B.S.; Flinn, M.A.; Poole, R.J.; Bader, J.R.; Besharse, J.C.; Wilson, S.W.; Link, B.A. Yap and Taz regulate retinal pigment epithelial cell fate. J. Cell Sci. 2015, 128, 3021–3032. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, Q.; Pignoni, F. Yki/YAP, Sd/TEAD and Hth/MEIS Control Tissue Specification in the Drosophila Eye Disc Epithelium. PLoS ONE 2011, 6, e22278. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-C.; Graves, H.K.; Moya, I.M.; Tao, C.; Hamaratoglu, F.; Gladden, A.B.; Halder, G. Differential regulation of the Hippo pathway by adherens junctions and apical–basal cell polarity modules. Proc. Natl. Acad. Sci. USA 2015, 112, 1785–1790. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, L.; Lei, Q.; Guan, K.-L. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010, 24, 862–874. [Google Scholar] [CrossRef]
- Skouloudaki, K.; Walz, G. YAP1 Recruits c-Abl to Protect Angiomotin-Like 1 from Nedd4-Mediated Degradation. PLoS ONE 2012, 7, e35735. [Google Scholar] [CrossRef]
- Park, R.; Moon, U.Y.; Park, J.Y.; Hughes, L.J.; Johnson, R.L.; Cho, S.-H.; Kim, S. Yap is required for ependymal integrity and is suppressed in LPA-induced hydrocephalus. Nat. Commun. 2016, 7, 10329. [Google Scholar] [CrossRef]
- Williamson, K.A.; Rainger, J.; Floyd, J.A.; Ansari, M.; Meynert, A.; Aldridge, K.V.; Rainger, J.K.; Anderson, C.A.; Moore, A.T.; Hurles, M.E.; et al. Heterozygous Loss-of-Function Mutations in YAP1 Cause Both Isolated and Syndromic Optic Fissure Closure Defects. Am. J. Hum. Genet. 2014, 94, 295–302. [Google Scholar] [CrossRef]
- Liu, Y.; Xin, Y.; Ye, F.; Wang, W.; Lu, Q.; Kaplan, H.J.; Dean, D.C. Taz-Tead1 Links Cell-Cell Contact to Zeb1 Expression, Proliferation, and Dedifferentiation in Retinal Pigment Epithelial Cells. Investig. Opthalmol. Vis. Sci. 2010, 51, 3372–3378. [Google Scholar] [CrossRef]
- Lyubasyuk, V.; Ouyang, H.; Yu, F.-X.; Guan, K.-L.; Zhang, K. YAP inhibition blocks uveal melanogenesis driven by GNAQ or GNA11 mutations. Mol. Cell. Oncol. 2014, 2, e970957. [Google Scholar] [CrossRef]
- Markby, D.W.; Onrust, R.; Bourne, H.R. Separate GTP binding and GTPase activating domains of a G alpha subunit. Science 1993, 262, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.-X.; Zhang, K.; Guan, K.-L. YAP as oncotarget in uveal melanoma. Oncoscience 2014, 1, 480–481. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giovannini, M.; Robanus-Maandag, E.; Van Der Valk, M.; Niwa-Kawakita, M.; Abramowski, V.; Goutebroze, L.; Woodruff, J.M.; Berns, A.; Thomas, G. Conditional biallelic Nf2 mutation in the mouse promotes manifestations of human neurofibromatosis type 2. Genome Res. 2000, 14, 1617–1630. [Google Scholar]
- E McLaughlin, M.; Pepin, S.M.; MacCollin, M.; Choopong, P.; Lessell, S. Ocular Pathologic Findings of Neurofibromatosis Type 2. Arch. Ophthalmol. 2007, 125, 389. [Google Scholar] [CrossRef] [PubMed]
- Wiley, L.A.; Dattilo, L.K.; Kang, K.B.; Giovannini, M.; Beebe, D.C. The Tumor Suppressor Merlin Is Required for Cell Cycle Exit, Terminal Differentiation, and Cell Polarity in the Developing Murine Lens. Investig. Opthalmol. Vis. Sci. 2010, 51, 3611–3618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Bai, H.; David, K.K.; Dong, J.; Zheng, Y.; Cai, J.; Giovannini, M.; Liu, P.; Anders, R.A.; Pan, D. The Merlin/NF2 Tumor Suppressor Functions through the YAP Oncoprotein to Regulate Tissue Homeostasis in Mammals. Dev. Cell 2010, 19, 27–38. [Google Scholar] [CrossRef]
- Masson, C.; García-García, D.; Bitard, J.; Grellier, E.-K.; Roger, J.E.; Perron, M. Yap haploinsufficiency leads to Müller cell dysfunction and late-onset cone dystrophy. Cell Death Dis. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Ai, L.-Q.-Y.; Zhu, J.-Y.; Chen, X.; Li, X.; Luo, L.-L.; Hu, Q.-M.; Lin, S.; Ye, J. Endothelial Yes-Associated Protein 1 Promotes Astrocyte Proliferation and Maturation via Cytoplasmic Leukemia Inhibitory Factor Secretion in Oxygen-Induced Retinopathy. Investig. Opthalmol. Vis. Sci. 2020, 61, 1. [Google Scholar] [CrossRef]
- Pham, H.; Yu, H.; Laski, F.A. Cofilin/ADF is required for retinal elongation and morphogenesis of the Drosophila rhabdomere. Dev. Biol. 2008, 318, 82–91. [Google Scholar] [CrossRef]
- Arber, S.; Barbayannis, F.A.; Hanser, H.; Schneider, C.; Stanyon, C.A.; Bernard, O.; Caroni, P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nat. Cell Biol. 1998, 393, 805–809. [Google Scholar] [CrossRef]
- Yang, N.; Higuchi, O.; Ohashi, K.; Nagata, K.; Wada, A.; Kangawa, K.; Nishida, E.; Mizuno, K. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nat. Cell Biol. 1998, 393, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Rogers, E.M.; Hsiung, F.; Rodrigues, A.B.; Moses, K. Slingshot cofilin phosphatase localization is regulated by Receptor Tyrosine Kinases and regulates cytoskeletal structure in the developing Drosophila eye. Mech. Dev. 2005, 122, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Niwa, R.; Nagata-Ohashi, K.; Takeichi, M.; Mizuno, K.; Uemura, T. Control of Actin Reorganization by Slingshot, a Family of Phosphatases that Dephosphorylate ADF/Cofilin. Cell 2002, 108, 233–246. [Google Scholar] [CrossRef]
- Lin, C.-M.; Lin, P.-Y.; Li, Y.-C.; Hsu, J.-C. Capulet and Slingshot share overlapping functions during Drosophila eye morphogenesis. J. Biomed. Sci. 2012, 19, 46. [Google Scholar] [CrossRef]
- Delalle, I.; Pfleger, C.M.; Buff, E.; Lueras, P.; Hariharan, I.K. Mutations in the Drosophila orthologs of the F-actin capping protein alpha- and beta-subunits cause actin accumulation and subsequent retinal degeneration. Genetics 2005, 171, 1757–1765. [Google Scholar] [CrossRef]
- Nie, J.; Mahato, S.; Zelhof, A.C. The Actomyosin Machinery Is Required for Drosophila Retinal Lumen Formation. PLoS Genet. 2014, 10, e1004608. [Google Scholar] [CrossRef][Green Version]
- Matsumoto, H.; Isono, K.; Pye, Q.; Pak, W.L. Gene encoding cytoskeletal proteins in Drosophila rhabdomeres. Proc. Natl. Acad. Sci. USA 1987, 84, 985–989. [Google Scholar] [CrossRef]
- A Porter, J.; Hicks, J.; Williams, D.S.; Montell, C. Differential localizations of and requirements for the two Drosophila ninaC kinase/myosins in photoreceptor cells. J. Cell Biol. 1992, 116, 683–693. [Google Scholar] [CrossRef]
- Fehon, R.G.; McClatchey, A.I.; Bretscher, A. Organizing the cell cortex: the role of ERM proteins. Nat. Rev. Mol. Cell Biol. 2010, 11, 276–287. [Google Scholar] [CrossRef]
- Klebes, A.; Knust, E. A conserved motif in Crumbs is required for E-cadherin localisation and zonula adherens formation in Drosophila. Curr. Biol. 2000, 10, 76–85. [Google Scholar] [CrossRef]
- Laprise, P.; Beronja, S.; Silva-Gagliardi, N.F.; Pellikka, M.; Jensen, A.M.; McGlade, C.J.; Tepass, U. The FERM Protein Yurt Is a Negative Regulatory Component of the Crumbs Complex that Controls Epithelial Polarity and Apical Membrane Size. Dev. Cell 2006, 11, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Polesello, C.; Delon, I.; Valenti, P.; Ferrer, P.; Payre, F. Dmoesin controls actin-based cell shape and polarity during Drosophila melanogaster oogenesis. Nat. Cell Biol. 2002, 4, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Tepass, U. FERM proteins in animal morphogenesis. Curr. Opin. Genet. Dev. 2009, 19, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Pellikka, M.; Tanentzapf, G.; Pinto, M.; Smith, C.; McGlade, C.J.; Ready, D.F.; Tepass, U. Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature 2002, 416, 143–149. [Google Scholar] [CrossRef]
- Kumar, R.; Janjanam, J.; Singh, N.K.; Rao, G.N. A new role for cofilin in retinal neovascularization. J. Cell Sci. 2016, 129, 1234–1249. [Google Scholar] [CrossRef]

| Gene | YAP effects | Disease | Phenotype | Evidence |
|---|---|---|---|---|
| YAP Arg124* | YAP premature stop | Coloboma | Gap in iris, cornea, or eyelid | [42,48] |
| YAP Glu356* | YAP premature stop | Coloboma | Gap in iris, cornea, or eyelid | [42,48] |
| TEAD1 | Abolished interaction with YAP | Sveinsson’s chorioretinal atrophy | Loss of RPE, choroid, and photoreceptor | [49] |
| GNAQ | YAP upregulation | Uveal melanoma | Tumour growth | [50,51,52] |
| GNA11 | YAP upregulation | Uveal melanoma | Tumour growth | [50,51,52] |
| NF2 | YAP upregulation | Neurofibromatosis 2 | Subcapsular cataract, disorganised lens | [53,54,55,56] |
| Ctgf | YAP increased transcription and protein levels | Retinal degeneration | Muller cell gliosis | [32] |
| Cyr61 | YAP increased transcription and protein levels | Retinal degeneration | Muller cell gliosis | [32] |
| Yap+/− | YAP haploinsufficiency | Retinal degeneration | Muller cell dysfunction, cone degeneration | [57] |
| Yap−/− | YAP endothelial deficiency | Pathologic retinal vascularisation | Retinal vasculature defects | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skouloudaki, K.; Papadopoulos, D.K.; Hurd, T.W. The Molecular Network of YAP/Yorkie at the Cell Cortex and their Role in Ocular Morphogenesis. Int. J. Mol. Sci. 2020, 21, 8804. https://doi.org/10.3390/ijms21228804
Skouloudaki K, Papadopoulos DK, Hurd TW. The Molecular Network of YAP/Yorkie at the Cell Cortex and their Role in Ocular Morphogenesis. International Journal of Molecular Sciences. 2020; 21(22):8804. https://doi.org/10.3390/ijms21228804
Chicago/Turabian StyleSkouloudaki, Kassiani, Dimitrios K. Papadopoulos, and Toby W. Hurd. 2020. "The Molecular Network of YAP/Yorkie at the Cell Cortex and their Role in Ocular Morphogenesis" International Journal of Molecular Sciences 21, no. 22: 8804. https://doi.org/10.3390/ijms21228804
APA StyleSkouloudaki, K., Papadopoulos, D. K., & Hurd, T. W. (2020). The Molecular Network of YAP/Yorkie at the Cell Cortex and their Role in Ocular Morphogenesis. International Journal of Molecular Sciences, 21(22), 8804. https://doi.org/10.3390/ijms21228804




