Tissue-Specific Ferritin- and GFP-Based Genetic Vectors Visualize Neurons by MRI in the Intact and Post-Ischemic Rat Brain
Abstract
1. Introduction
2. Results
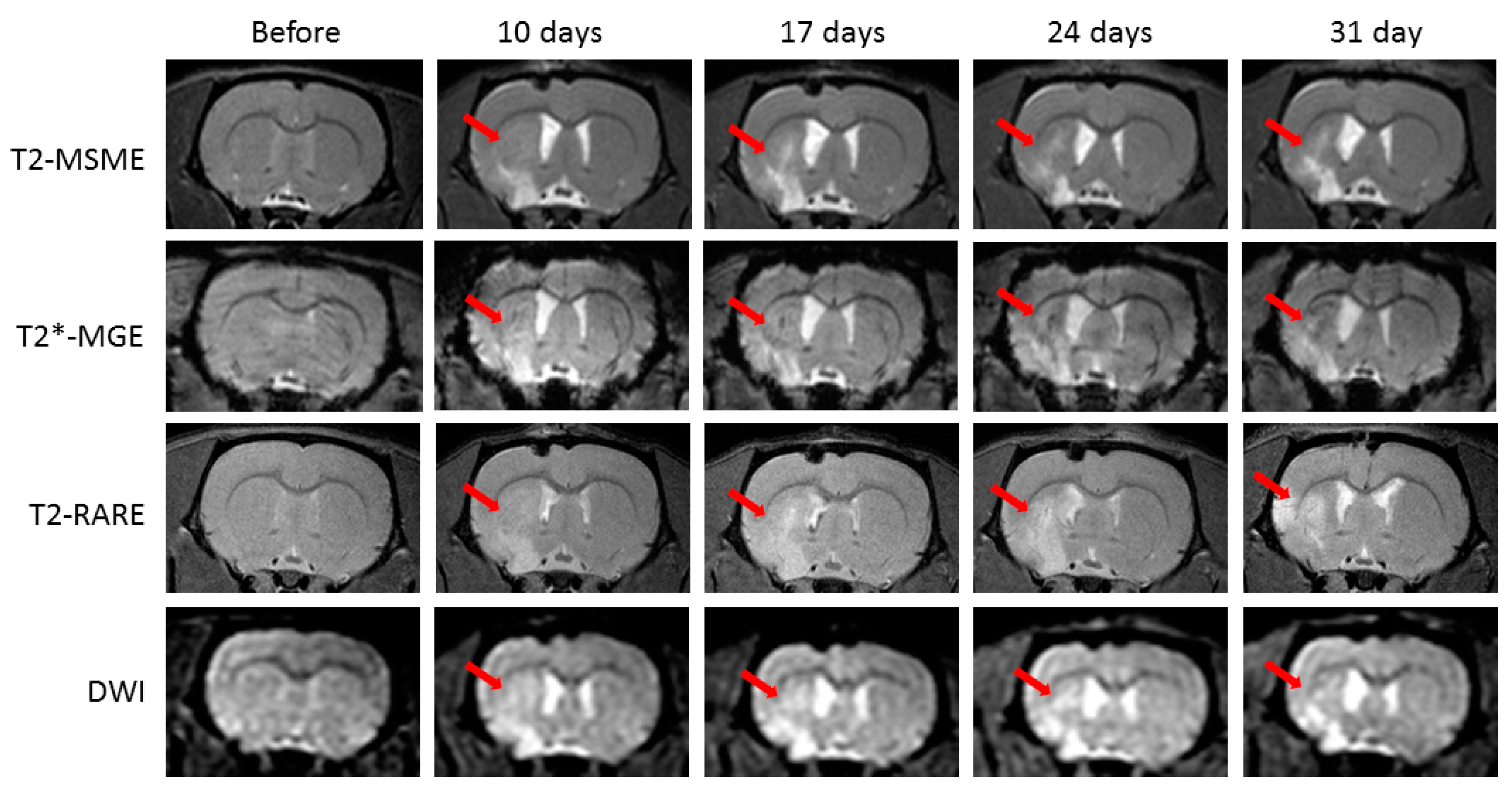
2.1. Neurological Deficit and Time Course of Ischemic Lesions
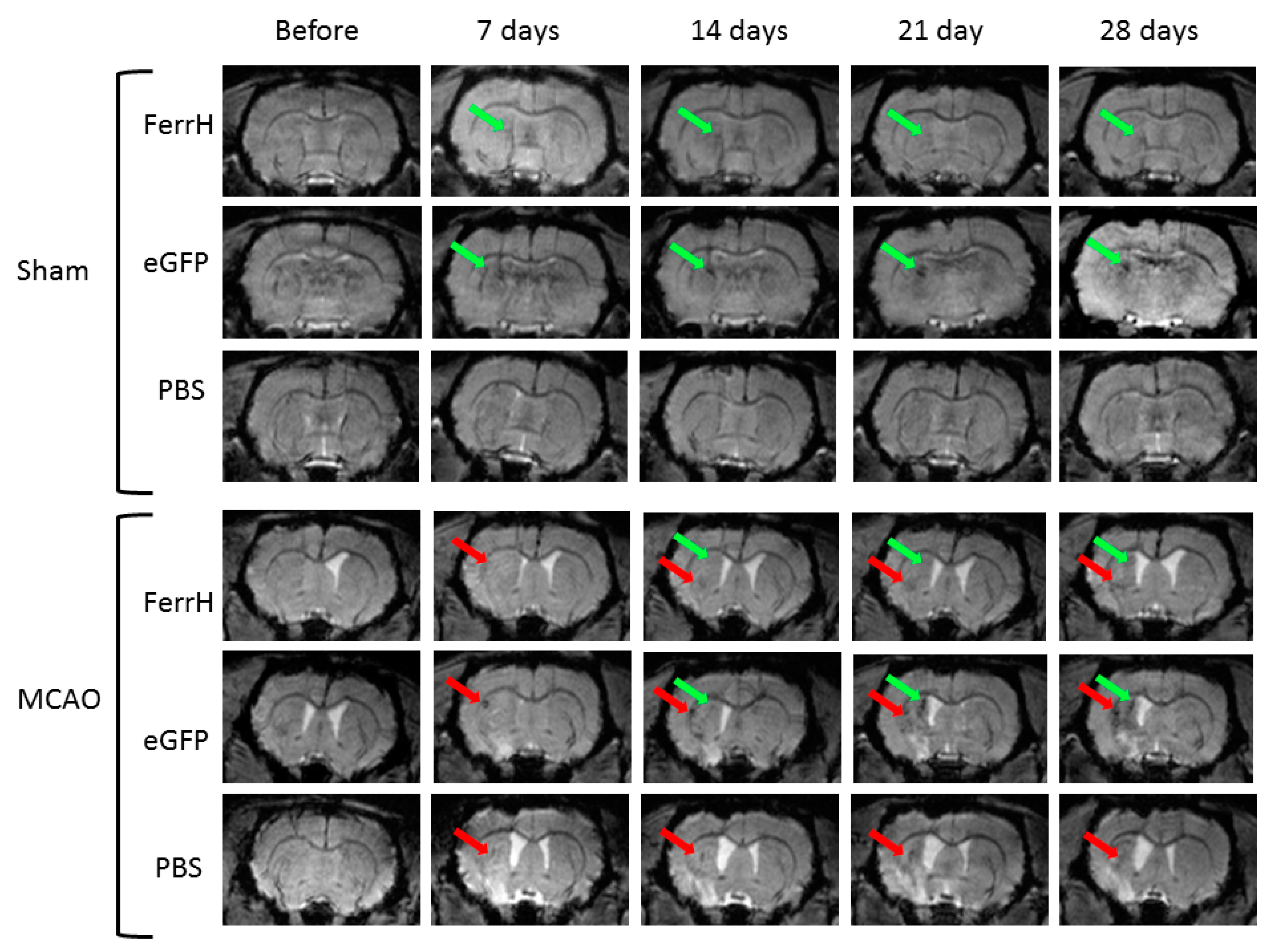
2.2. Injections of Both AAV-pDCX-FerrH and AAV-pDCX-eGFP Cause Signal Hypointensity on T2*-MGE Images in the Sham-Operated and Post-Ischemic Animals
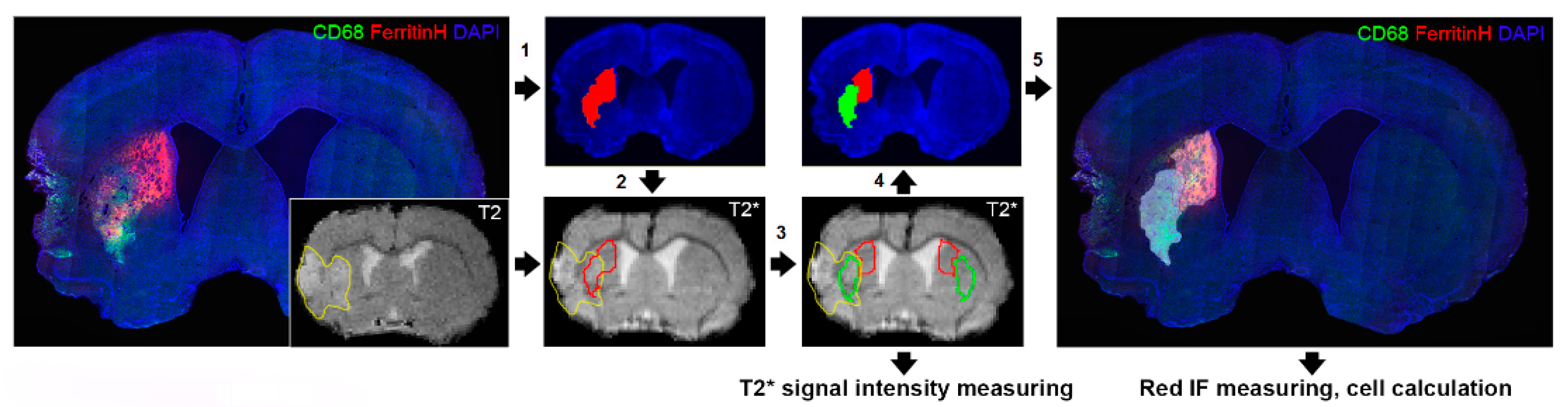
2.3. T2* Signal Hypointensity Correlates with FerrH and eGFP Accumulation
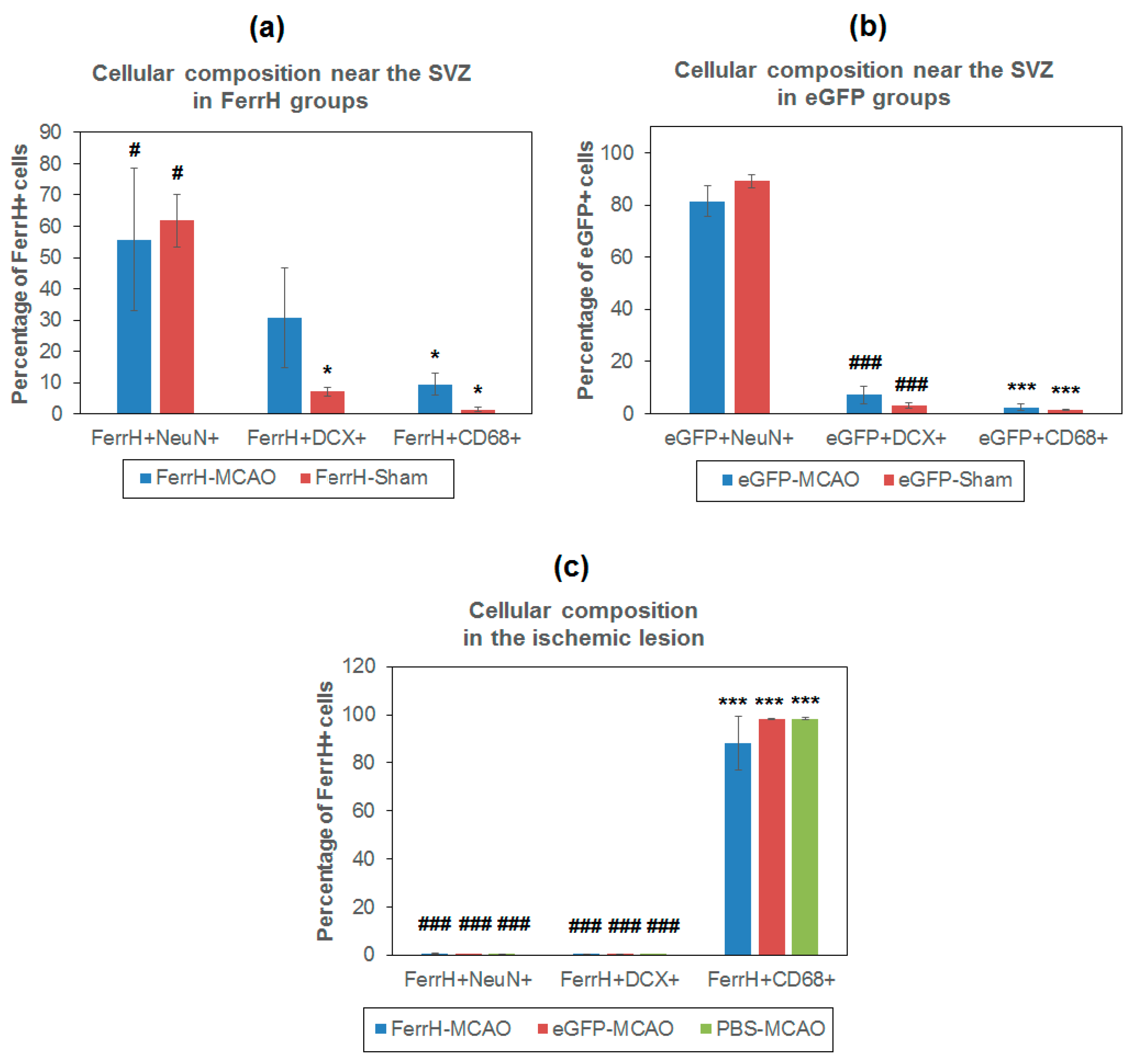
2.4. Iron Accumulation and Cellular Composition of FerrH- and eGFP-Positive Areas
2.5. RT-PCR Results
3. Discussion
- Results of our study showed that rat brain could be successfully infected with AAV-pDCX-FerrH and AAV-pDCX-eGFP viral vectors for expression of either ferritin or eGFP. Both vectors caused at about 20% decrease in signal hypointensity in the areas near the SVZ on T2*-weighted MRI at one month after intracranial injection of the viral constructs.
- The location of the signal hypointensity areas coincides with zones of ferritin and eGFP accumulation in immunohistochemical slides and zones of iron accumulation in Prussian blue staining a month after viral injection. RT-PCR data confirmed upregulated expression of the ferritin in the corpus callosum and caudoputamen in the left hemisphere of the rat brain on day 7 after intracerebral injected of the adenoviral vector construct.
- The main source of the signal hypointensity near SVZs in the AAV-pDCX-FerrH and AAV-pDCX-eGFP groups are mature neurons with a small percentage of young neurons.
- The main source of the signal hypointensity in the ischemic lesion area in AAV-pDCX-FerrH, AAV-pDCX-eGFP, and PBS-injected groups is macrophages.
4. Materials and Methods
4.1. Vector Constructs
4.2. Animals and Experimental Design
4.3. MCAO Model
4.4. Viral Microinjections
4.5. In vivo MRI Studies
- (1)
- T2-weighted multislice multiecho (T2-MSME): TR = 2700 ms, TE = 7.3ms, FOV = 36 × 36 mm, image resolution 0.2 × 0.2 mm2, slice thickness 1 mm, matrix 180 × 180, 1 signal average, scan time 6 min 24 s.
- (2)
- T2-weighted turbo rapid acquisition with relaxation enhancement (T2 TURBO RARE): TR = 2 s, TE = 7.6 ms, FOV = 36 × 36 mm, image resolution 0.12 × 0.12 mm2, slice thickness 1 mm, matrix 300 × 300, 3 signal averages, turbo factor 4, scan time 7 min 30 s.
- (3)
- T2*-weighted multiple gradient echo (T2*-MGE): number of echoes = 9, first echo TE = 2.718 ms, echo spacing 2.9 ms, TR = 950 ms, flip angle 20 degree, FOV = 36 × 36 mm, slice thickness 1 mm, image resolution 0.2 × 0.2 mm2, matrix 180 × 180, 4 signal averages, scan time 8 min.
- (4)
- Diffusion-weighted imaging (DWI): TR/TE = 3200/19 ms, 120 × 120 matrix, FOV = 36 × 36 mm2, matrix size = 128 × 128, section thickness = 0.9 mm, number of diffusion gradient directions = 6, one signal average, scan time 1 min 4 s.
4.6. RT-PCR
4.7. Immunochemistry and Histology
4.8. Image Processing
4.9. Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AAV | Adeno-associated viral backbone |
| ANOVA | Analysis of variance |
| BrdU | Bromodeoxyuridine |
| CC | Corpus callosum |
| CCA | Common carotid artery |
| cDNA | Complementary deoxyribonucleic acid |
| CD68 | Cluster of differentiation 68 |
| CPu | Caudoputamen |
| CT | Cycle threshold |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DCX | Doublecortin |
| DWI | Diffusion-weighted imaging |
| eGFP | Enhanced green fluorescent protein |
| FA | Flip angle |
| FerrH | Ferritin heavy chain |
| FLASH-3D | Fast low-angle shot three-dimensional |
| FOV | Field of view |
| GRE | Gradient echo |
| IF | Fluorescent intensity |
| LSD | Least significant difference |
| LV | Lentivirus |
| MCA | Middle cerebral artery |
| MCAO | Middle-cerebral-artery occlusion |
| MIP | Maximum intensity projection |
| MRI | Magnetic resonance imaging |
| MT | Magnetization transfer |
| MTC | Magnetization transfer contrast |
| NCBI | U.S. National Library of Medicine |
| NeuN | Neuronal nuclear antigen |
| NG2 | Neural/glial antigen 2 |
| NPCs | Neuronal precursors |
| NS | Neurological scores |
| OD | Optical density |
| PBS | Phosphate-buffered saline |
| pDCX | Doublecortin promoter |
| QPCR | Quantitative polymerase chain reaction |
| rAAV | Recombinant adeno-associated viral vectors |
| ROI | Region of interest |
| RT-PCR | Reverse-transcription polymerase chain reaction |
| SPF | Specific pathogen-free |
| SPIOs | Superparamagnetic iron oxide particles |
| SVZ | Subventricular zone |
| T2 TURBO RARE | T2-weighted turbo rapid acquisition with relaxation enhancement |
| T2*-MGE | T2*-weighted multiple gradient echo |
| T2-MSME | T2-weighted multislice multiecho |
| TE | Echo time |
| TR | Repetition time |
| WPRE | Woodchuck hepatitis virus posttranscriptional regulatory element |
References
- Mallett, C.L.; Shuboni-Mulligan, D.D.; Shapiro, E.M. Tracking neural progenitor cell migration in the rodent brain using magnetic resonance imaging. Front. Neurosci. 2019, 13, 995. [Google Scholar] [CrossRef] [PubMed]
- Manganas, L.N.; Zhang, X.; Li, Y.; Li, Y.; Hazel, R.D.; Smith, D.; Wagshul, M.E.; Henn, F.; Benveniste, H.; Djurić, P.M.; et al. Magnetic Resonance Spectroscopy Identifies Neural Progenitor Cells in the Live Human Brain. Science 2007, 318, 980–985. [Google Scholar] [CrossRef]
- Vreys, R.; Velde, G.V.; Krylychkina, O.; Vellema, M.; Verhoye, M.; Timmermans, J.P.; Baekelandt, V.; Van der Linden, A. MRI visualization of endogenous neural progenitor cell migration along the RMS in the adult mouse brain: Validation of various MPIO labeling strategies. NeuroImage 2010, 49, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Granot, D.; Nkansah, M.K.; Bennewitz, M.F.; Tang, K.S.; Markakis, E.A.; Shapiro, E.M. Clinically viable magnetic poly(lactide-co-glycolide) particles for MRI-based cell tracking. Magn. Reson. Med. 2014, 71, 1238–1250. [Google Scholar] [CrossRef] [PubMed]
- Pothayee, N.; Cummings, D.M.; Schoenfeld, T.J.; Schoenfeld, T.J.; Dodd, S.; Cameron, H.A.; Belluscio, L.; Koretsky, A.P. Magnetic resonance imaging of odorant activity-dependent migration of neural precursor cells and olfactory bulb growth. NeuroImage 2017, 158, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Shuboni-Mulligan, D.D.; Chakravarty, S.; Mallett, C.L.; Wolf, A.M.; Forton, S.; Shapiro, E.M. Age-dependent visualization of neural progenitor cells within the rostral migratory stream via MRI and endogenously labeled micron-sized iron oxide particles. bioRxiv 2018. [Google Scholar] [CrossRef]
- Nieman, B.J.; Shyu, J.Y.; Rodriguez, J.J.; Garcia, A.D.; Joyner, A.L.; Turnbull, D.H. In vivo MRI of neural cell migration dynamics in the mouse brain. Neuroimage 2010, 50, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Sumner, J.P.; Shapiro, E.M.; Maric, D.; Conroy, R.; Koretsky, A.P. In vivo Labeling of Adult Neural Progenitors for MRI with Micron Sized Particles of Iron Oxide: Quantitation of Labeled Cell Phenotype. NeuroImage 2009, 44, 671–678. [Google Scholar] [CrossRef]
- Zhang, F.; Duan, X.; Lu, L.; Lu, L.; Zhang, X.; Zhong, X.; Mao, J.; Chen, M.; Shen, J. In vivo Targeted MR Imaging of Endogenous Neural Stem Cells in Ischemic Stroke. Molecules 2016, 21, 1143. [Google Scholar] [CrossRef]
- Zhong, X.-M.M.; Zhang, F.; Yang, M.; Wen, X.-H.H.; Zhang, X.; Duan, X.-H.H.; Shen, J. In vivo Targeted Magnetic Resonance Imaging of Endogenous Neural Stem Cells in the Adult Rodent Brain. BioMed Res. Int. 2015, 2015, 131054. [Google Scholar] [CrossRef]
- Iordanova, B.; Ahrens, E.T. In vivo magnetic resonance imaging of ferritin-based reporter visualizes native neuroblast migration. NeuroImage 2012, 59, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Granot, D.; Scheinost, D.; Markakis, E.A.; Papademetris, X.; Shapiro, E.M. Serial Monitoring of Endogenous Neuroblast Migration by Cellular MRI. NeuroImage 2011, 57, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Granot, D.; Shapiro, E.M. Accumulation of micron sized iron oxide particles in endothelin-1 induced focal cortical ischemia in rats is independent of cell migration. Magn. Reson. Med. 2014, 71, 1568–1574. [Google Scholar] [CrossRef]
- Vande Velde, G.; Raman Rangarajan, J.; Vreys, R.; Guglielmetti, C.; Dresselaers, T.; Verhoye, M.; Van der Linden, A.; Debyser, Z.; Baekelandt, V.; Maes, F.; et al. Quantitative evaluation of MRI-based tracking of ferritin-labeled endogenous neural stem cell progeny in rodent brain. NeuroImage 2012, 62, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Guglielmetti, C.; Praet, J.; Rangarajan, J.R.; Vreys, R.; De Vocht, N.; Maes, F.; Verhoye, M.; Ponsaerts, P.; Van der Linden, A. Multimodal imaging of subventricular zone neural stem/progenitor cells in the cuprizone mouse model reveals increased neurogenic potential for the olfactory bulb pathway, but no contribution to remyelination of the corpus callosum. NeuroImage 2014, 86, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Elvira, G.; García, I.; Benito, M.; Gallo, J.; Desco, M.; Penadés, S.; Garcia-Sanz, J.A.; Silva, A. Live Imaging of Mouse Endogenous Neural Progenitors Migrating in Response to an Induced Tumor. PLoS ONE 2012, 7, e44466. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, E.M.; Gonzalez-Perez, O.; Manuel García-Verdugo, J.; Alvarez-Buylla, A.; Koretsky, A.P. Magnetic resonance imaging of the migration of neuronal precursors generated in the adult rodent brain. NeuroImage 2006, 32, 1150–1157. [Google Scholar] [CrossRef]
- Panizzo, R.A.; Kyrtatos, P.G.; Price, A.N.; Gadian, D.G.; Ferretti, P.; Lythgoe, M.F. In vivo magnetic resonance imaging of endogenous neuroblasts labelled with a ferumoxide-polycation complex. NeuroImage 2009, 44, 1239–1246. [Google Scholar] [CrossRef]
- Vreys, R.; Soenen, S.J.H.; De Cuyper, M.; Van Der Linden, A. Background migration of USPIO/MLs is a major drawback for in situ labeling of endogenous neural progenitor cells. Contrast Media Mol. Imaging 2011, 6, 1–6. [Google Scholar] [CrossRef]
- Williamson, M.R.; Jones, T.A.; Drew, M.R. Functions of subventricular zone neural precursor cells in stroke recovery. Behav. Brain Res. 2019, 376, 112209. [Google Scholar] [CrossRef]
- Nemirovich-Danchenko, N.M.; Khodanovich, M.Y. New neurons in the post-ischemic and injured brain: Migrating or resident? Front. Neurosci. 2019, 13, 588. [Google Scholar] [CrossRef] [PubMed]
- Ramm, P.; Couillard-Despres, S.; Plötz, S.; Rivera, F.J.; Krampert, M.; Lehner, B.; Kremer, W.; Bogdahn, U.; Kalbitzer, H.R.; Aigner, L. A Nuclear Magnetic Resonance Biomarker for Neural Progenitor Cells: Is It All Neurogenesis? Stem Cells 2009, 27, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Naumova, A.V.; Vande Velde, G. Genetically encoded iron-associated proteins as MRI reporters for molecular and cellular imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotech. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Karl, C.; Couillard-Despres, S.; Prang, P.; Munding, M.; Kilb, W.; Brigadski, T.; Plötz, S.; Mages, W.; Luhmann, H.; Winkler, J.; et al. Neuronal precursor-specific activity of a human doublecortin regulatory sequence. J. Neurochem. 2005, 92, 264–282. [Google Scholar] [CrossRef] [PubMed]
- Couillard-Despres, S.; Finkl, R.; Winner, B.; Ploetz, S.; Wiedermann, D.; Aigner, R.; Bogdahn, U.; Winkler, J.; Hoehn, M.; Aigner, L. In vivo optical imaging of neurogenesis: Watching new neurons in the intact brain. Mol. Imaging 2008, 7, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Marschallinger, J.; Schäffner, I.; Klein, B.; Gelfert, R.; Rivera, F.J.; Illes, S.; Grassner, L.; Janssen, M.; Rotheneichner, P.; Schmuckermair, C.; et al. Structural and functional rejuvenation of the aged brain by an approved anti-asthmatic drug. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef]
- Adamczak, J.; Aswendt, M.; Kreutzer, C.; Rotheneichner, P.; Riou, A.; Selt, M.; Beyrau, A.; Uhlenküken, U.; Diedenhofen, M.; Nelles, M.; et al. Neurogenesis upregulation on the healthy hemisphere after stroke enhances compensation for age-dependent decrease of basal neurogenesis. Neurobiol. Disease 2017, 99, 47–57. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, R.; Tsark, W.; Lu, Q. Rapid promoter analysis in developing mouse brain and genetic labeling of young neurons by doublecortin-DsRed-express. J. Neurosci. Res. 2007, 85, 3567–3573. [Google Scholar] [CrossRef]
- Walker, T.L.; Yasuda, T.; Adams, D.J.; Bartlett, P.F. The doublecortin-expressing population in the developing and adult brain contains multipotential precursors in addition to neuronal-lineage cells. J. Neurosci. 2007, 27, 3734–3742. [Google Scholar] [CrossRef]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef]
- Bederson, J.B.; Pitts, L.H.; Tsuji, M.; Nishimura, M.C.; Davis, R.L.; Bartkowski, H. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke 1986, 17, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Wojtowicz, J.M.; Kee, N. BrdU assay for neurogenesis in rodents. Nat. Protoc. 2006, 1, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Bordiuk, O.L.; Smith, K.; Morin, P.J.; Semënov, M.V. Cell proliferation and neurogenesis in adult mouse brain. PLoS ONE 2014, 9, e111453. [Google Scholar] [CrossRef]
- Zhang, F.; Duan, X.; Lu, L.; Zhang, X.; Chen, M.; Mao, J.; Cao, M.; Shen, J. In vivo Long-Term Tracking of Neural Stem Cells Transplanted into an Acute Ischemic Stroke model with Reporter Gene-Based Bimodal MR and Optical Imaging. Cell Transplant. 2017, 26, 1648–1662. [Google Scholar] [CrossRef] [PubMed]
- Vande Velde, G.; Rangarajan, J.R.; Toelen, J.; Dresselaers, T.; Ibrahimi, A.; Krylychkina, O.; Vreys, R.; Van Der Linden, A.; Maes, F.; Debyser, Z.; et al. Evaluation of the specificity and sensitivity of ferritin as an MRI reporter gene in the mouse brain using lentiviral and adeno-associated viral vectors. Gene Ther. 2011, 18, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Ninomiya, M.; Hernandez Acosta, P.; Garcia-Verdugo, J.M.; Sunabori, T.; Sakaguchi, M.; Adachi, K.; Kojima, T.; Hirota, Y.; Kawase, T.; et al. Subventricular Zone-Derived Neuroblasts Migrate and Differentiate into Mature Neurons in the Post-Stroke Adult Striatum. J. Neurosci. 2006, 26, 6627–6636. [Google Scholar] [CrossRef]
- Ghosh, H.S. Adult Neurogenesis and the Promise of Adult Neural Stem Cells. J. Exp. Neurosci. 2019, 13. [Google Scholar] [CrossRef]
- Ge, W.; He, F.; Kim, K.J.; Blanchi, B.; Coskun, V.; Nguyen, L.; Wu, X.; Zhao, J.; Heng, J.I.T.; Martinowich, K.; et al. Coupling of cell migration with neurogenesis by proneural bHLH factors. Proc. Natl. Acad. Sci. USA 2006, 103, 1319–1324. [Google Scholar] [CrossRef]
- Hagihara, H.; Murano, T.; Ohira, K.; Miwa, M.; Nakamura, K.; Miyakawa, T. Expression of progenitor cell/immature neuron markers does not present definitive evidence for adult neurogenesis. Mol. Brain 2019, 12, 1–6. [Google Scholar] [CrossRef]
- Nairz, M.; Theurl, I.; Swirski, F.K.; Weiss, G. “Pumping iron”—How macrophages handle iron at the systemic, microenvironmental, and cellular levels. Pflugers Arch. Eur. J. Physiol. 2017, 469, 397–418. [Google Scholar] [CrossRef]
- Amsalem, Y.; Mardor, Y.; Feinberg, M.S.; Landa, N.; Miller, L.; Daniels, D.; Ocherashvilli, A.; Holbova, R.; Yosef, O.; Barbash, I.M.; et al. Iron-oxide labeling and outcome of transplanted mesenchymal stem cells in the infarcted myocardium. Circulation 2007, 116 (Suppl. 1), 38–45. [Google Scholar] [CrossRef]
- Terrovitis, J.; Stuber, M.; Youssef, A.; Preece, S.; Leppo, M.; Kizana, E.; Schär, M.; Gerstenblith, G.; Weiss, R.G.; Marbán, E.; et al. Magnetic resonance imaging overestimates ferumoxide-labeled stem cell survival after transplantation in the heart. Circulation 2008, 117, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, D.S.; Granziera, C.; Hooker, J.M.; Loggia, M.L. In vivo Imaging of Human Neuroinflammation. ACS Chem. Neurosci. 2016, 7, 470–483. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.; Schroeter, M.; Jonkmanns, C.; Hartung, H.P.; Mödder, U.; Jander, S. In vivo MRI of brain inflammation in human ischaemic stroke. Brain 2004, 127, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz, J.; Panter, S.S.; Neema, M.; Arora, A.; Batt, C.E.; Bakshi, R. Iron in Chronic Brain Disorders: Imaging and Neurotherapeutic Implications. Neurotherapeutics 2007, 4, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Xi, G.; Hua, Y.; Sagher, O. T2* Magnetic Resonance Imaging Sequences Reflect Brain Tissue Iron Deposition Following Intracerebral Hemorrhage. Transl. Stroke Res. 2010, 1, 31–34. [Google Scholar] [CrossRef]
- Justicia, C.; Ramos-Cabrer, P.; Hoehn, M. MRI detection of secondary damage after stroke: Chronic iron accumulation in the thalamus of the rat brain. Stroke 2008, 39, 1541–1547. [Google Scholar] [CrossRef]
- Li, X.; Wolf, M.E. Visualization of virus-infected brain regions using a GFP-illuminating flashlight enables accurate and rapid dissection for biochemical analysis. J. Neurosci. Methods 2011, 201, 177–179. [Google Scholar] [CrossRef][Green Version]
- Zheng, N.; Su, P.; Liu, Y.; Wang, H.; Nie, B.; Fang, X.; Xu, Y.; Lin, K.; Lv, P.; He, X.; et al. Detection of neural connections with ex vivo MRI using a ferritin-encoding trans-synaptic virus. NeuroImage 2019, 197, 133–142. [Google Scholar] [CrossRef]
- Jiang, C.; Wu, D.; Haacke, E.M. Ferritin-EGFP Chimera as an Endogenous Dual-Reporter for Both Fluorescence and Magnetic Resonance Imaging in Human Glioma U251 Cells. Tomography 2017, 3, 1–8. [Google Scholar] [CrossRef]
- Pérez-Torres, C.J.; Massaad, C.A.; Hilsenbeck, S.G.; Serrano, F.; Pautler, R.G. In Vitro and In vivo Magnetic Resonance Imaging (MRI) Detection of GFP through Magnetization Transfer Contrast (MTC). NeuroImage 2010, 50, 375–382. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gerits, A.; Vancraeyenest, P.; Vreysen, S.; Laramée, M.-E.; Michiels, A.; Gijsbers, R.; Van den Haute, C.; Moons, L.; Debyser, Z.; Baekelandt, V.; et al. Serotype-dependent transduction efficiencies of recombinant adeno-associated viral vectors in monkey neocortex. Neurophotonics 2015, 2, 031209. [Google Scholar] [CrossRef] [PubMed]
- Van Der Perren, A.; Toelen, J.; Carlon, M.; Van Den Haute, C.; Coun, F.; Heeman, B.; Reumers, V.; Vandenberghe, L.H.; Wilson, J.M.; Debyser, Z.; et al. Efficient and stable transduction of dopaminergic neurons in rat substantia nigra by rAAV 2/1, 2/2, 2/5, 2/6.2, 2/7, 2/8 and 2/9. Gene Ther. 2011, 18, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Khodanovich, M.Y.; Kisel, A.A.; Akulov, A.E.; Atochin, D.N.; Kudabaeva, M.S.; Glazacheva, V.Y.; Svetlik, M.V.; Medvednikova, Y.A.; Mustafina, L.R.; Yarnykh, V.L. Quantitative assessment of demyelination in ischemic stroke in vivo using macromolecular proton fraction mapping. J. Cereb. Blood Flow Metab. 2018, 38, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press: San Diego, CA, USA, 2007. [Google Scholar]
- Weaver, J.B. Simultaneous multislice acquisition of MR images. Magn. Reson. Med. 1988, 8, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Hennig, J.; Nauerth, A.; Friedburg, H. RARE imaging: A fast imaging method for clinical MR. Magn. Reson. Med. 1986, 3, 823–833. [Google Scholar] [CrossRef]
- Chavhan, G.; Babyn, P.; Thomas, B.; Shroff, M.; Haacke, M. Principles, Techniques, and Applications of T2*-based MR Imaging and Its Special Applications. Radiographics 2009, 29, 1433–1446. [Google Scholar] [CrossRef]
- Schaefer, P.W.; Grant, E.; Gonzalez, G. Diffusion-weighted MR imaging of the brain. Radiology 2000, 217, 331–345. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]







| Surgery | Group | Total Number | Neurological Scores | ||
|---|---|---|---|---|---|
| 1 Day | 10 Days | 31 Day | |||
| Sham-operated | AAV-pDCX-FerrH | 3 | 0 | 0 | 0 |
| Sham-operated | AAV-pDCX-eGFP | 3 | 0 | 0 | 0 |
| Sham-operated | PBS | 3 | 0 | 0 | 0 |
| MCAO | AAV-pDCX-FerrH | 3 | 3 (1–4) | 2 (0–3) | 2 (0–3) |
| MCAO | AAV-pDCX-eGFP | 3 | 3 (2–4) | 2 (1–3) | 2 (2–3) |
| MCAO | PBS | 3 | 3 (3–4) | 3 (2–4) | 2 (2–3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khodanovich, M.Y.; Akulov, A.E.; Anan’ina, T.V.; Kudabaeva, M.S.; Pishchelko, A.O.; Krutenkova, E.P.; Nemirovich-Danchenko, N.M.; Svetlik, M.V.; Tumentceva, Y.A.; Van den Haute, C.; et al. Tissue-Specific Ferritin- and GFP-Based Genetic Vectors Visualize Neurons by MRI in the Intact and Post-Ischemic Rat Brain. Int. J. Mol. Sci. 2020, 21, 8951. https://doi.org/10.3390/ijms21238951
Khodanovich MY, Akulov AE, Anan’ina TV, Kudabaeva MS, Pishchelko AO, Krutenkova EP, Nemirovich-Danchenko NM, Svetlik MV, Tumentceva YA, Van den Haute C, et al. Tissue-Specific Ferritin- and GFP-Based Genetic Vectors Visualize Neurons by MRI in the Intact and Post-Ischemic Rat Brain. International Journal of Molecular Sciences. 2020; 21(23):8951. https://doi.org/10.3390/ijms21238951
Chicago/Turabian StyleKhodanovich, Marina Y., Andrey E. Akulov, Tatyana V. Anan’ina, Marina S. Kudabaeva, Anna O. Pishchelko, Elena P. Krutenkova, Nikolay M. Nemirovich-Danchenko, Mikhail V. Svetlik, Yana A. Tumentceva, Chris Van den Haute, and et al. 2020. "Tissue-Specific Ferritin- and GFP-Based Genetic Vectors Visualize Neurons by MRI in the Intact and Post-Ischemic Rat Brain" International Journal of Molecular Sciences 21, no. 23: 8951. https://doi.org/10.3390/ijms21238951
APA StyleKhodanovich, M. Y., Akulov, A. E., Anan’ina, T. V., Kudabaeva, M. S., Pishchelko, A. O., Krutenkova, E. P., Nemirovich-Danchenko, N. M., Svetlik, M. V., Tumentceva, Y. A., Van den Haute, C., Gijsbers, R., Daniëls, V., Thiry, I., Pershina, A. G., Shadrina, M. M., & Naumova, A. V. (2020). Tissue-Specific Ferritin- and GFP-Based Genetic Vectors Visualize Neurons by MRI in the Intact and Post-Ischemic Rat Brain. International Journal of Molecular Sciences, 21(23), 8951. https://doi.org/10.3390/ijms21238951







