Cortisol Metabolism in Carp Macrophages: A Role for Macrophage-Derived Cortisol in M1/M2 Polarization
Abstract
1. Introduction
2. Results
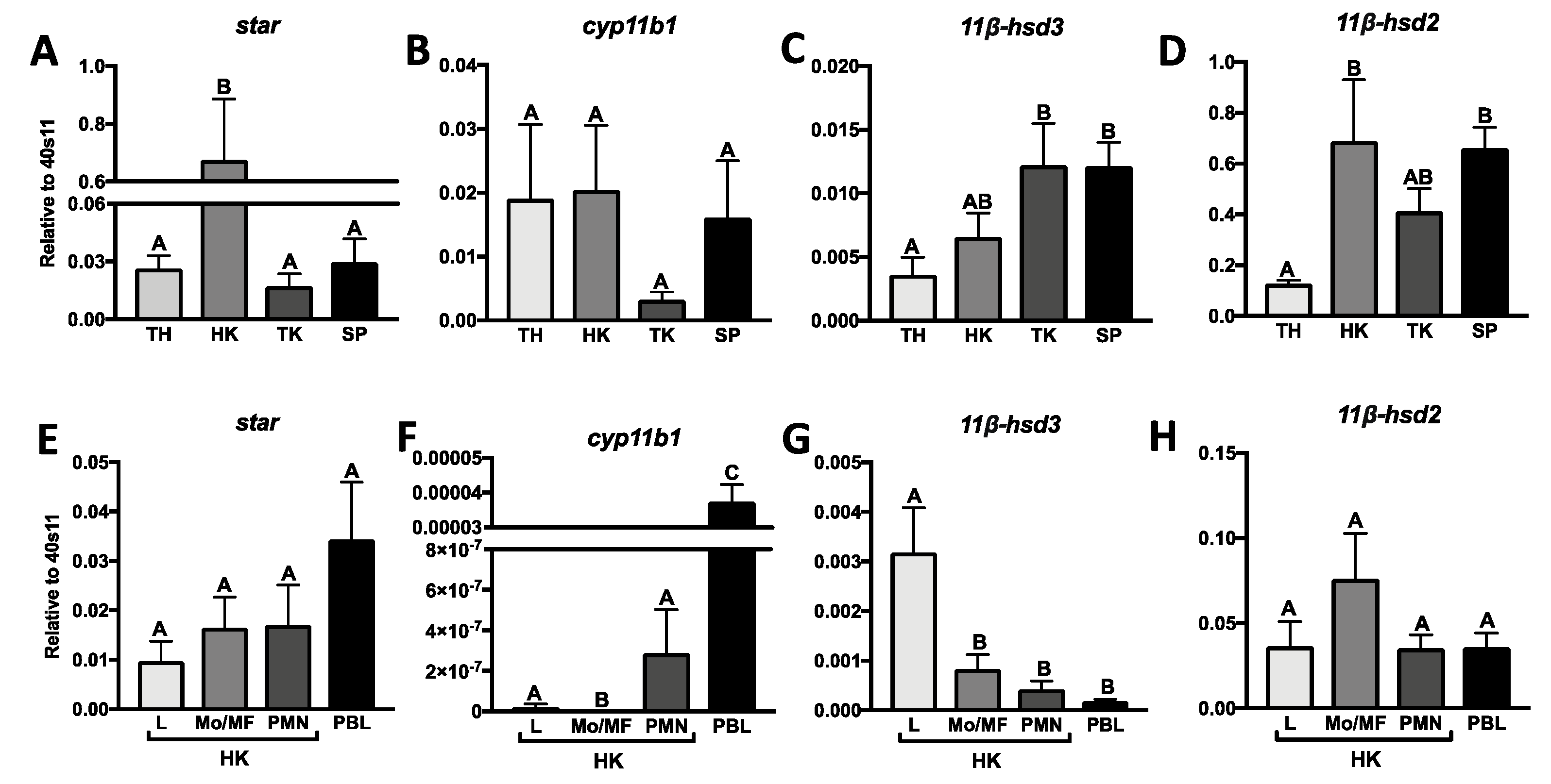
2.1. Constitutive Expression of Genes Involved in Cortisol Conversion in Lymphoid Organs and Leukocytes
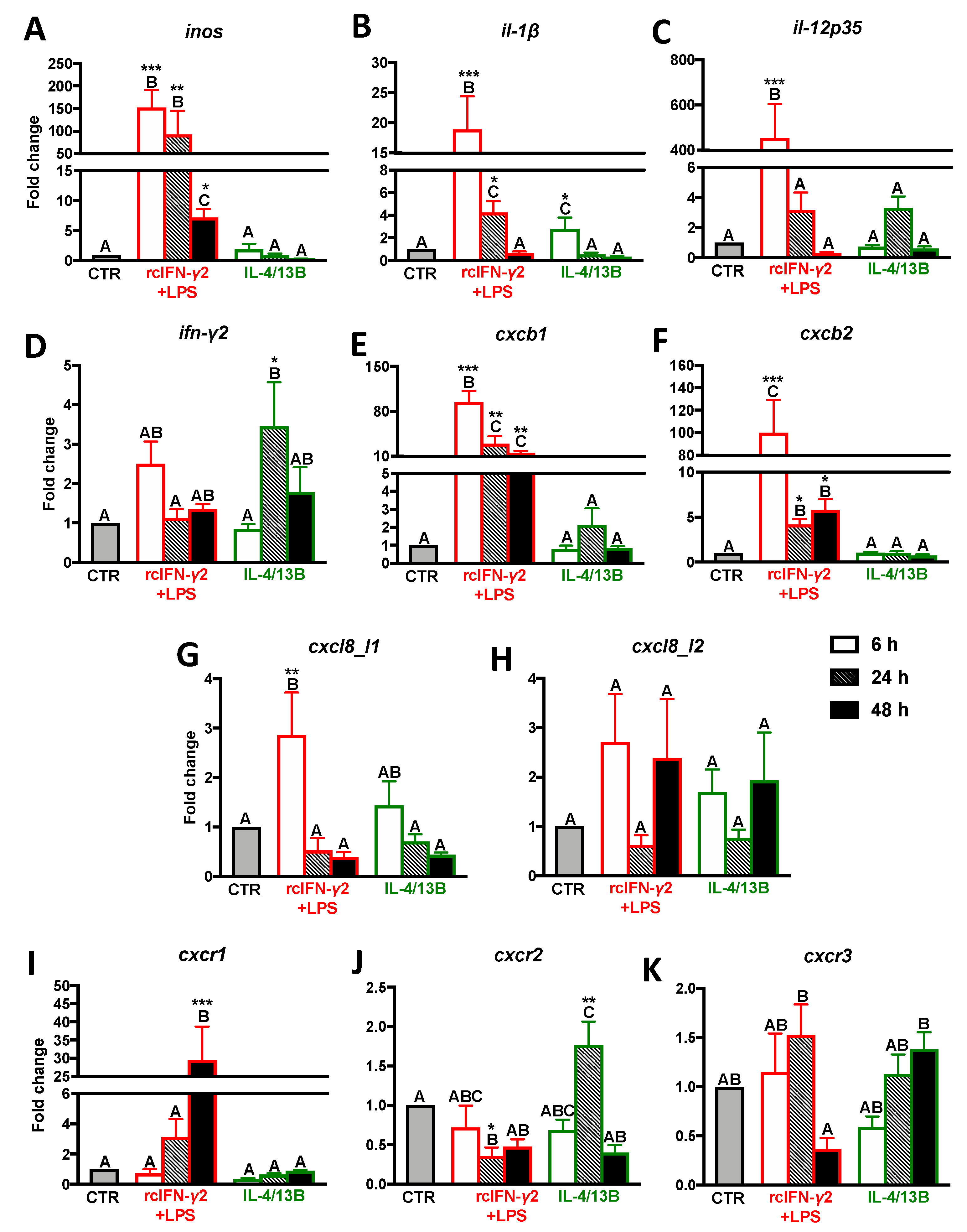
2.2. Expression of Proinflammatory Cytokines/Chemokines and CXC Receptors in Monocytes/Macrophages Stimulated with rcIFN-γ2 + LPS and IL-4/13b
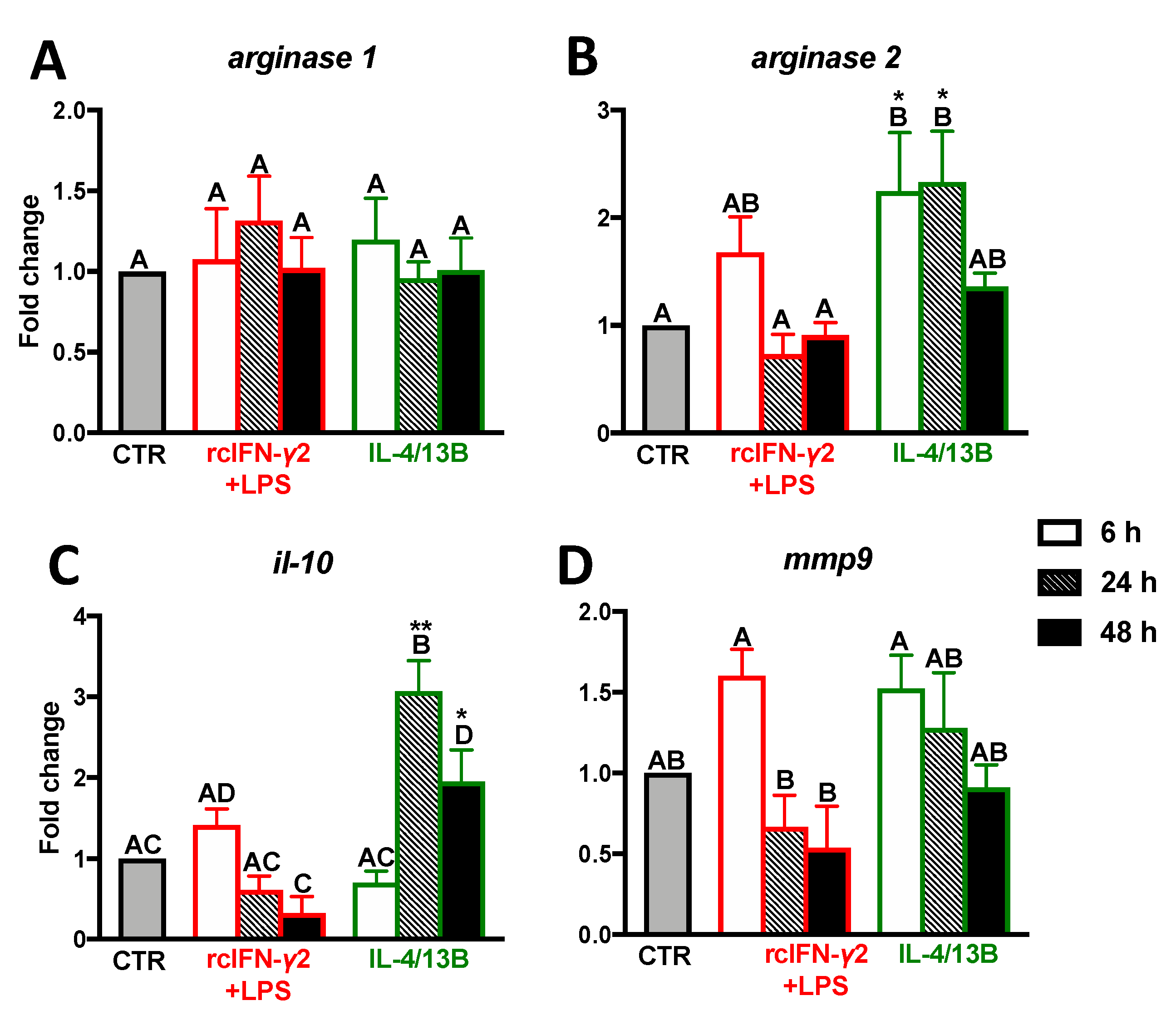
2.3. Expression of Anti-Inflammatory Mediators in Monocytes/Macrophages Stimulated with IFN-γ2 + LPS and IL-4/13b
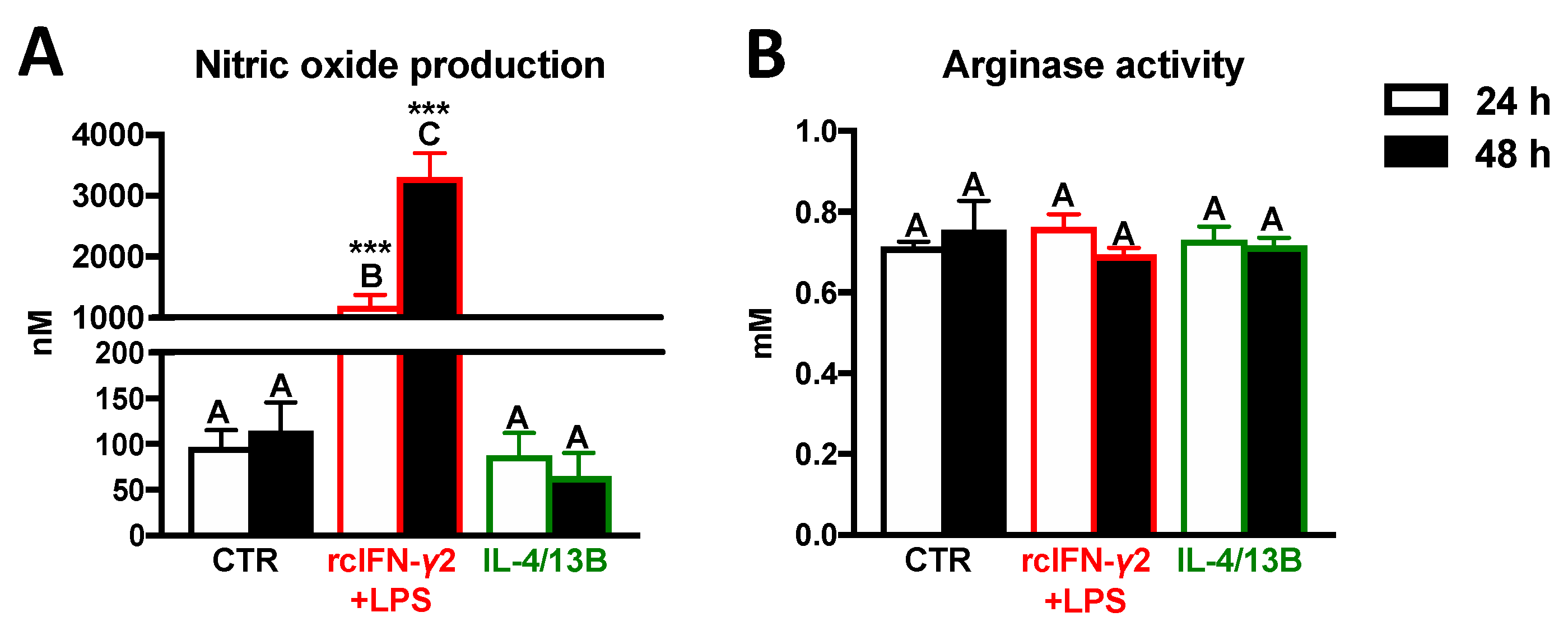
2.4. Activity of iNOS and Arginase in Monocytes/Macrophages after Stimulation with rcIFN-γ2 + LPS or IL-4/13B
2.5. Expression of Genes Encoding Molecules Involved in Cortisol Conversion and Binding in Monocytes/Macrophages Stimulated with rcIFN-γ2+LPS and IL-4/13B
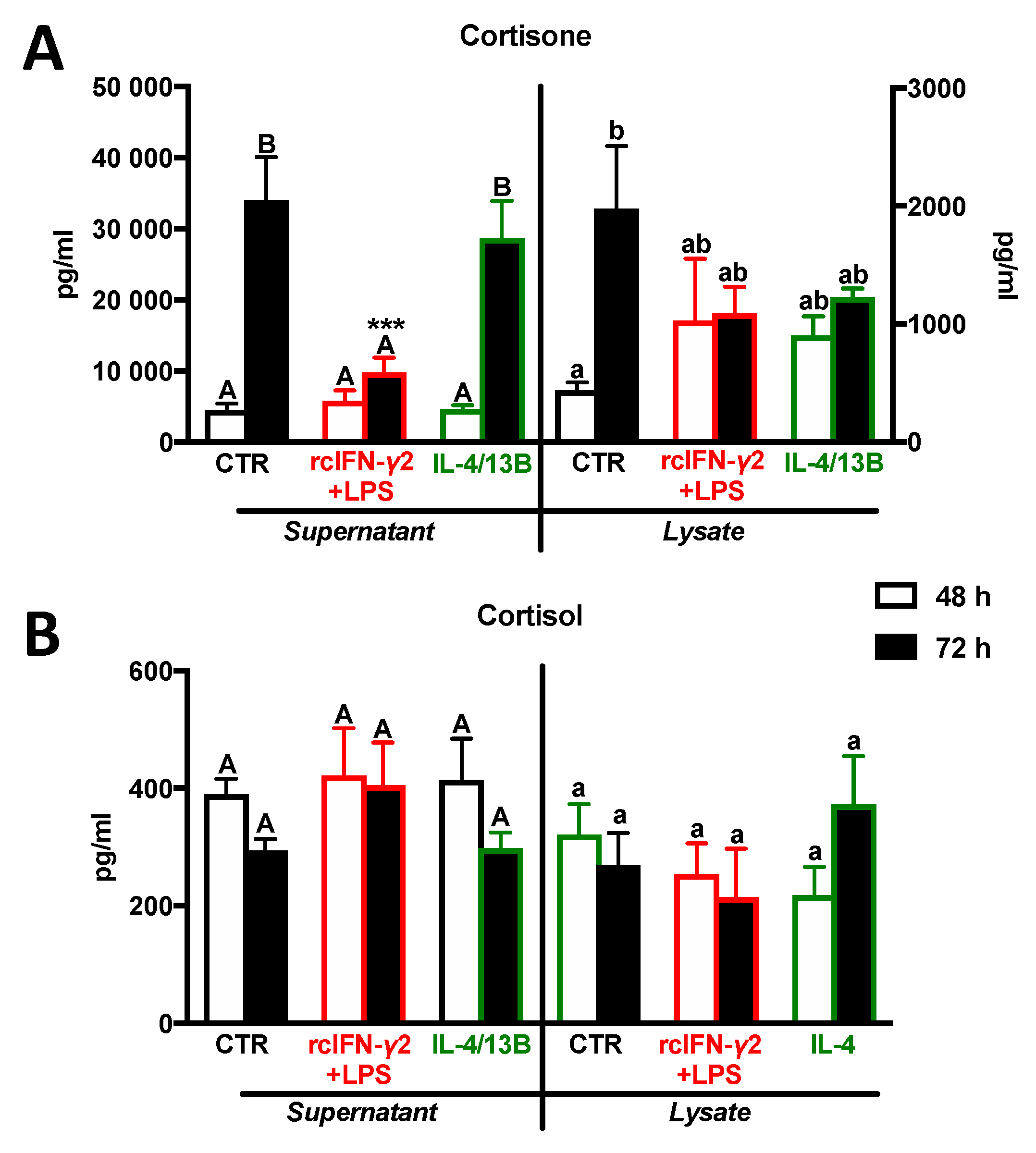
2.6. Cortisone and Cortisol Levels in Monocytes/Macrophages after Stimulation with rcIFN-γ2 + LPS or IL-4/13B
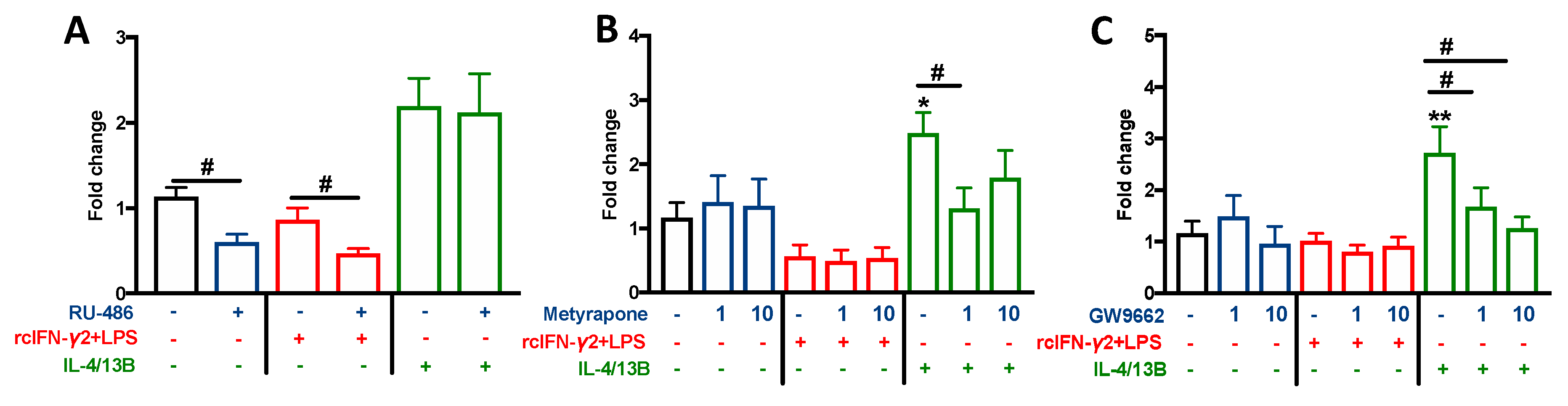
2.7. The Effect of Cortisol Conversion/Inhibition and GR and PPARγ Blocking on the Monocyte/Macrophage Polarization
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Isolation of Lymphoid Organs, Peripheral Blood Leukocytes, and Head Kidney Leukocytes
4.3. Monocytes/Macrophages Culture and Polarization
4.4. Inhibition of Cortisol Conversion and Blocking of GR and PPARγ
4.5. Arginase Activity Assay
4.6. Nitric Oxide Assay
4.7. Enzyme Immunoassays
4.8. Gene Expression
4.8.1. Isolation of RNA and Complementary DNA (cDNA) Synthesis
4.8.2. RQ-PCR
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Plowden, J.; Renshaw-Hoelscher, M.; Engleman, C.; Katz, J.; Sambhara, S. Innate immunity in aging: Impact on macrophage function. Aging Cell 2004, 3, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Chittezhath, M.; Shalova, I.N.; Lim, J.Y. Macrophage polarization and plasticity in health and disease. Immunol. Res. 2012, 53, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.; Natoli, G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat. Rev. Immunol. 2011, 11, 750–761. [Google Scholar] [CrossRef]
- Verburg-van Kemenade, B.M.L.; Cohen, N.; Chadzinska, M. Neuroendocrine-immune interaction: Evolutionarily conserved mechanisms that maintain allostasis in an ever-changing environment. Dev. Comp. Immunol. 2017, 66, 2–23. [Google Scholar] [CrossRef]
- Thieringer, R.; Le Grand, C.B.; Carbin, L.; Cai, T.-Q.; Wong, B.; Wright, S.D.; Hermanowski-Vosatka, A. 11β-hydroxysteroid dehydrogenase type 1 is induced in human monocytes upon differentiation to macrophages. J. Immunol. 2001, 167, 30–35. [Google Scholar] [CrossRef]
- Chapman, K.; Holmes, M.; Seckl, J. 11β-Hydroxysteroid dehydrogenases: Intracellular gate-keepers of tissue glucocorticoid action. Physiol. Rev. 2013, 93, 1139–1206. [Google Scholar] [CrossRef]
- Payne, A.H.; Hales, D.B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef]
- Tokarz, J.; Möller, G.; Hrabě de Angelis, M.; Adamski, J. Steroids in teleost fishes: A functional point of view. Steroids 2015, 103, 123–144. [Google Scholar] [CrossRef]
- Arakane, F.; King, S.R.; Du, Y.; Kallen, C.B.; Walsh, L.P.; Watari, H.; Stocco, D.M.; Strauss, J.F. Phosphorylation of steroidogenic acute regulatory protein (StAR) modulates its steroidogenic activity. J. Biol. Chem. 1997, 272, 32656–32662. [Google Scholar] [CrossRef] [PubMed]
- Lawson, A.J.; Walker, E.A.; Lavery, G.G.; Bujalska, I.J.; Hughes, B.; Arlt, W.; Stewart, P.M.; Ride, J.P. Cortisone-reductase deficiency associated with heterozygous mutations in 11β-hydroxysteroid dehydrogenase type 1. Proc. Natl. Acad. Sci. USA 2011, 108, 4111–4116. [Google Scholar] [CrossRef] [PubMed]
- White, P.C. Alterations of cortisol metabolism in human disorders. Horm. Res. Paediatr. 2018, 89, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ren, S.; Pandak, W.M.; Li, X.; Ning, Y.; Lu, C.; Zhao, F.; Yin, L. The effects of inflammatory cytokines on steroidogenic acute regulatory protein expression in macrophages. Inflamm. Res. 2007, 56, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, F.; Taylor, J.M.; Bartholomew, C.; Graham, A. Differential regulation of the STARD1 subfamily of START lipid trafficking proteins in human macrophages. FEBS Lett. 2009, 583, 1147–1153. [Google Scholar] [CrossRef]
- Ning, Y.; Bai, Q.; Lu, H.; Li, X.; Pandak, W.M.; Zhao, F.; Chen, S.; Ren, S.; Yin, L. Overexpression of mitochondrial cholesterol delivery protein, StAR, decreases intracellular lipids and inflammatory factors secretion in macrophages. Atherosclerosis 2009, 204, 114–120. [Google Scholar] [CrossRef]
- Taylor, J.M.W.; Borthwick, F.; Bartholomew, C.; Graham, A. Overexpression of steroidogenic acute regulatory protein increases macrophage cholesterol efflux to apolipoprotein AI. Cardiovasc. Res. 2010, 86, 526–534. [Google Scholar] [CrossRef]
- Graham, A. Mitochondrial regulation of macrophage cholesterol homeostasis. Free Radic. Biol. Med. 2015, 89, 982–992. [Google Scholar] [CrossRef]
- Baker, M.E. Evolution of 11β-hydroxysteroid dehydrogenase-type 1 and 11β-hydroxysteroid dehydrogenase-type 3. FEBS Lett. 2010, 584, 2279–2284. [Google Scholar] [CrossRef]
- Baker, M.E. Evolutionary analysis of 11β-hydroxysteroid dehydrogenase-type 1, -type 2, -type 3 and 17β-hydroxysteroid dehydrogenase-type 2 in fish. FEBS Lett. 2004, 574, 167–170. [Google Scholar] [CrossRef]
- Chadzinska, M.; Leon-Kloosterziel, K.M.; Plytycz, B.; Verburg-van Kemenade, B.M.L. In vivo kinetics of cytokine expression during peritonitis in carp: Evidence for innate and alternative macrophage polarization. Dev. Comp. Immunol. 2008, 32, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Forlenza, M.; Fink, I.R.; Raes, G.; Wiegertjes, G.F. Heterogeneity of macrophage activation in fish. Dev. Comp. Immunol. 2011, 35, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Joerink, M.; Ribeiro, C.M.S.; Stet, R.J.M.; Hermsen, T.; Savelkoul, H.F.J.; Wiegertjes, G.F. Head kidney-derived macrophages of common carp (Cyprinus carpio L.) show plasticity and functional polarization upon differential stimulation. J. Immunol. 2006, 177, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, J.W.; Fibke, C.; Belosevic, M. Recombinant IL-4/13A and IL-4/13B induce arginase activity and down-regulate nitric oxide response of primary goldfish (Carassius auratus L.) macrophages. Dev. Comp. Immunol. 2017, 67, 377–384. [Google Scholar] [CrossRef]
- Fink, I.R.; Elks, P.M.; Wentzel, A.S.; Spaink, H.P.; Wiegertjes, G.F. Polarization of immune responses in fish: The ‘macrophages first’ point of view. Mol. Immunol. 2015, 69, 146–156. [Google Scholar] [CrossRef]
- Mao, K.; Chen, W.; Mu, Y.; Ao, J.; Chen, X. Identification of two IL-4/13 homologues in large yellow croaker (Larimichthys crocea) revealed their similar roles in inducing alternative activation of monocytes/macrophages. Fish Shellfish Immunol. 2018, 80, 180–190. [Google Scholar] [CrossRef]
- Montero, J.; Gómez-Abellán, V.; Arizcun, M.; Mulero, V.; Sepulcre, M.P. Prostaglandin E2 promotes M2 polarization of macrophages via a cAMP/CREB signaling pathway and deactivates granulocytes in teleost fish. Fish Shellfish Immunol. 2016, 55, 632–641. [Google Scholar] [CrossRef]
- Wentzel, A.S.; Janssen, J.J.E.; de Boer, V.C.J.; van Veen, W.G.; Forlenza, M.; Wiegertjes, G.F. Fish macrophages show distinct metabolic signatures upon polarization. Front. Immunol. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Wentzel, A.S.; Petit, J.; van Veen, W.G.; Fink, I.R.; Scheer, M.H.; Piazzon, M.C.; Forlenza, M.; Spaink, H.P.; Wiegertjes, G.F. Transcriptome sequencing supports a conservation of macrophage polarization in fish. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Joerink, M.; Forlenza, M.; Ribeiro, C.M.S.; de Vries, B.J.; Savelkoul, H.F.J.; Wiegertjes, G.F. Differential macrophage polarisation during parasitic infections in common carp (Cyprinus carpio L.). Fish Shellfish Immunol. 2006, 21, 561–571. [Google Scholar] [CrossRef]
- Joerink, M.; Savelkoul, H.F.J.; Wiegertjes, G.F. Evolutionary conservation of alternative activation of macrophages: Structural and functional characterization of arginase 1 and 2 in carp (Cyprinus carpio L.). Mol. Immunol. 2006, 43, 1116–1128. [Google Scholar] [CrossRef] [PubMed]
- Maciuszek, M.; Rydz, L.; Świtakowska, I.; Verburg-van Kemenade, B.M.L.; Chadzińska, M. Effects of stress and cortisol on the polarization of carp macrophages. Fish Shellfish Immunol. 2019, 94, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Kostadinova, F.; Hostettler, N.; Bianchi, P.; Brunner, T. Extra-adrenal glucocorticoid synthesis in mucosal tissues and its implication in mucosal immune homeostasis and tumor development. In Glucocorticoids—New Recognition of Our Familiar Friend; InTech: London, UK, 2012; ISBN 9789535108726. [Google Scholar]
- Taves, M.D.; Hamden, J.E.; Soma, K.K. Local glucocorticoid production in lymphoid organs of mice and birds: Functions in lymphocyte development. Horm. Behav. 2017, 88, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Terao, M.; Katayama, I. Local cortisol/corticosterone activation in skin physiology and pathology. J. Dermatol. Sci. 2016, 84, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Bessout, R.; Semont, A.; Demarquay, C.; Charcosset, A.; Benderitter, M.; Mathieu, N. Mesenchymal stem cell therapy induces glucocorticoid synthesis in colonic mucosa and suppresses radiation-activated T cells: New insights into MSC immunomodulation. Mucosal Immunol. 2014, 7, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Ashwell, J.D.; Vacchio, M.S.; Galon, J. Do glucocorticoids participate in thymocyte development? Immunol. Today 2000, 21, 644–645. [Google Scholar] [CrossRef]
- Lechner, O.; Dietrich, H.; Wiegers, G.J.; Vacchio, M.; Wick, G. Glucocorticoid production in the chicken bursa and thymus. Int. Immunol. 2001, 13, 769–776. [Google Scholar] [CrossRef]
- Böhm, M.; Zmijewski, M.A.; Wasiewicz, T.; Straub, R.H.; Raap, U.; Luger, T.A.; Slominski, A. KU812 basophils express urocortin, CRH-R, MC1R and steroidogenic enzymes and secrete progesterone. Exp. Dermatol. 2012, 21, 541–543. [Google Scholar] [CrossRef]
- Xiong, X.; Liang, Q.; Chen, J.; Fan, R.; Cheng, T. Proteomics profiling of pituitary, adrenal gland, and splenic lymphocytes in rats with middle cerebral artery occlusion. Biosci. Biotechnol. Biochem. 2009, 73, 657–664. [Google Scholar] [CrossRef][Green Version]
- Bai, Q.; Li, X.; Ning, Y.; Zhao, F.; Yin, L. Mitochondrial cholesterol transporter, StAR, inhibits human THP-1 monocyte-derived macrophage apoptosis. Lipids 2010, 45, 29–36. [Google Scholar] [CrossRef]
- Kusakabe, M.; Todo, T.; James Mcquillan, H.; Goetz, F.W.; Young, G. Characterization and expression of steroidogenic acute regulatory protein and MLN64 cDNAs in trout. Endocrinology 2002, 143, 2062–2070. [Google Scholar] [CrossRef] [PubMed]
- Taves, M.D.; Plumb, A.W.; Sandkam, B.A.; Ma, C.; Van Der Gugten, J.G.; Holmes, D.T.; Close, D.A.; Abraham, N.; Soma, K.K. Steroid profiling reveals widespread local regulation of glucocorticoid levels during mouse development. Endocrinology 2015, 156, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.E.; Coutinho, A.E.; Zhang, Z.; Kipari, T.; Savill, J.S.; Seckl, J.R. Changing glucocorticoid action: 11β-Hydroxysteroid dehydrogenase type 1 in acute and chronic inflammation. J. Steroid Biochem. Mol. Biol. 2013, 137, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, A.E.; Brown, J.K.; Yang, F.; Brownstein, D.G.; Gray, M.; Seckl, J.R.; Savill, J.S.; Chapman, K.E. Mast cells express 11β-hydroxysteroid dehydrogenase type 1: A role in restraining mast cell degranulation. PLoS ONE 2013, 8, e54640. [Google Scholar] [CrossRef]
- Freeman, L.; Hewison, M.; Hughes, S.V.; Evans, K.M.; Hardie, D.; Means, T.K.; Chakraverty, R. Expression of 11β-hydroxysteroid dehydrogenase type 1 permits regulation of glucocorticoid bioavailability by human dendritic cells. Blood 2005, 106, 2042–2049. [Google Scholar] [CrossRef]
- Gilmour, J.S.; Coutinho, A.E.; Cailhier, J.-F.; Man, T.Y.; Clay, M.; Thomas, G.; Harris, H.J.; Mullins, J.J.; Seckl, J.R.; Savill, J.S.; et al. Local amplification of glucocorticoids by 11β-hydroxysteroid dehydrogenase type 1 promotes macrophage phagocytosis of apoptotic leukocytes. J. Immunol. 2006, 176, 7605–7611. [Google Scholar] [CrossRef]
- Fuzzen, M.L.M.; Van Der Kraak, G.; Bernier, N.J. Stirring up new ideas about the regulation of the hypothalamic-pituitary-interrenal axis in zebrafish (Danio rerio). Zebrafish 2010, 7, 349–358. [Google Scholar] [CrossRef]
- Nematollahi, M.A.; van Pelt-Heerschap, H.; Komen, J. Transcript levels of five enzymes involved in cortisol synthesis and regulation during the stress response in common carp: Relationship with cortisol. Gen. Comp. Endocrinol. 2009, 164, 85–90. [Google Scholar] [CrossRef]
- Jiang, J.Q.; Wang, D.S.; Senthilkumaran, B.; Kobayashi, T.; Kobayashi, H.K.; Yamaguchi, A.; Ge, W.; Young, G.; Nagahama, Y. Isolation, characterization and expression of 11β-hydroxysteroid dehydrogenase type 2 cDNAs from the testes of Japanese eel (Anguilla japonica) and Nile tilapia (Oreochromis niloticus). J. Mol. Endocrinol. 2003, 31, 305–315. [Google Scholar] [CrossRef]
- Kusakabe, M.; Nakamura, I.; Young, G. Enzymatic activity of 11β-hydroxysteroid dehydrogenase in rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2003, 28, 197–198. [Google Scholar] [CrossRef]
- Chapman, K.E.; Coutinho, A.E.; Gray, M.; Gilmour, J.S.; Savill, J.S.; Seckl, J.R. The role and regulation of 11β-hydroxysteroid dehydrogenase type 1 in the inflammatory response. Mol. Cell. Endocrinol. 2009, 301, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Hennebold, J.D.; Ryu, S.Y.; Mu, H.H.; Galbraith, A.; Daynes, R.A. 11β-hydroxysteroid dehydrogenase modulation of glucocorticoid activities in lymphoid organs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1996, 270. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, A.E.; Kipari, T.M.J.; Zhang, Z.; Esteves, C.L.; Lucas, C.D.; Gilmour, J.S.; Webster, S.P.; Walker, B.R.; Hughes, J.; Savill, J.S.; et al. 11β-Hydroxysteroid dehydrogenase type 1 is expressed in neutrophils and restrains an inflammatory response in male mice. Endocrinology 2016, 157, 2928–2936. [Google Scholar] [CrossRef] [PubMed]
- Kardon, T.; Senesi, S.; Marcolongo, P.; Legeza, B.; Bánhegyi, G.; Mandl, J.; Fulceri, R.; Benedetti, A. Maintenance of luminal NADPH in the endoplasmic reticulum promotes the survival of human neutrophil granulocytes. FEBS Lett. 2008, 582, 1809–1815. [Google Scholar] [CrossRef][Green Version]
- Zhang, T.Y.; Daynes, R.A. Macrophages from 11β-hydroxysteroid dehydrogenase type 1-deficient mice exhibit an increased sensitivity to lipopolysaccharide stimulation due to TGF-β-Mediated up-regulation of SHIP1 expression. J. Immunol. 2007, 179, 6325–6335. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Ding, X.; Daynes, R.A. The expression of 11β-hydroxysteroid dehydrogenase type i by lymphocytes provides a novel means for intracrine regulation of glucocorticoid activities. J. Immunol. 2005, 174, 879–889. [Google Scholar] [CrossRef]
- Huang, C.; Wan, B.; Gao, B.; Hexige, S.; Yu, L. Isolation and characterization of novel human short-chain dehydrogenase/reductase SCDR10B which is highly expressed in the brain and acts as hydroxysteroid dehydrogenase. Acta Biochim. Pol. 2009, 56, 279–289. [Google Scholar] [CrossRef]
- Tsachaki, M.; Meyer, A.; Weger, B.; Kratschmar, D.V.; Tokarz, J.; Adamski, J.; Belting, H.G.; Affolter, M.; Dickmeis, T.; Odermatt, A. Absence of 11-keto reduction of cortisone and 11-ketotestosterone in the model organism zebrafish. J. Endocrinol. 2017, 232, 323–335. [Google Scholar] [CrossRef][Green Version]
- Tokarz, J.; Mindnich, R.; Norton, W.; Möller, G.; Hrabé de Angelis, M.; Adamski, J. Discovery of a novel enzyme mediating glucocorticoid catabolism in fish: 20beta-Hydroxysteroid dehydrogenase type 2. Mol. Cell. Endocrinol. 2012, 349, 202–213. [Google Scholar] [CrossRef]
- Arts, J.A.J.; Tijhaar, E.J.; Chadzinska, M.; Savelkoul, H.F.J.; Verburg-van Kemenade, B.M.L. Functional analysis of carp interferon-γ: Evolutionary conservation of classical phagocyte activation. Fish Shellfish Immunol. 2010, 29, 793–802. [Google Scholar] [CrossRef]
- Pijanowski, L.; Scheer, M.; Verburg-van Kemenade, B.M.L.; Chadzinska, M. Production of inflammatory mediators and extracellular traps by carp macrophages and neutrophils in response to lipopolysaccharide and/or interferon-γ2. Fish Shellfish Immunol. 2015, 42, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Tumbol, R.A.; Baiano, J.C.F.; Barnes, A.C. Differing cell population structure reflects differing activity of Percoll-separated pronephros and peritoneal leucocytes from barramundi (Lates calcarifer). Aquaculture 2009, 292, 180–188. [Google Scholar] [CrossRef]
- Chen, T.; Hu, Y.; Zhou, J.; Hu, S.; Xiao, X.; Liu, X.; Su, J.; Yuan, G. Chitosan reduces the protective effects of IFN-γ2 on grass carp (Ctenopharyngodon idella) against Flavobacterium columnare infection due to excessive inflammation. Fish Shellfish Immunol. 2019, 95, 305–313. [Google Scholar] [CrossRef]
- Wang, T.; Johansson, P.; Abós, B.; Holt, A.; Tafalla, C.; Jiang, Y.; Wang, A.; Xu, Q.; Qi, Z.; Huang, W.; et al. First in-depth analysis of the novel Th2-type cytokines in salmonid fish reveals distinct patterns of expression and modulation but overlapping bioactivities. Oncotarget 2016, 7, 10917–10946. [Google Scholar] [CrossRef] [PubMed]
- Chinetti-Gbaguidi, G.; Bouhlel, M.A.; Copin, C.; Duhem, C.; Derudas, B.; Neve, B.; Noel, B.; Eeckhoute, J.; Lefebvre, P.; Seckl, J.R.; et al. Peroxisome proliferator-activated receptor-γ activation induces 11β-hydroxysteroid dehydrogenase type 1 activity in human alternative macrophages. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Masuzaki, H.; Tanaka, T.; Arai, N.; Yasue, S.; Kobayashi, N.; Tomita, T.; Noguchi, M.; Fujikura, J.; Ebihara, K.; et al. Augmentation of 11β-hydroxysteroid dehydrogenase type 1 in LPS-activated J774.1 macrophages—Role of 11β-HSD1 in pro-inflammatory properties in macrophages. FEBS Lett. 2007, 581, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S.; Locati, M.; Mantovani, A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. J. Immunol. 2006, 177, 7303–7311. [Google Scholar] [CrossRef]
- Ergang, P.; Vytáčková, K.; Švec, J.; Bryndová, J.; Mikšík, I.; Pácha, J. Upregulation of 11β-hydroxysteroid dehydrogenase 1 in lymphoid organs during inflammation in the rat. J. Steroid Biochem. Mol. Biol. 2011, 126, 19–25. [Google Scholar] [CrossRef]
- Olsen, N.; Sokka, T.; Seehorn, C.; Kraft, B.; Maas, K.; Moore, J.; Aune, T. A gene expression signature for recent onset rheumatoid arthritis in peripheral blood mononuclear cells. Ann. Rheum. Dis. 2004, 63, 1387–1392. [Google Scholar] [CrossRef]
- Suzuki, S.; Tsubochi, H.; Ishibashi, H.; Suzuki, T.; Kondo, T.; Sasano, H. Increased expression of 11β-hydroxysteroid dehydrogenase type 2 in the lungs of patients with acute respiratory distress syndrome. Pathol. Int. 2003, 53, 751–756. [Google Scholar] [CrossRef]
- Stolte, E.H.; Nabuurs, S.B.; Bury, N.R.; Sturm, A.; Flik, G.; Savelkoul, H.F.J.; Verburg-van Kemenade, B.M.L. Stress and innate immunity in carp: Corticosteroid receptors and pro-inflammatory cytokines. Mol. Immunol. 2008, 46, 70–79. [Google Scholar] [CrossRef]
- Stolte, E.H.; Chadzinska, M.; Przybylska, D.; Flik, G.; Savelkoul, H.F.J.; Verburg-van Kemenade, B.M.L. The immune response differentially regulates Hsp70 and glucocorticoid receptor expression in vitro and in vivo in common carp (Cyprinus carpio L.). Fish Shellfish Immunol. 2009, 27, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Desgeorges, T.; Caratti, G.; Mounier, R.; Tuckermann, J.; Chazaud, B. Glucocorticoids shape macrophage phenotype for tissue repair. Front. Immunol. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Oh, K.-S.; Patel, H.; Gottschalk, R.A.; Lee, W.S.; Baek, S.; Fraser, I.D.C.; Hager, G.L.; Sung, M.-H. Anti-inflammatory chromatinscape suggests alternative mechanisms of glucocorticoid receptor action. Immunity 2017, 47, 298–309.e5. [Google Scholar] [CrossRef] [PubMed]
- Smyth, G.P.; Stapleton, P.P.; Freeman, T.A.; Concannon, E.M.; Mestre, J.R.; Duff, M.; Maddali, S.; Daly, J.M. Glucocorticoid pretreatment induces cytokine overexpression and nuclear factor-κB activation in macrophages. J. Surg. Res. 2004, 116, 253–261. [Google Scholar] [CrossRef]
- Van de Garde, M.D.B.; Martinez, F.O.; Melgert, B.N.; Hylkema, M.N.; Jonkers, R.E.; Hamann, J. Chronic exposure to glucocorticoids shapes gene expression and modulates innate and adaptive activation pathways in macrophages with distinct changes in leukocyte attraction. J. Immunol. 2014, 192, 1196–1208. [Google Scholar] [CrossRef]
- Abumaree, M.H.; Al Jumah, M.A.; Kalionis, B.; Jawdat, D.; Al Khaldi, A.; Abomaray, F.M.; Fatani, A.S.; Chamley, L.W.; Knawy, B.A. Human placental mesenchymal stem cells (pMSCs) Play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell Rev. Rep. 2013, 9, 620–641. [Google Scholar] [CrossRef]
- Terao, M.; Murota, H.; Kimura, A.; Kato, A.; Ishikawa, A.; Igawa, K.; Miyoshi, E.; Katayama, I. 11β-hydroxysteroid dehydrogenase-1 is a novel regulator of skin homeostasis and a candidate target for promoting tissue repair. PLoS ONE 2011, 6, e25039. [Google Scholar] [CrossRef]
- Emmerich, J.; van Koppen, C.J.; Burkhart, J.L.; Engeli, R.T.; Hu, Q.; Odermatt, A.; Hartmann, R.W. Accelerated skin wound healing by selective 11β-Hydroxylase (CYP11B1) inhibitors. Eur. J. Med. Chem. 2018, 143, 591–597. [Google Scholar] [CrossRef]
- Noti, M.; Corazza, N.; Tuffin, G.; Schoonjans, K.; Brunner, T. Lipopolysaccharide induces intestinal glucocorticoid synthesis in a TNFα-dependent manner. FASEB J. 2010, 24, 1340–1346. [Google Scholar] [CrossRef]
- Park, S.B.; Park, J.S.; Jung, W.H.; Kim, H.Y.; Kwak, H.J.; Ahn, J.H.; Choi, K.J.; Na, Y.J.; Choi, S.; Dal Rhee, S.; et al. Anti-inflammatory effect of a selective 11β-hydroxysteroid dehydrogenase type 1 inhibitor via the stimulation of heme oxygenase-1 in LPS-activated mice and J774.1 murine macrophages. J. Pharmacol. Sci. 2016, 131, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage M1–M2 polarization balance. Front. Immunol. 2014, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.S.; Ricote, M.; Akiyama, T.E.; Gonzalez, F.J.; Glass, C.K. PPARγ and PPARδ negatively regulate specific subsets of lipopolysaccharide and IFN- target genes in macrophages. Proc. Natl. Acad. Sci. USA 2003, 100, 6712–6717. [Google Scholar] [CrossRef] [PubMed]
- Bouhlel, M.A.; Derudas, B.; Rigamonti, E.; Dièvart, R.; Brozek, J.; Haulon, S.; Zawadzki, C.; Jude, B.; Torpier, G.; Marx, N.; et al. PPARγ Activation Primes Human Monocytes into Alternative M2 Macrophages with Anti-inflammatory Properties. Cell Metab. 2007, 6, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Ting, A.T.; Seed, B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature 1998, 391, 82–86. [Google Scholar] [CrossRef]
- Saqib, U.; Sarkar, S.; Suk, K.; Mohammad, O.; Baig, M.S.; Savai, R. Phytochemicals as modulators of M1-M2 macrophages in inflammation. Oncotarget 2018, 9, 17937–17950. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, C.; Yao, Q.; Qian, L.; Liu, J.; Xie, X.; Ma, W.; Nie, X.; Lai, B.; Xiao, L.; et al. Procyanidin B2 activates PPARγ to induce M2 polarization in mouse macrophages. Front. Immunol. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Luo, S.; Huang, Y.; Xie, F.; Huang, X.; Liu, Y.; Wang, W.; Qin, Q. Molecular cloning, characterization and expression analysis of PPAR gamma in the orange-spotted grouper (Epinephelus coioides) after the Vibrio alginolyticus challenge. Fish Shellfish Immunol. 2015, 43, 310–324. [Google Scholar] [CrossRef]
- Zizzo, G.; Cohen, P.L. The PPAR-γ antagonist GW9662 elicits differentiation of M2c-like cells and upregulation of the MerTK/Gas6 axis: A key role for PPAR-γ in human macrophage polarization. J. Inflamm. 2015, 12, 1–16. [Google Scholar] [CrossRef]
- Verburg-van Kemenade, B.M.L.; Groeneveld, A.; van Rens, B.T.T.M.; Rombout, J.H.W.M. Characterization of macrophages and neutrophilic granulocytes from the pronephros of carp (Cyprinus carpio). J. Exp. Biol. 1994, 187, 143–158. [Google Scholar]
- Szwejser, E.; Maciuszek, M.; Casanova-Nakayama, A.; Segner, H.; Verburg-van Kemenade, B.M.L.; Chadzinska, M. A role for multiple estrogen receptors in immune regulation of common carp. Dev. Comp. Immunol. 2017, 66, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Weyts, F.A.A.; Verburg-Van Kemenade, B.M.L.; Flik, G. Characterisation of glucocorticoid receptors in peripheral blood leukocytes of carp, Cyprinus carpio L. Gen. Comp. Endocrinol. 1998, 111, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Corraliza, I.M.; Campo, M.L.; Soler, G.; Modolell, M. Determination of arginase activity in macrophages: A micromethod. J. Immunol. Methods 1994, 174, 231–235. [Google Scholar] [CrossRef]
- Chadzinska, M.; Savelkoul, H.F.J.; Verburg-van Kemenade, B.M.L. Morphine affects the inflammatory response in carp by impairment of leukocyte migration. Dev. Comp. Immunol. 2009, 33, 88–96. [Google Scholar] [CrossRef]
- Kepka, M.; Verburg-van Kemenade, B.M.L.; Chadzinska, M. Neuroendocrine modulation of the inflammatory response in common carp: Adrenaline regulates leukocyte profile and activity. Gen. Comp. Endocrinol. 2013, 188, 102–109. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maciuszek, M.; Klak, K.; Rydz, L.; Verburg-van Kemenade, B.M.L.; Chadzinska, M. Cortisol Metabolism in Carp Macrophages: A Role for Macrophage-Derived Cortisol in M1/M2 Polarization. Int. J. Mol. Sci. 2020, 21, 8954. https://doi.org/10.3390/ijms21238954
Maciuszek M, Klak K, Rydz L, Verburg-van Kemenade BML, Chadzinska M. Cortisol Metabolism in Carp Macrophages: A Role for Macrophage-Derived Cortisol in M1/M2 Polarization. International Journal of Molecular Sciences. 2020; 21(23):8954. https://doi.org/10.3390/ijms21238954
Chicago/Turabian StyleMaciuszek, Magdalena, Katarzyna Klak, Leszek Rydz, B. M. Lidy Verburg-van Kemenade, and Magdalena Chadzinska. 2020. "Cortisol Metabolism in Carp Macrophages: A Role for Macrophage-Derived Cortisol in M1/M2 Polarization" International Journal of Molecular Sciences 21, no. 23: 8954. https://doi.org/10.3390/ijms21238954
APA StyleMaciuszek, M., Klak, K., Rydz, L., Verburg-van Kemenade, B. M. L., & Chadzinska, M. (2020). Cortisol Metabolism in Carp Macrophages: A Role for Macrophage-Derived Cortisol in M1/M2 Polarization. International Journal of Molecular Sciences, 21(23), 8954. https://doi.org/10.3390/ijms21238954





