The Crosstalk between FAK and Wnt Signaling Pathways in Cancer and Its Therapeutic Implication
Abstract
:1. Introduction
2. The Focal Adhesion Kinase (FAK) Signaling Pathway
3. The Wnt Signaling Pathway
4. FAK–Wnt Pathways Crosstalk in Development
5. FAK–Wnt Pathways Crosstalk in Cancer
5.1. Colorectal and Intestinal Cancers
5.2. Malignant Mesothelioma and Lung Cancer
5.3. Ovarian and Breast Cancer
5.4. Other Malignancies
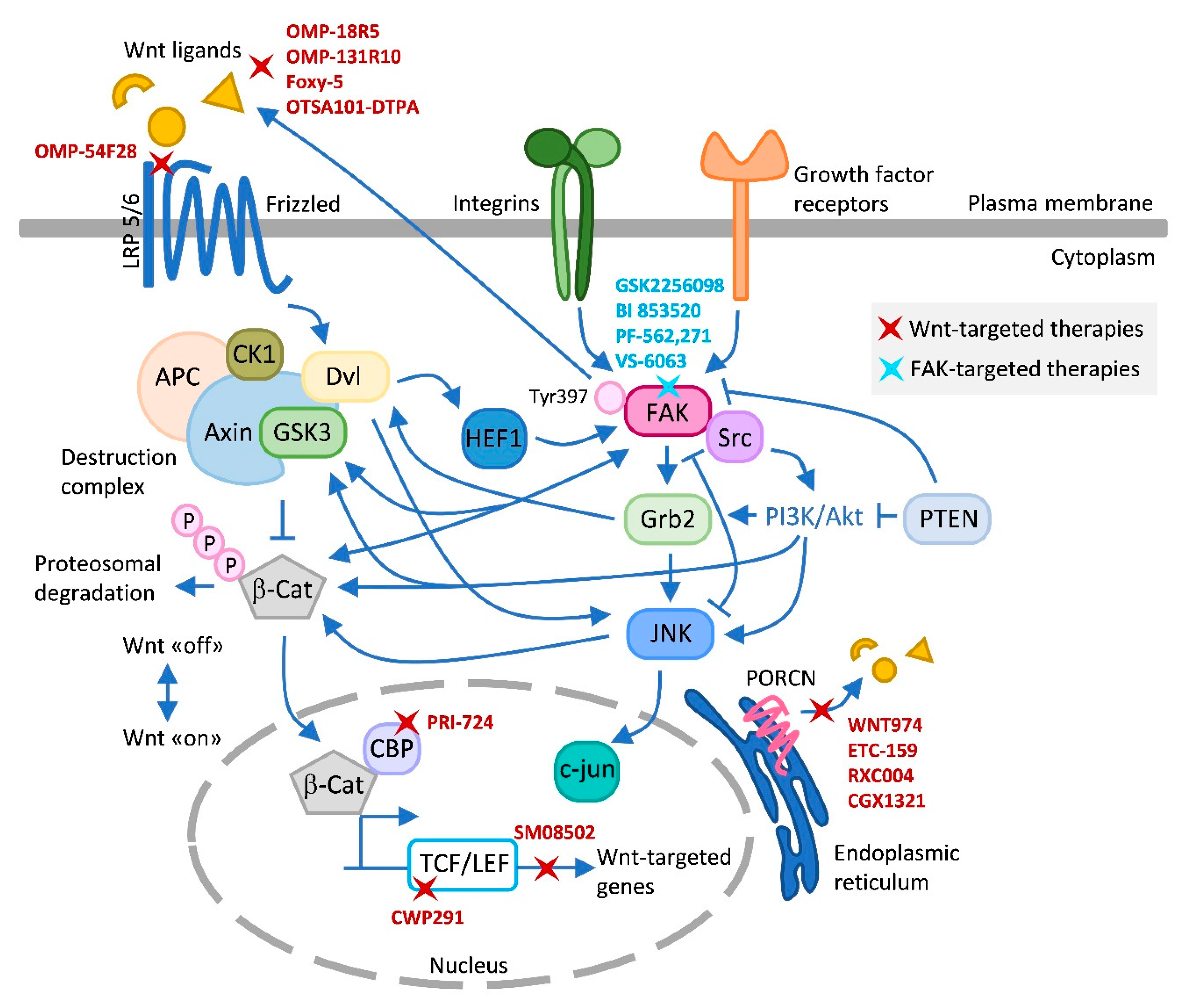
6. Current Landscape of Wnt- and FAK-Targeted Therapies in Clinical Trials
7. Potential Benefits of Targeting the Crosstalk Wnt–FAK
8. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sulzmaier, F.J.; Jean, C.; Schlaepfer, D.D. FAK in cancer: Mechanistic findings and clinical applications. Nat. Rev. Cancer 2014, 14, 598–610. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.Y.; Timpson, P.; Horvath, L.G.; Daly, R.J. FAK signaling in human cancer as a target for therapeutics. Pharmacol. Ther. 2015, 146, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M.D. Cellular functions of FAK kinases: Insight into molecular mechanisms and novel functions. J. Cell Sci. 2010, 123, 1007–1013. [Google Scholar] [CrossRef] [Green Version]
- Golubovskaya, V.M. Targeting FAK in human cancer: From finding to first clinical trials. Front. Biosci. 2014, 19, 687–706. [Google Scholar] [CrossRef] [Green Version]
- Kahn, M. Can we safely target the WNT pathway? Nat. Rev. Drug Discov. 2014, 13, 513–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nusse, R. Wnt signaling. Cold Spring Harb. Perspect. Biol. 2012, 4, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Harb, J.; Lin, P.-J.; Hao, J. Recent Development of Wnt Signaling Pathway Inhibitors for Cancer Therapeutics. Curr. Oncol. Rep. 2019, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Vert, G.; Chory, J. Crosstalk in Cellular Signaling: Background Noise or the Real Thing? Dev. Cell 2011, 21, 985–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowland, M.A.; Greenbaum, J.M.; Deeds, E.J. Crosstalk and the evolvability of intracellular communication. Nat. Commun. 2017, 8, 16009. [Google Scholar] [CrossRef] [Green Version]
- Gossage, L.; Eisen, T. Targeting multiple kinase pathways: A change in paradigm. Clin. Cancer Res. 2010, 16, 1973–1978. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.-T.; Chen, X.L.; Lim, Y.; Hanson, D.A.; Vo, T.-T.; Howerton, K.; Larocque, N.; Fisher, S.J.; Schlaepfer, D.D.; Ilic, D. Nuclear FAK Promotes Cell Proliferation and Survival through FERM-Enhanced p53 Degradation. Mol. Cell 2008, 29, 9–22. [Google Scholar] [CrossRef] [Green Version]
- Gabarra-Niecko, V.; Schaller, M.D.; Dunty, J.M. FAK regulates biological processes important for the pathogenesis of cancer. Cancer Metastasis Rev. 2003, 22, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M.D. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim. Biophys. Acta (BBA) Bioenergy 2001, 1540, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Fiedorek, F.T.; Kay, E.S. Mapping of the focal adhesion kinase (Fadk) gene to mouse chromosome 15 and human chromosome 8. Mamm. Genome 1995, 6, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M.D.; Borgman, C.A.; Cobb, B.S.; Vines, R.R.; Reynolds, A.B.; Parsons, J.T. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc. Natl. Acad. Sci. USA 1992, 89, 5192–5196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanks, S.K.; Calalb, M.B.; Harper, M.C.; Patel, S.K. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc. Natl. Acad. Sci. USA 1992, 89, 8487–8491. [Google Scholar] [CrossRef] [Green Version]
- Andre, E.; Beckerandre, M. Expression of an N-Terminally Truncated Form of Human Focal Adhesion Kinase in Brain. Biochem. Biophys. Res. Commun. 1993, 190, 140–147. [Google Scholar] [CrossRef]
- Zhang, X.; Wright, C.V.; Hanks, S.K. Cloning of a Xenopus laevis cDNA encoding focal adhesion kinase (FAK) and expression during early development. Gene 1995, 160, 219–222. [Google Scholar] [CrossRef]
- Schaller, M.D.; Hildebrand, J.D.; Shannon, J.D.; Fox, J.W.; Vines, R.R.; Parsons, J.T. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol. Cell. Biol. 1994, 14, 1680–1688. [Google Scholar] [CrossRef] [Green Version]
- Polte, T.R.; Hanks, S.K. Interaction between focal adhesion kinase and Crk-associated tyrosine kinase substrate p130Cas. Proc. Natl. Acad. Sci. USA 1995, 92, 10678–10682. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.C.; Appeddu, P.A.; Isoda, H.; Guan, J.L. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J. Biol. Chem. 1996, 271, 26329–26334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, S.K.; Schlaepfer, D.D. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 2006, 18, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Avizienyte, E.; Frame, M.C. Src and FAK signalling controls adhesion fate and the epithelial-to-mesenchymal transition. Curr. Opin. Cell Biol. 2005, 17, 542–547. [Google Scholar] [CrossRef]
- Owens, L.V.; Xu, L.; Craven, R.J.; Dent, G.A.; Weiner, T.M.; Kornberg, L.; Liu, E.T.; Cance, W.G. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 1995, 55, 2752–2755. [Google Scholar] [PubMed]
- Owens, L.V.; Xu, L.; Dent, G.A.; Yang, X.; Sturge, G.C.; Craven, R.J.; Cance, W. Focal adhesion kinase as a marker of invasive potential in differentiated human thyroid cancer. Ann. Surg. Oncol. 1996, 3, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, L.; Hauck, W.; Aprikian, A.G.; Begin, L.R.; Chapdelaine, A.; Chevalier, S. Focal adhesion kinase (pp125FAK) expression, activation and association with paxillin and p50CSK in human metastatic prostate carcinoma. Int. J. Cancer 1996, 68, 164–171. [Google Scholar] [CrossRef]
- Lark, A.L.; Livasy, C.A.; Calvo, B.; Caskey, L.; Moore, D.T.; Yang, X.; Cance, W.G. Overexpression of focal adhesion kinase in primary colorectal carcinomas and colorectal liver metastases: Immunohistochemistry and real-time PCR analyses. Clin. Cancer Res. 2003, 9, 215–222. [Google Scholar] [PubMed]
- Judson, P.L.; He, X.; Cance, W.G.; Van Le, L. Overexpression of focal adhesion kinase, a protein tyrosine kinase, in ovarian carcinoma. Cancer 1999, 86, 1551–1556. [Google Scholar] [CrossRef]
- Wörthmüller, J.; Blum, W.; Pecze, L.; Salicio, V.; Schwaller, B. Calretinin promotes invasiveness and EMT in malignant mesothelioma cells involving the activation of the FAK signaling pathway. Oncotarget 2018, 9, 36256–36272. [Google Scholar] [CrossRef] [Green Version]
- Yom, C.K.; Noh, D.-Y.; Kim, W.H.; Kim, H.S. Clinical significance of high focal adhesion kinase gene copy number and overexpression in invasive breast cancer. Breast Cancer Res. Treat. 2011, 128, 647–655. [Google Scholar] [CrossRef]
- Canel, M.; Secades, P.; Rodrigo, J.-P.; Cabanillas, R.; Herrero, A.; Suarez, C.; Chiara, M.-D. Overexpression of Focal Adhesion Kinase in Head and Neck Squamous Cell Carcinoma Is Independent of fak Gene Copy Number. Clin. Cancer Res. 2006, 12, 3272–3279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.T.; Chen, X.L.; Tomar, A.; Miller, N.L.G.; Yoo, J.; Schlaepfer, D.D. Knock-in mutation reveals an essential role for focal adhesion kinase activity in blood vessel morphogenesis and cell motility-polarity but not cell proliferation. J. Biol. Chem. 2010, 285, 21526–21536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, S.K.; Hanson, D.A.; Schlaepfer, D.D. Focal adhesion kinase: In command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005, 6, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Brami-Cherrier, K.; Gervasi, N.; Arsenieva, D.; Walkiewicz, K.; Boutterin, M.; Ortega, A.; Leonard, P.G.; Seantier, B.; Gasmi, L.; Bouceba, T.; et al. FAK dimerization controls its kinase-dependent functions at focal adhesions. EMBO J. 2014, 33, 356–370. [Google Scholar] [CrossRef]
- Cary, L.A.; Chang, J.F.; Guan, J.L. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J. Cell Sci. 1996, 109, 1787–1794. [Google Scholar]
- Parsons, J.T. Focal adhesion kinase: The first ten years. J. Cell Sci. 2003, 116, 1409–1416. [Google Scholar] [CrossRef] [Green Version]
- Golubovskaya, V.M.; Finch, R.; Cance, W.G. Direct Interaction of the N-terminal Domain of Focal Adhesion Kinase with the N-terminal Transactivation Domain of p53. J. Biol. Chem. 2005, 280, 25008–25021. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Yi, Q.; Tang, L. The roles of nuclear focal adhesion kinase (FAK) on Cancer: A focused review. J. Exp. Clin. Cancer Res. 2019, 38, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Komiya, Y.; Habas, R. Wnt signal transduction pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Clevers, H.; Nusse, R. Wnt/beta-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef] [Green Version]
- Ilyas, M. Wnt signalling and the mechanistic basis of tumour development. J. Pathol. 2005, 205, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Florian, M.C.; Nattamai, K.J.; Dörr, K.; Marka, G.; Überle, B.; Vas, V.; Eckl, C.; Andrä, I.; Schiemann, M.; Oostendorp, R.A.J.; et al. A canonical to non-canonical Wnt signalling switch in haematopoietic stem-cell ageing. Nat. Cell Biol. 2013, 503, 392–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, M.D.; Nusse, R. Wnt Signaling: Multiple Pathways, Multiple Receptors, and Multiple Transcription Factors. J. Biol. Chem. 2006, 281, 22429–22433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luna-Ulloa, L.B.; Hernández-Maqueda, J.G.; Castañeda-Patlán, M.C.; Robles-Flores, M. Protein kinase C in Wnt signaling: Implications in cancer initiation and progression. IUBMB Life 2011, 63, 915–921. [Google Scholar] [CrossRef]
- Lim, X.; Nusse, R. Wnt Signaling in Skin Development, Homeostasis, and Disease. Cold Spring Harb. Perspect. Biol. 2012, 5, a008029. [Google Scholar] [CrossRef] [Green Version]
- Simons, M.; Mlodzik, M. Planar Cell Polarity Signaling: From Fly Development to Human Disease. Annu. Rev. Genet. 2008, 42, 517–540. [Google Scholar] [CrossRef] [Green Version]
- Kurayoshi, M.; Oue, N.; Yamamoto, H.; Kishida, M.; Inoue, A.; Asahara, T.; Yasui, W.; Kikuchi, A. Expression of Wnt-5a Is Correlated with Aggressiveness of Gastric Cancer by Stimulating Cell Migration and Invasion. Cancer Res. 2006, 66, 10439–10448. [Google Scholar] [CrossRef] [Green Version]
- Corda, G.; Sala, G.; Lattanzio, R.; Iezzi, M.; Sallese, M.; Fragassi, G.; Lamolinara, A.; Mirza, H.; Barcaroli, D.; Ermler, S.; et al. Functional and prognostic significance of the genomic amplification of frizzled 6 (FZD6) in breast cancer. J. Pathol. 2017, 241, 350–361. [Google Scholar] [CrossRef]
- Koval, A.; Katanaev, V.L. Dramatic dysbalancing of the Wnt pathway in breast cancers. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Ilić, D.; Furuta, Y.; Kanazawa, S.; Takeda, N.; Sobue, K.; Nakatsuji, N.; Nomura, S.; Fujimoto, J.; Okada, M.; Yamamoto, T.; et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nat. Cell Biol. 1995, 377, 539–544. [Google Scholar] [CrossRef]
- Shen, T.L.; Park, A.Y.; Alcaraz, A.; Peng, X.; Jang, I.; Koni, P.; Flavell, R.A.; Gu, H.; Guan, J.L. Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J Cell Biol. 2005, 169, 941–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Lee, J.; Vikis, H.G.; Lee, S.H.; Liu, G.; Aurandt, J.; Shen, T.L.; Fearon, E.R.; Guan, J.L.; Han, M.; et al. Activation of FAK and Src are receptor-proximal events required for netrin signaling. Nat. Neurosci. 2004, 7, 1213–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatzizacharias, N.A.; Kouraklis, G.P.; Theocharis, S.E. The role of focal adhesion kinase in early development. Histol. Histopathol. 2010, 25, 1039–1055. [Google Scholar] [PubMed]
- Steinhart, Z.; Angers, S. Wnt signaling in development and tissue homeostasis. Development 2018, 145, dev146589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clevers, H.; Loh, K.M.; Nusse, R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346, 1248012. [Google Scholar] [CrossRef] [PubMed]
- Fonar, Y.; Frank, D. FAK and WNT Signaling: The Meeting of Two Pathways in Cancer and Development. Anti-Cancer Agents Med. Chem. 2011, 11, 600–606. [Google Scholar] [CrossRef]
- Cohen, E.D.; Mariol, M.-C.; Wallace, R.M.; Weyers, J.; Kamberov, Y.G.; Pradel, J.; Wilder, E.L. DWnt4 Regulates Cell Movement and Focal Adhesion Kinase during Drosophila Ovarian Morphogenesis. Dev. Cell 2002, 2, 437–448. [Google Scholar] [CrossRef] [Green Version]
- Fonar, Y.; Gutkovich, Y.E.; Root, H.; Malyarova, A.; Aamar, E.; Golubovskaya, V.M.; Elias, S.; Elkouby, Y.M.; Frank, D. Focal adhesion kinase protein regulates Wnt3a gene expression to control cell fate specification in the developing neural plate. Mol. Biol. Cell 2011, 22, 2409–2421. [Google Scholar] [CrossRef]
- Sun, C.; Yuan, H.; Wang, L.; Wei, X.; Williams, L.; Krebsbach, P.H.; Guan, J.-L.; Liu, F. FAK Promotes Osteoblast Progenitor Cell Proliferation and Differentiation by Enhancing Wnt Signaling. J. Bone Miner. Res. 2016, 31, 2227–2238. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Gu, X.; Sun, X.; Wu, Q.; Dan, H. FAK mediates BMP9-induced osteogenic differentiation via Wnt and MAPK signaling pathway in synovial mesenchymal stem cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2641–2649. [Google Scholar] [CrossRef] [Green Version]
- Yuan, C.; Gou, X.; Deng, J.; Dong, Z.; Ye, P.; Hu, Z. FAK and BMP-9 synergistically trigger osteogenic differentiation and bone formation of adipose derived stem cells through enhancing Wnt-beta-catenin signaling. Biomed. Pharm. 2018, 105, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Ashton, G.H.; Morton, J.P.; Myant, K.; Phesse, T.J.; Ridgway, R.A.; Marsh, V.; Wilkins, J.A.; Athineos, D.; Muncan, V.; Kemp, R.; et al. Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling. Dev. Cell. 2010, 19, 259–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oshima, H.; Kok, S.-Y.; Nakayama, M.; Murakami, K.; Voon, D.C.-C.; Kimura, T.; Oshima, M. Stat3 is indispensable for damage-induced crypt regeneration but not for Wnt-driven intestinal tumorigenesis. FASEB J. 2019, 33, 1873–1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridgway, R.A.; Serrels, B.; Mason, S.; Kinnaird, A.; Muir, M.; Patel, H.; Muller, W.J.; Sansom, O.J.; Brunton, V.G. Focal adhesion kinase is required for beta-catenin-induced mobilization of epidermal stem cells. Carcinogenesis 2012, 33, 2369–2376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jemal, A.; Siegel, R.; Ward, E.; Murray, T.; Xu, J.; Smigal, C.; Thun, M.J. Cancer Statistics, 2006. CA: A Cancer J. Clin. 2006, 56, 106–130. [Google Scholar] [CrossRef] [Green Version]
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, N.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Chien, A.J.; Moore, E.C.; Lonsdorf, A.S.; Kulikauskas, R.M.; Rothberg, B.G.; Berger, A.J.; Major, M.B.; Hwang, S.T.; Rimm, D.L.; Moon, R.T. Activated Wnt/beta-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc. Natl. Acad. Sci. USA 2009, 106, 1193–1198. [Google Scholar] [CrossRef] [Green Version]
- Nusse, R.; Clevers, H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- Kwong, L.N.; Dove, W.F. APC and Its Modifiers in Colon Cancer. Adv. Exp. Med. Biol. 2009, 656, 85–106. [Google Scholar] [CrossRef] [Green Version]
- Parker, T.W.; Neufeld, K.L. APC controls Wnt-induced beta-catenin destruction complex recruitment in human colonocytes. Sci. Rep. 2020, 10, 2957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, V.S.; Ng, S.S.; Boersema, P.J.; Low, T.Y.; Karthaus, W.R.; Gerlach, J.P.; Mohammed, S.; Heck, A.J.; Maurice, M.M.; Mahmoudi, T.; et al. Wnt signaling through inhibition of beta-catenin degradation in an intact Axin1 complex. Cell 2012, 149, 1245–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tejeda-Muñoz, N.; Gonzalez-Aguilar, H.; Santoyo-Ramos, P.; Castañeda-Patlan, M.C.; Robles-Flores, M. Glycogen Synthase Kinase 3beta Is Positively Regulated by Protein Kinase Czeta-Mediated Phosphorylation Induced by Wnt Agonists. Mol. Cell Biol. 2015, 36, 731–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, C.; Chen, G.; Kuan, S.F.; Zhang, D.H.; Schlaepfer, D.D.; Hu, J. FAK/PYK2 promotes the Wnt/beta-catenin pathway and intestinal tumorigenesis by phosphorylating GSK3beta. Elife 2015, 4, e10072. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Gao, C.; Gao, X.; Zhang, D.H.; Kuan, S.F.; Burns, T.F.; Hu, J. Wnt/beta-Catenin Pathway Activation Mediates Adaptive Resistance to BRAF Inhibition in Colorectal Cancer. Mol. Cancer Ther. 2018, 17, 806–813. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Hu, J. Targeting parallel bypass signaling to combat adaptive resistance to BRAF inhibition in colorectal Cancer. Oncoscience 2018, 5, 57–58. [Google Scholar] [CrossRef]
- Heffler, M.; Golubovskaya, V.M.; Conroy, J.; Liu, S.; Wang, D.; Cance, W.G.; Dunn, K.M.B. FAK and HAS inhibition synergistically decrease colon cancer cell viability and affect expression of critical genes. Anti-Cancer Agents Med. Chem. 2013, 13, 584–594. [Google Scholar] [CrossRef] [Green Version]
- van Seventer, G.A.; Salmen, H.J.; Law, S.F.; .O’Neill, G.M.; Mullen, M.M.; Franz, A.M.; Kanner, S.B.; Golemis, E.A.; van Seventer, J.M. Focal adhesion kinase regulates beta1 integrin-dependent T cell migration through an HEF1 effector pathway. Eur. J. Immunol. 2001, 31, 1417–1427. [Google Scholar] [CrossRef]
- Natarajan, M.; Stewart, J.E.; Golemis, E.A.; Pugacheva, E.N.; Alexandropoulos, K.; Cox, B.D.; Wang, W.; Grammer, J.R.; Gladson, C.L. HEF1 is a necessary and specific downstream effector of FAK that promotes the migration of glioblastoma cells. Oncogene 2005, 25, 1721–1732. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Bavarva, J.H.; Wang, Z.; Guo, J.; Qian, C.; Thibodeau, S.N.; Golemis, E.A.; Liu, W. HEF1, a novel target of Wnt signaling, promotes colonic cell migration and cancer progression. Oncogene 2011, 30, 2633–2643. [Google Scholar] [CrossRef] [Green Version]
- Morton, J.P.; Myant, K.B.; Sansom, O.J. A FAK-PI-3K-mTOR axis is required for Wnt-Myc driven intestinal regeneration and tumorigenesis. Cell Cycle 2011, 10, 173–175. [Google Scholar] [CrossRef]
- McLean, G.W.; Carragher, N.O.; Avizienyte, E.; Evans, J.A.; Brunton, V.G.; Frame, M.C. The role of focal-adhesion kinase in cancer—A new therapeutic opportunity. Nat. Rev. Cancer 2005, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Testa, J.R.; Carbone, M. Mesothelioma Epidemiology, Carcinogenesis, and Pathogenesis. Curr. Treat. Options Oncol. 2008, 9, 147–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wörthmüller, J.; Salicio, V.; Oberson, A.; Blum, W.; Schwaller, B. Modulation of Calretinin Expression in Human Mesothelioma Cells Reveals the Implication of the FAK and Wnt Signaling Pathways in Conferring Chemoresistance towards Cisplatin. Int. J. Mol. Sci. 2019, 20, 5391. [Google Scholar] [CrossRef] [Green Version]
- Uematsu, K.; Kanazawa, S.; You, L.; He, B.; Xu, Z.; Li, K.; Peterlin, B.M.; McCormick, F.; Jablons, D.M. Wnt pathway activation in mesothelioma: Evidence of Dishevelled overexpression and transcriptional activity of beta-catenin. Cancer Res. 2003, 63, 4547–4551. [Google Scholar]
- Lee, A.Y.; He, B.; You, L.; Dadfarmay, S.; Xu, Z.; Mazieres, J.; Mikami, I.; McCormick, F.; Jablons, D.M. Expression of the secreted frizzled-related protein gene family is downregulated in human mesothelioma. Oncogene 2004, 23, 6672–6676. [Google Scholar] [CrossRef] [Green Version]
- Bai, D.; Cong, S.; Zhu, L.P. Attenuation of Focal Adhesion Kinase Reduces Lipopolysaccharide-Induced Inflammation Injury through Inactivation of the Wnt and NF-kappaB Pathways in A549 Cells. Biochemistry 2017, 82, 446–453. [Google Scholar]
- Ku, M.J.; Kim, J.H.; Lee, J.; Cho, J.Y.; Chun, T.; Lee, S.Y. Maclurin suppresses migration and invasion of human non-small-cell lung cancer cells via anti-oxidative activity and inhibition of the Src/FAK-ERK-beta-catenin pathway. Mol. Cel. Biochem. 2015, 402, 243–252. [Google Scholar] [CrossRef]
- Hsu, H.-Y.; Lin, J.-H.; Li, C.-J.; Tsang, S.-F.; Tsai, C.-H.; Chyuan, J.-H.; Chiu, S.-J.; Chuang, S.-E. Antimigratory Effects of the Methanol Extract from Momordica charantia on Human Lung Adenocarcinoma CL1 Cells. Evidence-Based Complement. Altern. Med. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, S.H.; Seo, J.; Chung, H.; Kwak, H.J.; Lee, S.K.; Yoon, H.J.; Shin, D.H.; Park, S.S.; Sohn, J.W. Blockade of the Wnt/beta-catenin pathway attenuates bleomycin-induced pulmonary fibrosis. Tohoku J. Exp. Med. 2011, 223, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Vittal, R.; Horowitz, J.C.; Moore, B.B.; Zhang, H.; Martinez, F.J.; Toews, G.B.; Standiford, T.J.; Thannickal, V.J. Modulation of Prosurvival Signaling in Fibroblasts by a Protein Kinase Inhibitor Protects against Fibrotic Tissue Injury. Am. J. Pathol. 2005, 166, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Osterman, C.J.; Ozmadenci, D.; Kleinschmidt, E.G.; Taylor, K.N.; Barrie, A.M.; Jiang, S.; Bean, L.M.; Sulzmaier, F.J.; Jean, C.; Tancioni, I.; et al. FAK activity sustains intrinsic and acquired ovarian cancer resistance to platinum chemotherapy. eLife 2019, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Villedieu, M.; Deslandes, E.; Duval, M.; Heron, J.-F.; Gauduchon, P.; Poulain, L. Acquisition of chemoresistance following discontinuous exposures to cisplatin is associated in ovarian carcinoma cells with progressive alteration of FAK, ERK and p38 activation in response to treatment. Gynecol. Oncol. 2006, 101, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, D.; Li, X.; Zhang, L.; Zhang, H.; Zhang, Y. Extracellular matrix protein ITGBL1 promotes ovarian cancer cell migration and adhesion through Wnt/PCP signaling and FAK/SRC pathway. Biomed. Pharmacother. 2016, 81, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Q.; Du, X.; Li, D.M.; Kong, P.Z.; Sun, Y.; Liu, P.F.; Wang, Q.S.; Feng, Y.M. ITGBL1 Is a Runx2 Transcriptional Target and Promotes Breast Cancer Bone Metastasis by Activating the TGFbeta Signaling Pathway. Cancer Res. 2015, 75, 3302–3313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leask, A. Focal Adhesion Kinase: A Key Mediator of Transforming Growth Factor Beta Signaling in Fibroblasts. Adv. Wound Care 2013, 2, 247–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, X.; Feng, J.-R.; Qiu, J.; Liu, L.; Xie, Y.; Zhang, Y.-P.; Liu, J.; Zhao, Q. ITGBL1 promotes migration, invasion and predicts a poor prognosis in colorectal cancer. Biomed. Pharmacother. 2018, 104, 172–180. [Google Scholar] [CrossRef]
- Yang, J.; Hou, Y.; Zhou, M.; Wen, S.; Zhou, J.; Xu, L.; Tang, X.; Du, Y.-E.; Hu, P.; Liu, M. Twist induces epithelial-mesenchymal transition and cell motility in breast cancer via ITGB1-FAK/ILK signaling axis and its associated downstream network. Int. J. Biochem. Cell Biol. 2016, 71, 62–71. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Lin, H.H.; Tang, M.J. A tale of two collagen receptors, integrin beta1 and discoidin domain receptor 1, in epithelial cell differentiation. Am. J. Physiol. Cell Physiol. 2012, C1207–C1217. [Google Scholar] [CrossRef] [Green Version]
- Prosperi, J.R.; Goss, K.H. A Wnt-ow of opportunity: Targeting the Wnt/beta-catenin pathway in breast cancer. Curr. Drug Targets 2010, 11, 1074–1088. [Google Scholar] [CrossRef]
- Prosperi, J.R.; Khramtsov, A.I.; Khramtsova, G.F.; Goss, K.H. Apc mutation enhances PyMT-induced mammary tumorigenesis. PLoS ONE 2011, 6, e29339. [Google Scholar] [CrossRef]
- Williams, K.E.; Bundred, N.J.; Landberg, G.; Clarke, R.B.; Farnie, G. Focal Adhesion Kinase and Wnt Signaling Regulate Human Ductal Carcinoma In Situ Stem Cell Activity and Response to Radiotherapy. Stem Cells 2015, 33, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Kolev, V.N.; Tam, W.F.; Wright, Q.G.; McDermott, S.P.; Vidal, C.M.; Shapiro, I.M.; Xu, Q.; Wicha, M.S.; Pachter, J.A.; Weaver, D.T. Inhibition of FAK kinase activity preferentially targets cancer stem cells. Oncotarget 2017, 8, 51733–51747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, N.; Arteaga, M.; Zaidi, A.; Stauffer, J.; Cotler, S.J.; Zeleznik-Le, N.J.; Zhang, J.; Qiu, W. FAK is required for c-Met/beta-catenin-driven hepatocarcinogenesis. Hepatology 2015, 61, 214–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, N.; Wang, H.; Bank, T.; Perera, A.; Joyce, C.; Kuffel, G.; Zilliox, M.J.; Cotler, S.J.; Ding, X.; Dhanarajan, A.; et al. Focal Adhesion Kinase and beta-Catenin Cooperate to Induce Hepatocellular Carcinoma. Hepatology 2019, 70, 1631–1645. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Duan, J.; Wang, L.; Xiao, S.; Li, L.; Yan, X.; Yao, W.; Wu, L.; Zhang, S.; Zhang, Y.; et al. PTK2 promotes cancer stem cell traits in hepatocellular carcinoma by activating Wnt/beta-catenin signaling. Cancer Lett. 2019, 450, 132–143. [Google Scholar] [CrossRef]
- Delgado-Bellido, D.; Fernández-Cortés, M.; Rodríguez, M.I.; Serrano-Saenz, S.; Carracedo, A.; Garcia-Diaz, A.; Oliver, F.J. VE-cadherin promotes vasculogenic mimicry by modulating kaiso-dependent gene expression. Cell Death Differ. 2018, 26, 348–361. [Google Scholar] [CrossRef] [Green Version]
- Despeaux, M.; Chicanne, G.; Rouer, E.; De Toni-Costes, F.; Bertrand, J.; Mansat-De Mas, V.; Vergnolle, N.; Eaves, C.; Payrastre, B.; Girault, J.-A.; et al. Focal Adhesion Kinase Splice Variants Maintain Primitive Acute Myeloid Leukemia Cells Through Altered Wnt Signaling. Stem Cells 2012, 30, 1597–1610. [Google Scholar] [CrossRef]
- He, M.; Wang, Z.; Hu, W.; Wang, K.; Wang, D.; Fang, Z.; Huang, A.; Gao, Y.; Xia, J.; Li, W. Golgi Phosphoprotein 3 Promotes Malignant Phenotypes via FAK/Raf/MEK and Wnt/beta-Catenin Signaling Pathways in Human Renal Cell Carcinoma. J. Biomed. Nanotechnol. 2019, 15, 1812–1823. [Google Scholar] [CrossRef]
- Tai, H.C.; Chang, A.C.; Yu, H.J.; Huang, C.Y.; Tsai, Y.C.; Lai, Y.W.; Sun, H.L.; Tang, C.H.; Wang, S.W. Osteoblast-derived WNT-induced secreted protein 1 increases VCAM-1 expression and enhances prostate cancer metastasis by down-regulating miR-126. Oncotarget 2014, 5, 7589–7598. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Shi, Z.; Xu, H.; Chen, R.; Xue, S.; Sun, X. Knockdown of Cripto-1 inhibits the proliferation, migration, invasion, and angiogenesis in prostate carcinoma cells. J. Biosci. 2017, 42, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.K.; Schiff, R. Mechanisms of Endocrine Resistance in Breast Cancer. Annu. Rev. Med. 2011, 62, 233–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammond, W.A.; Swaika, A.; Mody, K. Pharmacologic resistance in colorectal cancer: A review. Ther. Adv. Med Oncol. 2015, 8, 57–84. [Google Scholar] [CrossRef] [Green Version]
- Bronte, G.; Incorvaia, L.; Rizzo, S.; Passiglia, F.; Galvano, A.; Rizzo, F.; Rolfo, C.; Fanale, D.; Listì, A.; Natoli, C.; et al. The resistance related to targeted therapy in malignant pleural mesothelioma: Why has not the target been hit yet? Crit. Rev. Oncol. 2016, 107, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, S.; Igea, A.; Arroyo, R.; Alcalde, V.; Canovas, B.; Orozco, M.; Nebreda, A.R.; Aloy, P. Quantification of Pathway Cross-talk Reveals Novel Synergistic Drug Combinations for Breast Cancer. Cancer Res. 2016, 77, 459–469. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, R.; Muqbil, I.; Lowe, L.; Yedjou, C.; Hsu, H.-Y.; Lin, L.-T.; Siegelin, M.D.; Fimognari, C.; Kumar, N.B.; Dou, Q.P.; et al. Broad targeting of resistance to apoptosis in cancer. Semin. Cancer Biol. 2015, 35, S78–S103. [Google Scholar] [CrossRef]
- Sun, X.; Bao, J.; You, Z.; Chen, X.; Cui, J. Modeling of signaling crosstalk-mediated drug resistance and its implications on drug combination. Oncotarget 2016, 7, 63995–64006. [Google Scholar] [CrossRef]
- Martín-Orozco, E.; Sanchez-Fernandez, A.; Ortiz-Parra, I.; Ayala-San Nicolas, M. WNT Signaling in Tumors: The Way to Evade Drugs and Immunity. Front. Immunol. 2019, 10, 2854. [Google Scholar] [CrossRef]
- Shaw, H.V.; Koval, A.; Katanaev, V.L. Targeting the Wnt signalling pathway in cancer: Prospects and perils. Swiss Med Wkly. 2019, 149, w20129. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.S.; Park, J.I. Wnt signaling in cancer: Therapeutic targeting of Wnt signaling beyond beta-catenin and the destruction complex. Exp. Mol. Med. 2020, 52, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Gurney, A.; Axelrod, F.; Bond, C.J.; Cain, J.; Chartier, C.; Donigan, L.; Fischer, M.; Chaudhari, A.; Ji, M.; Kapoun, A.M.; et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 11717–11722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, M.M.; Cancilla, B.; Yeung, V.P.; Cattaruzza, F.; Chartier, C.; Murriel, C.L.; Cain, J.; Tam, R.; Cheng, C.-Y.; Evans, J.W.; et al. WNT antagonists exhibit unique combinatorial antitumor activity with taxanes by potentiating mitotic cell death. Sci. Adv. 2017, 3, e1700090. [Google Scholar] [CrossRef] [Green Version]
- Jimeno, A.; Gordon, M.; Chugh, R.; Messersmith, W.A.; Mendelson, D.; Dupont, J.; Stagg, R.; Kapoun, A.M.; Xu, L.; Uttamsingh, S.; et al. A First-in-Human Phase I Study of the Anticancer Stem Cell Agent Ipafricept (OMP-54F28), a Decoy Receptor for Wnt Ligands, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2017, 23, 7490–7497. [Google Scholar] [CrossRef] [Green Version]
- Diamond, J.R.; Becerra, C.; Richards, D.; Mita, A.; Osborne, C.; O’Shaughnessy, J.; Zhang, C.; Henner, R.; Kapoun, A.M.; Xu, L.; et al. Phase Ib clinical trial of the anti-frizzled antibody vantictumab (OMP-18R5) plus paclitaxel in patients with locally advanced or metastatic HER2-negative breast cancer. Breast Cancer Res. Treat. 2020, 184, 53–62. [Google Scholar] [CrossRef]
- Madan, B.; McDonald, M.J.; Foxa, G.E.; Diegel, C.R.; Williams, B.O.; Virshup, D.M. Bone loss from Wnt inhibition mitigated by concurrent alendronate therapy. Bone Res. 2018, 6, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OncoMed Pharmaceuticals, Inc. Annual Report. Available online: https://sec.report/Document/1302573/000156459019006795/omed-10k_20181231.htm (accessed on 23 November 2020).
- Säfholm, A.; Leandersson, K.; Dejmek, J.; Nielsen, C.K.; Villoutreix, B.O.; Andersson, T. A Formylated Hexapeptide Ligand Mimics the Ability of Wnt-5a to Impair Migration of Human Breast Epithelial Cells. J. Biol. Chem. 2005, 281, 2740–2749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-H.; Faderl, S.; Pagel, J.M.; Jung, C.W.; Yoon, S.-S.; Pardanani, A.D.; Becker, P.S.; Lee, H.; Choi, J.; Lee, K.; et al. Phase 1 study of CWP232291 in patients with relapsed or refractory acute myeloid leukemia and myelodysplastic syndrome. Blood Adv. 2020, 4, 2032–2043. [Google Scholar] [CrossRef] [PubMed]
- Tam, B.Y.; Chiu, K.; Chung, H.; Bossard, C.; Nguyen, J.D.; Creger, E.; Eastman, B.W.; Mak, C.C.; Ibanez, M.; Ghias, A.; et al. The CLK inhibitor SM08502 induces anti-tumor activity and reduces Wnt pathway gene expression in gastrointestinal cancer models. Cancer Lett. 2020, 473, 186–197. [Google Scholar] [CrossRef]
- Kadowaki, T.; Wilder, E.; Klingensmith, J.; Zachary, K.; Perrimon, N. The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in Wingless processing. Genes Dev. 1996, 10, 3116–3128. [Google Scholar] [CrossRef] [Green Version]
- Zimmerli, D.; Hausmann, G.; Cantù, C.; Basler, K. Pharmacological interventions in the Wnt pathway: Inhibition of Wnt secretion versus disrupting the protein-protein interfaces of nuclear factors. Br. J. Pharmacol. 2017, 174, 4600–4610. [Google Scholar] [CrossRef]
- Sebio, A.; Kahn, M.; Lenz, H.J. The potential of targeting Wnt/beta-catenin in colon cancer. Expert Opin. Ther. Targets 2014, 18, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Roy-Luzarraga, M.; Hodivala-Dilke, K. Molecular Pathways: Endothelial Cell FAK—A Target for Cancer Treatment. Clin. Cancer Res. 2016, 22, 3718–3724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soria, J.-C.; Gan, H.K.; Blagden, S.P.; Plummer, R.; Arkenau, H.T.; Ranson, M.; Evans, T.R.J.; Zalcman, G.; Bahleda, R.; Hollebecque, A.; et al. A phase I, pharmacokinetic and pharmacodynamic study of GSK2256098, a focal adhesion kinase inhibitor, in patients with advanced solid tumors. Ann. Oncol. 2016, 27, 2268–2274. [Google Scholar] [CrossRef] [PubMed]
- Mak, G.; Soria, J.-C.; Blagden, S.P.; Plummer, R.; Fleming, R.A.; Nebot, N.; Zhang, J.; Mazumdar, J.; Rogan, D.; Gazzah, A.; et al. A phase Ib dose-finding, pharmacokinetic study of the focal adhesion kinase inhibitor GSK2256098 and trametinib in patients with advanced solid tumours. Br. J. Cancer 2019, 120, 975–981. [Google Scholar] [CrossRef]
- De Jonge, M.; Steeghs, N.; Lolkema, M.; Hotte, S.J.; Hirte, H.; Van Der Biessen, D.A.J.; Razak, A.R.A.; De Vos, F.Y.; Verheijen, R.B.; Schnell, D.; et al. Phase I Study of BI 853520, an Inhibitor of Focal Adhesion Kinase, in Patients with Advanced or Metastatic Nonhematologic Malignancies. Target. Oncol. 2019, 14, 43–55. [Google Scholar] [CrossRef] [Green Version]
- Infante, J.R.; Camidge, D.R.; Mileshkin, L.R.; Chen, E.X.; Hicks, R.J.; Rischin, D.; Fingert, H.; Pierce, K.J.; Xu, H.; Roberts, W.G.; et al. Safety, Pharmacokinetic, and Pharmacodynamic Phase I Dose-Escalation Trial of PF-00562271, an Inhibitor of Focal Adhesion Kinase, in Advanced Solid Tumors. J. Clin. Oncol. 2012, 30, 1527–1533. [Google Scholar] [CrossRef]
- Lv, P.-C.; Jiang, A.-Q.; Zhang, W.-M.; Zhu, H.-L. FAK inhibitors in Cancer, a patent review. Expert Opin. Ther. Patents 2018, 28, 139–145. [Google Scholar] [CrossRef]
- Jones, S.F.; Siu, L.L.; Bendell, J.C.; Cleary, J.M.; Razak, A.R.A.; Infante, J.R.; Pandya, S.S.; Bedard, P.L.; Pierce, K.J.; Houk, B.; et al. A phase I study of VS-6063, a second-generation focal adhesion kinase inhibitor, in patients with advanced solid tumors. Investig. New Drugs 2015, 33, 1100–1107. [Google Scholar] [CrossRef]
- Shimizu, T.; Fukuoka, K.; Takeda, M.; Iwasa, T.; Yoshida, T.; Horobin, J.; Keegan, M.; Vaickus, L.; Chavan, A.; Padval, M.; et al. A first-in-Asian phase 1 study to evaluate safety, pharmacokinetics and clinical activity of VS-6063, a focal adhesion kinase (FAK) inhibitor in Japanese patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2016, 77, 997–1003. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, I.M.; Kolev, V.N.; Vidal, C.M.; Kadariya, Y.; Ring, J.E.; Wright, Q.; Weaver, D.T.; Menges, C.; Padval, M.; McClatchey, A.I.; et al. Merlin Deficiency Predicts FAK Inhibitor Sensitivity: A Synthetic Lethal Relationship. Sci. Transl. Med. 2014, 6, 237ra68. [Google Scholar] [CrossRef] [Green Version]
- Gerber, D.E.; Camidge, D.R.; Morgensztern, D.; Cetnar, J.; Kelly, R.; Ramalingam, S.S.; Spigel, D.R.; Jeong, W.; Scaglioni, P.P.; Zhang, S.; et al. Phase 2 study of the focal adhesion kinase inhibitor defactinib (VS-6063) in previously treated advanced KRAS mutant non-small cell lung cancer. Lung Cancer 2020, 139, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.; Pharaon, R.R.; Nam, A.; Salgia, S.; Kulkarni, P.; Massarelli, E. FAK-targeted and combination therapies for the treatment of cancer: An overview of phase I and II clinical trials. Expert Opin. Investig. Drugs 2020, 29, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Bernards, R. A Missing Link in Genotype-Directed Cancer Therapy. Cell 2012, 151, 465–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, P.-J.; Xu, L.H.; Lin, K.; Weng, W.-J.; Fang, J. Synergism between the mTOR inhibitor rapamycin and FAK down-regulation in the treatment of acute lymphoblastic leukemia. J. Hematol. Oncol. 2016, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Golubovskaya, V.; Beviglia, L.; Xu, L.H.; Earp, H.S., 3rd; Craven, R.; Cance, W. Dual inhibition of focal adhesion kinase and epidermal growth factor receptor pathways cooperatively induces death receptor-mediated apoptosis in human breast cancer cells. J. Biol. Chem. 2002, 277, 38978–38987. [Google Scholar] [CrossRef] [Green Version]
- Golubovskaya, V.M.; Gross, S.; Kaur, A.S.; Wilson, R.I.; Xu, L.-H.; Yang, X.H.; Cance, W.G. Simultaneous inhibition of focal adhesion kinase and SRC enhances detachment and apoptosis in colon cancer cell lines. Mol. Cancer Res. 2003, 1, 755–764. [Google Scholar]
- Tamura, M.; Gu, J.; Matsumoto, K.; Aota, S.; Parsons, R.; Yamada, K.M. Inhibition of Cell Migration, Spreading, and Focal Adhesions by Tumor Suppressor PTEN. Science 1998, 280, 1614–1617. [Google Scholar] [CrossRef]
- Alfieri, R.; Giovannetti, E.; Bonelli, M.; Cavazzoni, A. New Treatment Opportunities in Phosphatase and Tensin Homolog (PTEN)-Deficient Tumors: Focus on PTEN/Focal Adhesion Kinase Pathway. Front. Oncol. 2017, 7, 170. [Google Scholar] [CrossRef]
- Doglioni, C.; Dei Tos, A.P.; Laurino, L.; Iuzzolino, P.; Chiarelli, C.; Celio, M.R.; Viale, G. Calretinin: A Novel Immunocytochemical Marker for Mesothelioma. Am. J. Surg. Pathol. 1996, 20, 1037–1046. [Google Scholar] [CrossRef]
- Tang, J.; Karhinen, L.; Xu, T.; Szwajda, A.; Yadav, B.; Wennerberg, K.; Aittokallio, T. Target Inhibition Networks: Predicting Selective Combinations of Druggable Targets to Block Cancer Survival Pathways. PLoS Comput. Biol. 2013, 9, e1003226. [Google Scholar] [CrossRef]
- Ackermann, T.F.; Kempe, D.S.; Lang, F.; Lang, U.E. Hyperactivity and Enhanced Curiosity of Mice Expressing PKB/SGK-resistant Glycogen Synthase Kinase-3 (GSK-3). Cell. Physiol. Biochem. 2010, 25, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Crampton, S.P.; Wu, B.; Park, E.J.; Kim, J.H.; Solomon, C.; Waterman, M.L.; Hughes, C.C. Integration of the beta-catenin-dependent Wnt pathway with integrin signaling through the adaptor molecule Grb2. PLoS ONE 2009, 4, e7841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Yuan, H.; Xie, W.; Mao, J.; Caruso, A.M.; McMahon, A.; Sussman, D.J.; Wu, D. Dishevelled proteins lead to two signaling pathways. Regulation of LEF-1 and c-Jun N-terminal kinase in mammalian cells. J. Biol. Chem. 1999, 274, 129–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vivanco, I.; Palaskas, N.; Tran, C.; Finn, S.P.; Getz, G.; Kennedy, N.J.; Jiao, J.; Rose, J.; Xie, W.; Loda, M.; et al. Identification of the JNK Signaling Pathway as a Functional Target of the Tumor Suppressor PTEN. Cancer Cell 2007, 11, 555–569. [Google Scholar] [CrossRef] [Green Version]
- Lehar, J.; Krueger, A.S.; Avery, W.; Heilbut, A.M.; Johansen, L.M.; Price, E.R.; Rickles, R.J.; Short 3rd, G.F.; Staunton, J.E.; Jin, X.; et al. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat. Biotechnol. 2009, 27, 659–666. [Google Scholar] [CrossRef]
- Chen, S.-H.; Lahav, G. Two is better than one; toward a rational design of combinatorial therapy. Curr. Opin. Struct. Biol. 2016, 41, 145–150. [Google Scholar] [CrossRef] [Green Version]
| Name | Target/Mode of Action | Development Phase | Condition or Disease | Status | Trial Identifier |
|---|---|---|---|---|---|
| OMP-18R5 (Vantictumab) | Anti-Fzd7 antibody | Phase I | Advanced solid tumors; metastatic breast cancer; pancreatic cancer | Completed | NCT01345201 NCT01973309 NCT02005315 NCT01957007 |
| OMP-54F28 (Ipafricept) | Fzd8 decoy receptor | Phase I | Advanced solid tumors; ovarian cancer; hepatocellular cancer; pancreatic cancer | Completed | NCT01608867 NCT02092363 NCT02069145 NCT02050178 |
| OMP-131R10 (Rosmantuzumab) | Anti-R-spondin3 antibody | Phase I | Advanced relapsed tumors; refractory solid tumors | Completed | NCT02482441 |
| Foxy-5 | Wnt-5a mimicking peptide | Phase II | Colon cancer | Recruiting | NCT03883802 |
| OTSA 101-DPTA | Anti Fzd10 antibody | Phase I | Relapsed or refractory synovial sarcoma | Recruiting | NCT04176016 |
| PRI-724 | Inhibitor β-catenin-CBP | Phase I/II | Advances solid tumors; chronic/acute myeloid leukemia; pancreatic cancer | Terminated or Completed | NCT01302405 NCT01606579 NCT01764477 |
| CWP291 | Sam68 | Phase I | Acute/chronic myeloid leukemia | Completed | NCT01398462 |
| SM08502 | CLK | Phase I | Advanced solid tumors | Recruiting | NCT03355066 |
| Wnt974 (LGK974) | Porcupine inhibitor | Phase I | Advanced solid tumors | Recruiting | NCT01351103 |
| ETC-159 | Porcupine inhibitor | Phase I | Advanced solid tumors | Recruiting | NCT02521844 |
| RXC004 | Porcupine inhibitor | Phase I | Advanced solid tumors | Recruiting | NCT03447470 |
| CGX1321 | Porcupine inhibitor | Phase I | Advanced GI tumors | Recruiting | NCT02675946 |
| Name | Target/Mode of Action | Development Phase | Condition or Disease | Status | Trial Identifier |
|---|---|---|---|---|---|
| GSK2256098 | ATP-competitive kinase inhibitor | Phase II | Pancreatic cancer; adenocarcinoma | Active, not recruiting | NCT02428270 |
| Phase I | Mesothelioma; solid tumors | Completed | NCT01138033 NCT01938443 | ||
| Phase II | Meningioma | Suspended | NCT02523014 | ||
| BI 853520 | ATP-competitive kinase inhibitor | Phase I | Advanced or metastatic cancers | Completed | NCT01335269 NCT01905111 |
| PF-562271 (VS-6062) | ATP-competitive kinase inhibitor | Phase I | Head and neck cancer; prostatic cancer; pancreatic cancer | Completed | NCT00666926 |
| PND-1186 (VS-4718) | ATP-competitive kinase inhibitor | Phase I | Pancreatic cancer; non-hematologic or metastatic cancers; leukemia | Terminated or withdrawn | NCT02651727 NCT01849744 NCT02215629 |
| Defactinib (VS-6063; PF-04554878) | ATP-competitive kinase inhibitor | Phase I | Malignant pleural mesothelioma | Not yet recruiting | NCT04201145 |
| Phase I | NSCLC; solid tumors; low grade serous ovarian cancer; colorectal cancer | Recruiting | NCT03875820 | ||
| Phase I/II | Ovarian cancer | Recruiting | NCT03287271 | ||
| Phase I/II | Carcinoma; NSCLC; mesothelioma; pancreatic cancer | Recruiting | NCT02758587 | ||
| Phase II | Pancreatic ductal adenocarcinoma | Recruiting | NCT03727880 | ||
| Phase I | Advanced solid tumors; pancreatic cancer | Active, not recruiting | NCT02546531 | ||
| Phase II | Cancers with NF2 genetic changes (advanced lymphoma; advanced solid cancers; hematopoietic cancers, etc.) | Active, not recruiting | NCT04439331 | ||
| Phase II | Patients with KRAS mutations (NSCLC; lung cancer) | Completed | NCT01951690 | ||
| Phase I | Ovarian cancer | Completed | NCT01778803 | ||
| Phase I | Non-hematologic cancers | Completed | NCT01943292 | ||
| Phase I | Advanced non-hematologic malignancies | Completed | NCT00787033 | ||
| Phase I | Healthy subjects | Completed | NCT02913716 | ||
| Phase II | Malignant pleural mesothelioma | Terminated | NCT02004028 | ||
| Phase I | Epithelial ovarian cancer | Terminated | NCT02943317 | ||
| Phase II | Malignant pleural mesothelioma | Terminated | NCT01870609 | ||
| Phase I | Relapsed malignant mesothelioma | Terminated | NCT02372227 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wörthmüller, J.; Rüegg, C. The Crosstalk between FAK and Wnt Signaling Pathways in Cancer and Its Therapeutic Implication. Int. J. Mol. Sci. 2020, 21, 9107. https://doi.org/10.3390/ijms21239107
Wörthmüller J, Rüegg C. The Crosstalk between FAK and Wnt Signaling Pathways in Cancer and Its Therapeutic Implication. International Journal of Molecular Sciences. 2020; 21(23):9107. https://doi.org/10.3390/ijms21239107
Chicago/Turabian StyleWörthmüller, Janine, and Curzio Rüegg. 2020. "The Crosstalk between FAK and Wnt Signaling Pathways in Cancer and Its Therapeutic Implication" International Journal of Molecular Sciences 21, no. 23: 9107. https://doi.org/10.3390/ijms21239107
APA StyleWörthmüller, J., & Rüegg, C. (2020). The Crosstalk between FAK and Wnt Signaling Pathways in Cancer and Its Therapeutic Implication. International Journal of Molecular Sciences, 21(23), 9107. https://doi.org/10.3390/ijms21239107






