Dendritic Nanotheranostic for the Delivery of Infliximab: A Potential Carrier in Rheumatoid Arthritis Therapy
Abstract
:1. Introduction
2. Results
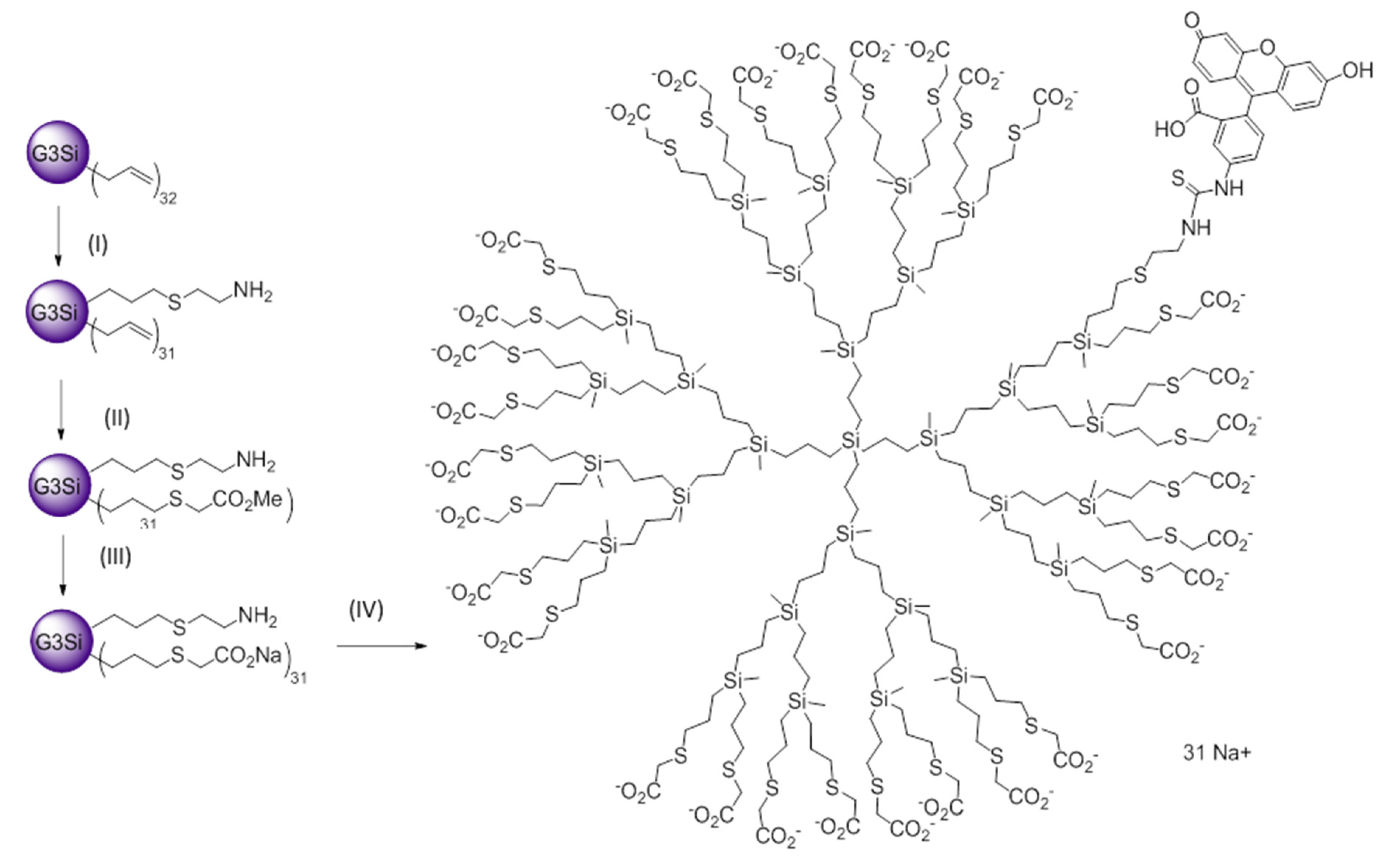
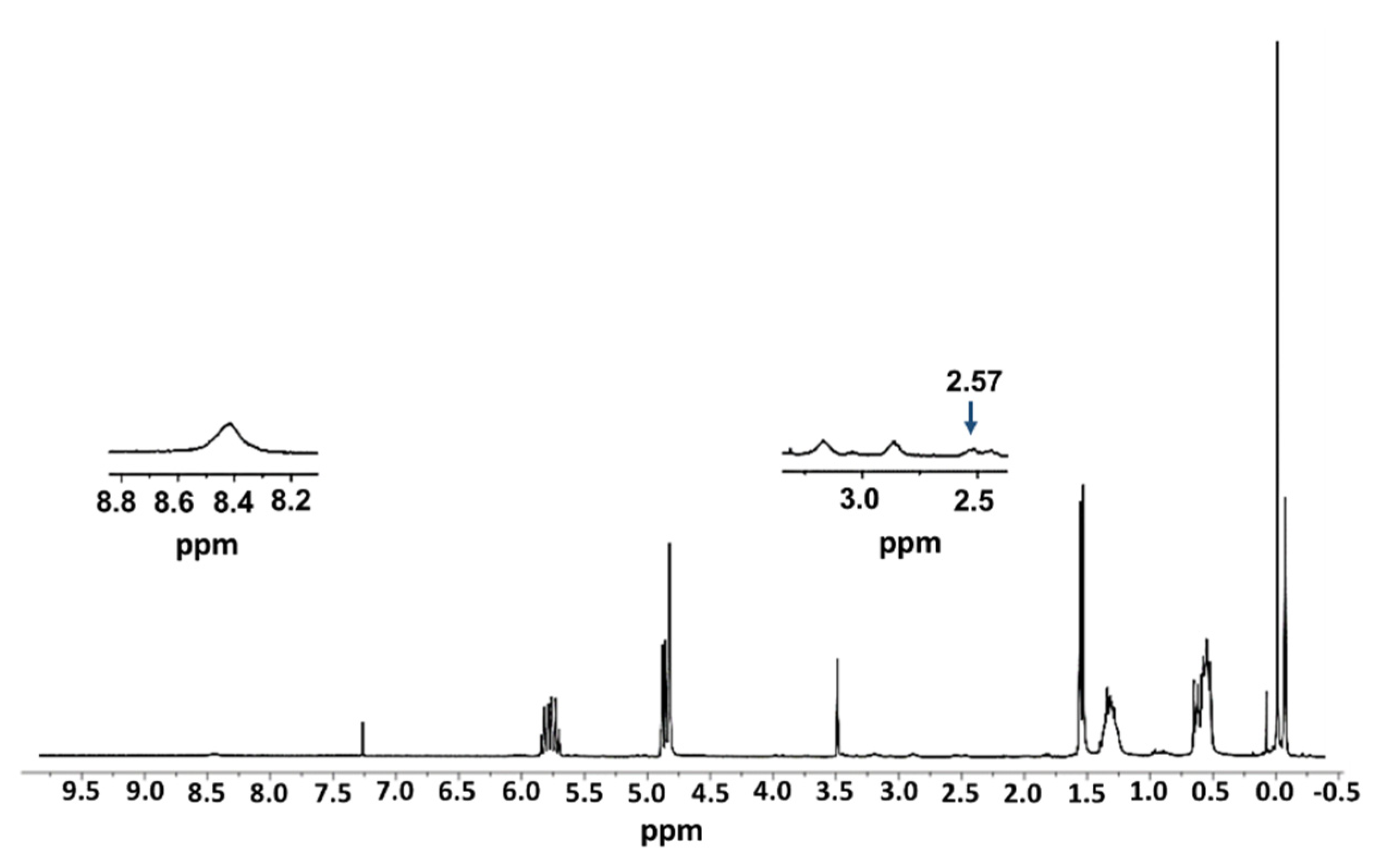
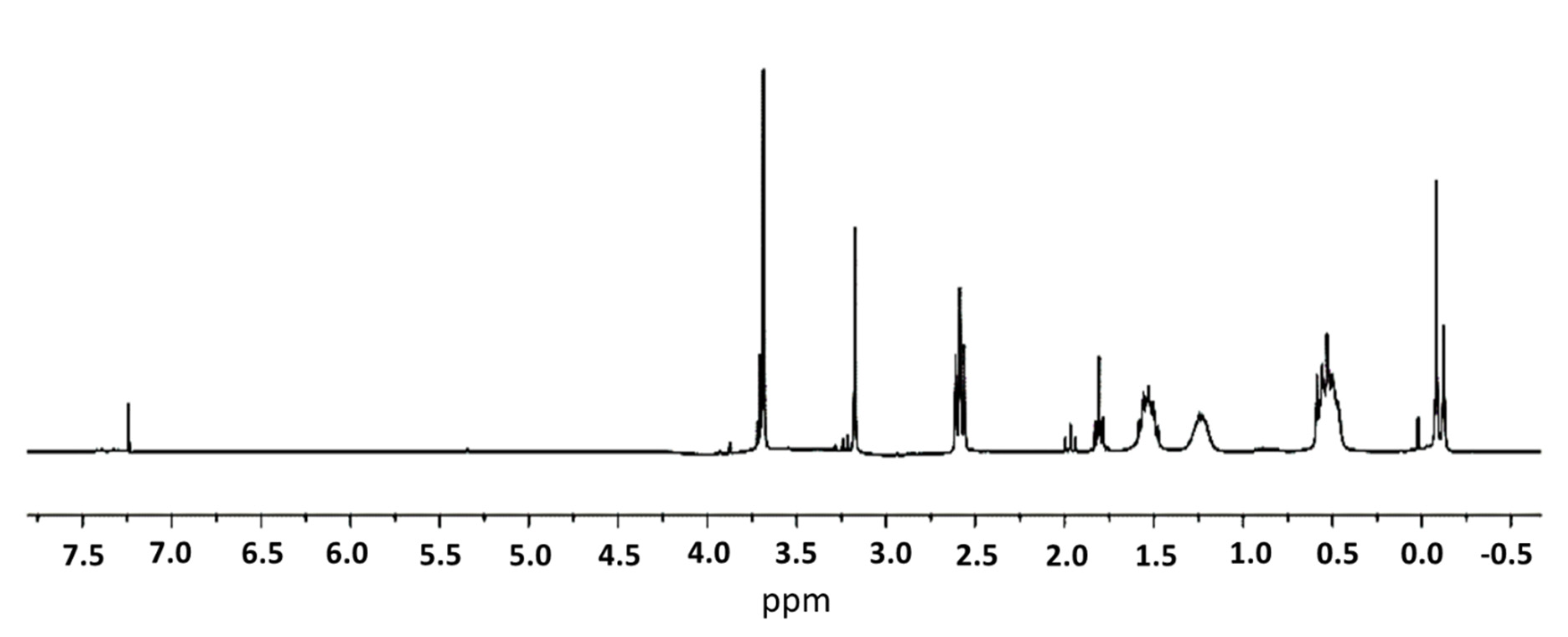
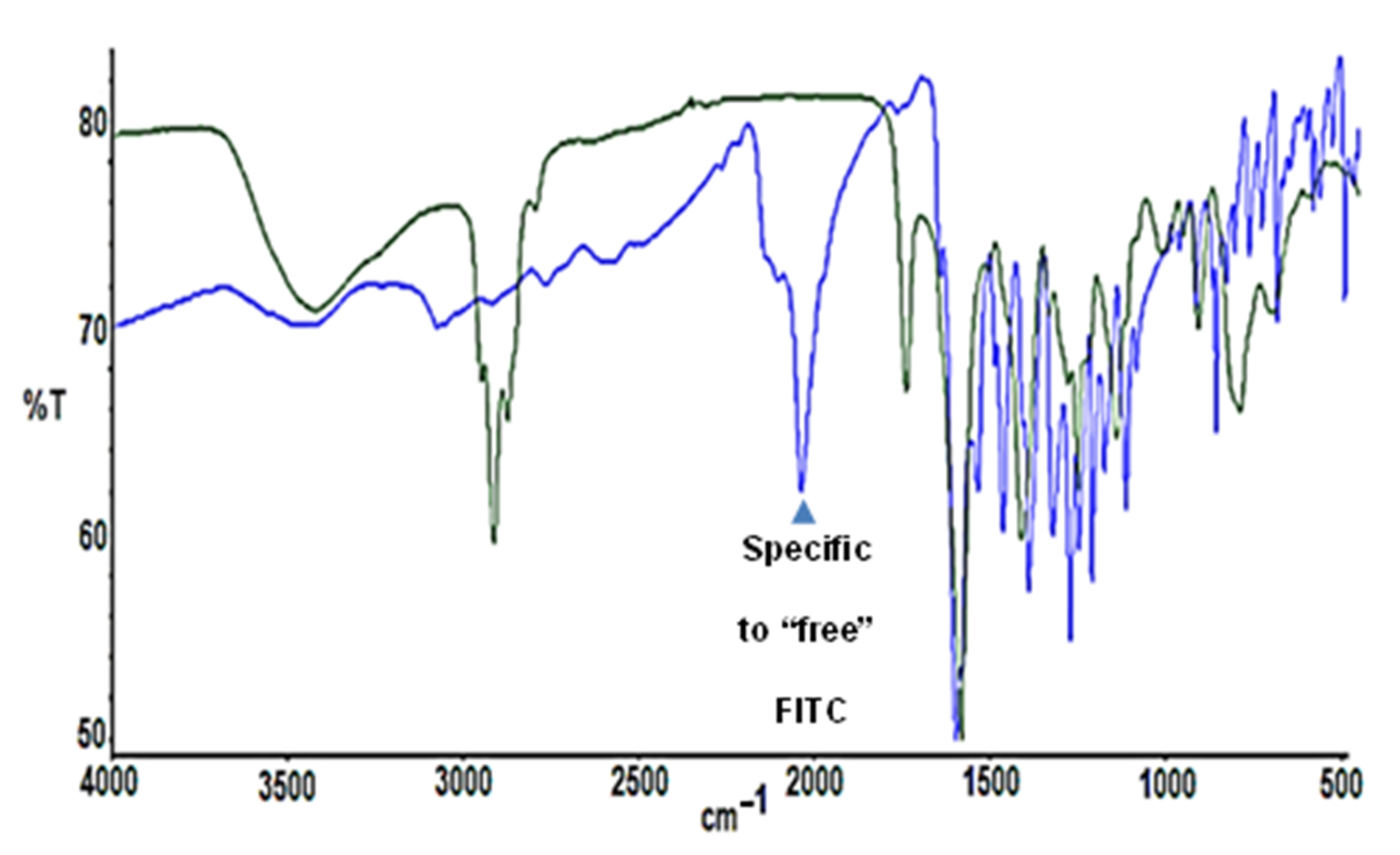
2.1. Synthesis and Characterization of FITC-Labelled Dendrimer
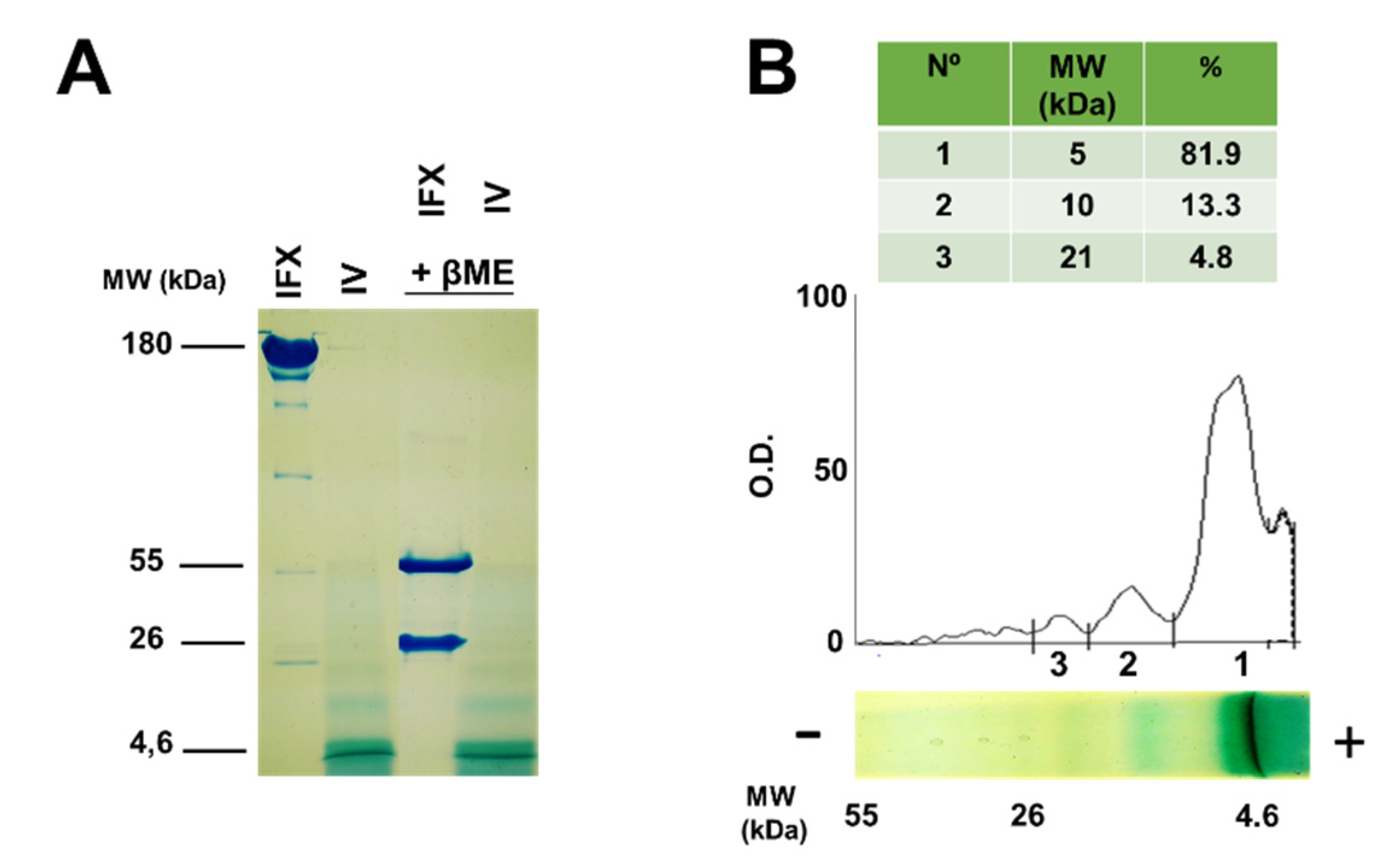
2.2. Molecular Characterization of Infliximab and Compound IV Using SDS-PAGE
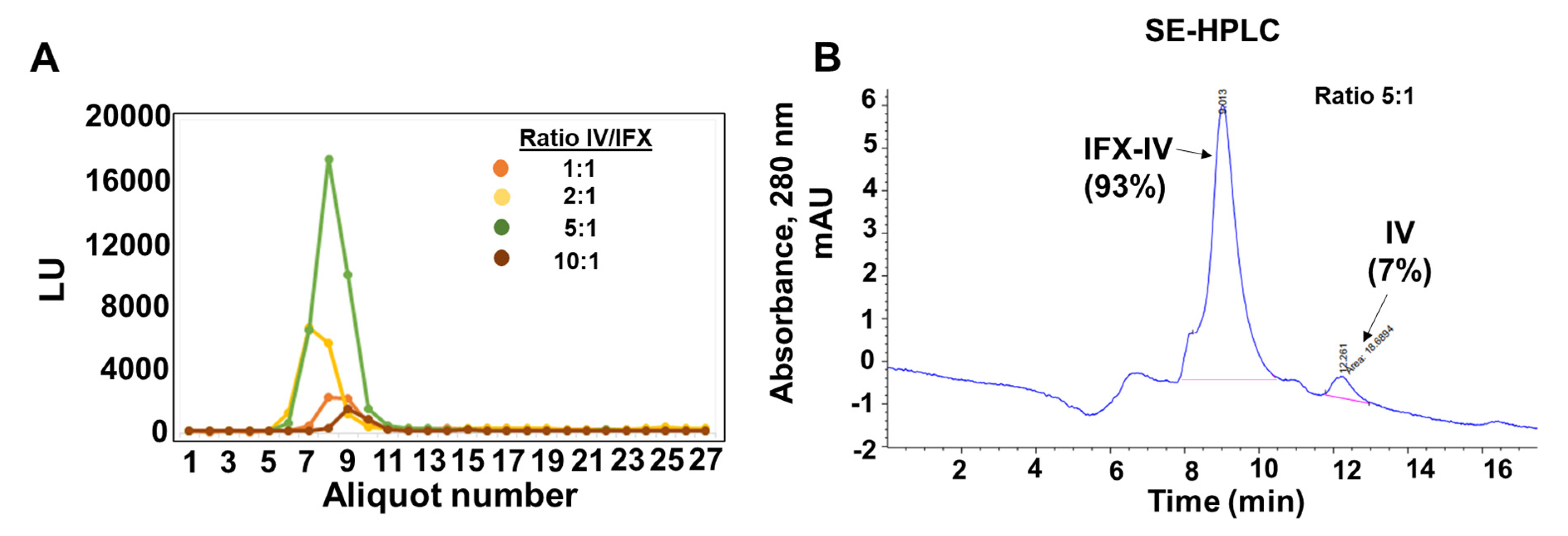
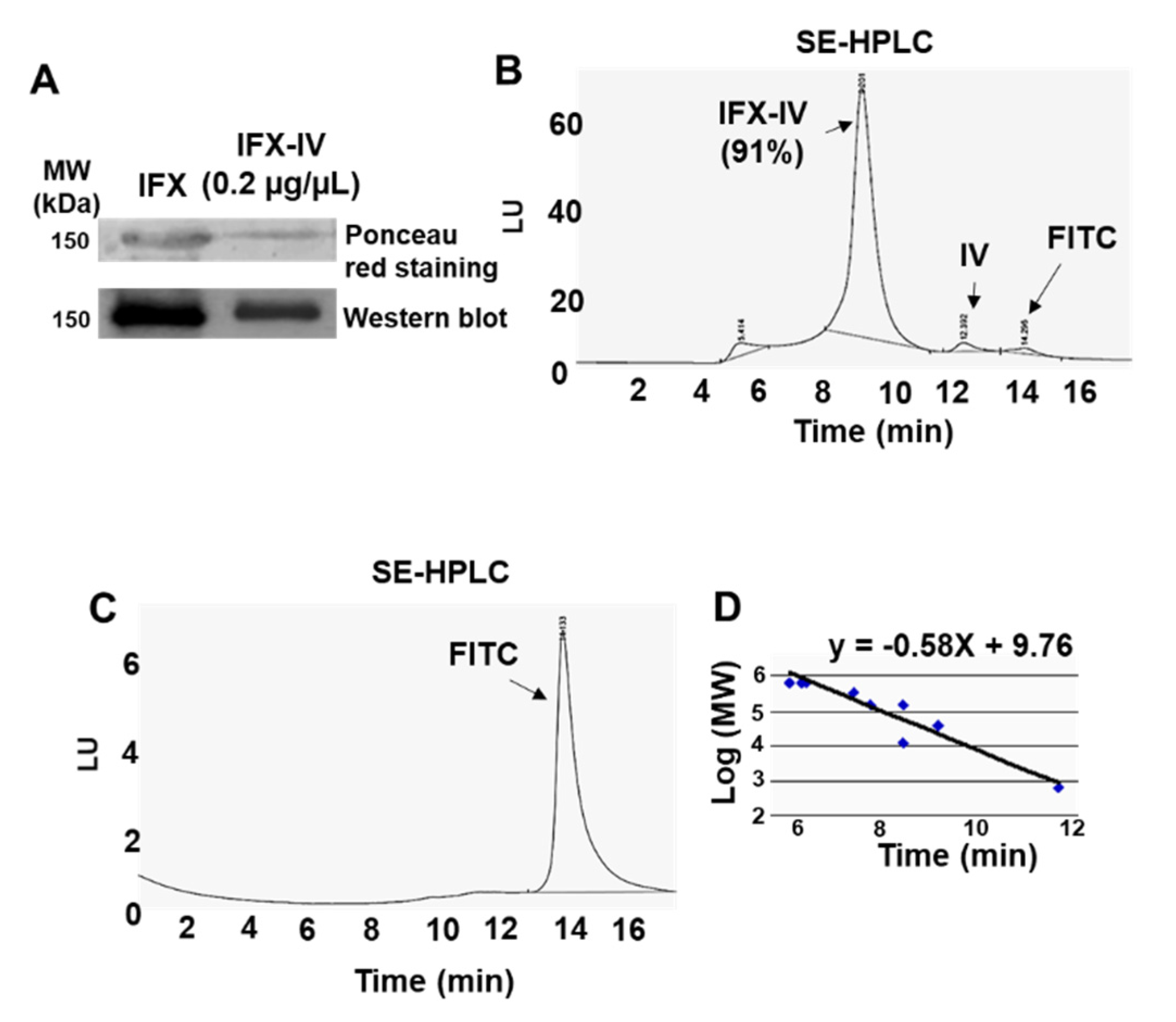
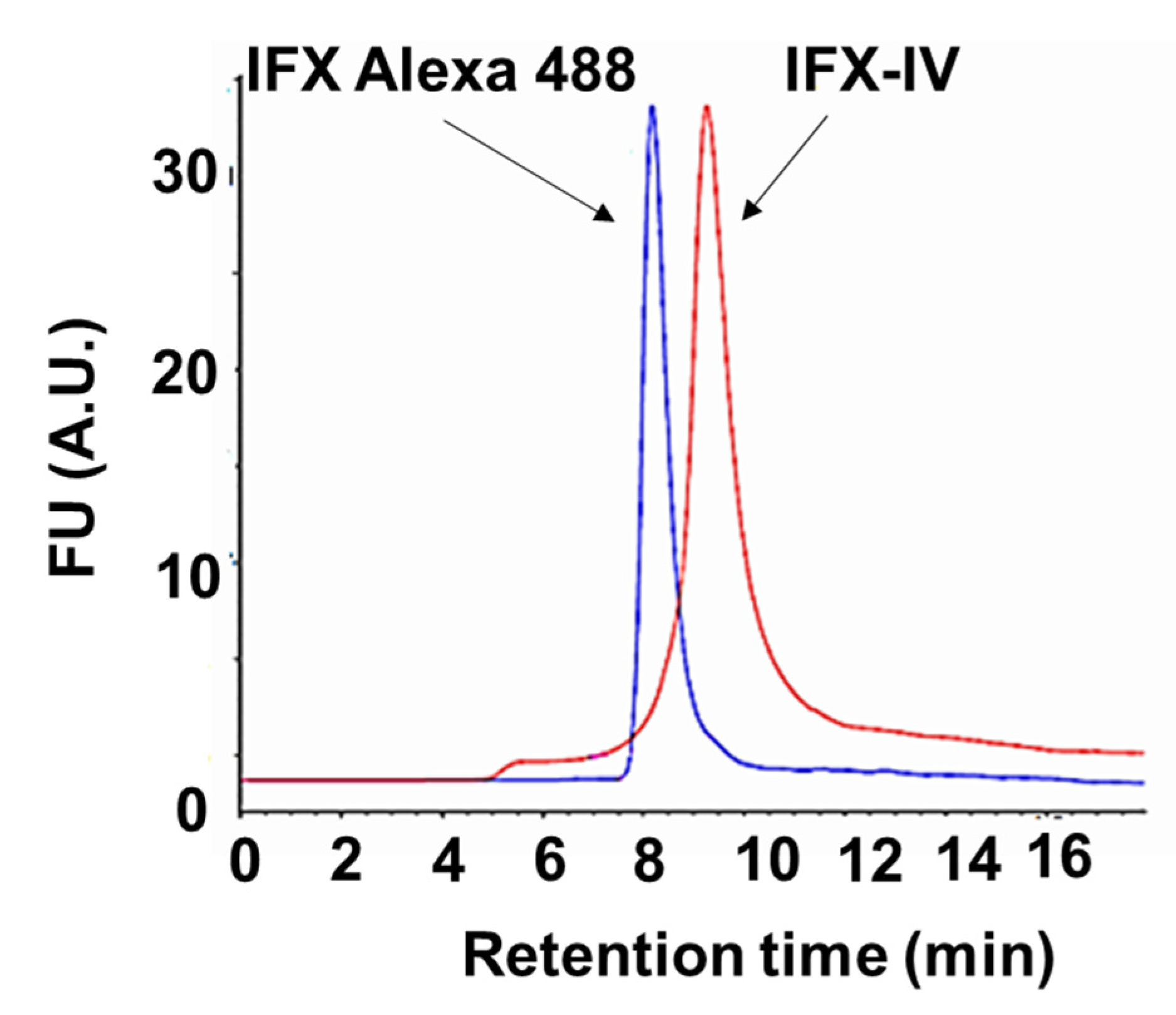
2.3. Conjugation of Infliximab to Labelled Dendrimer
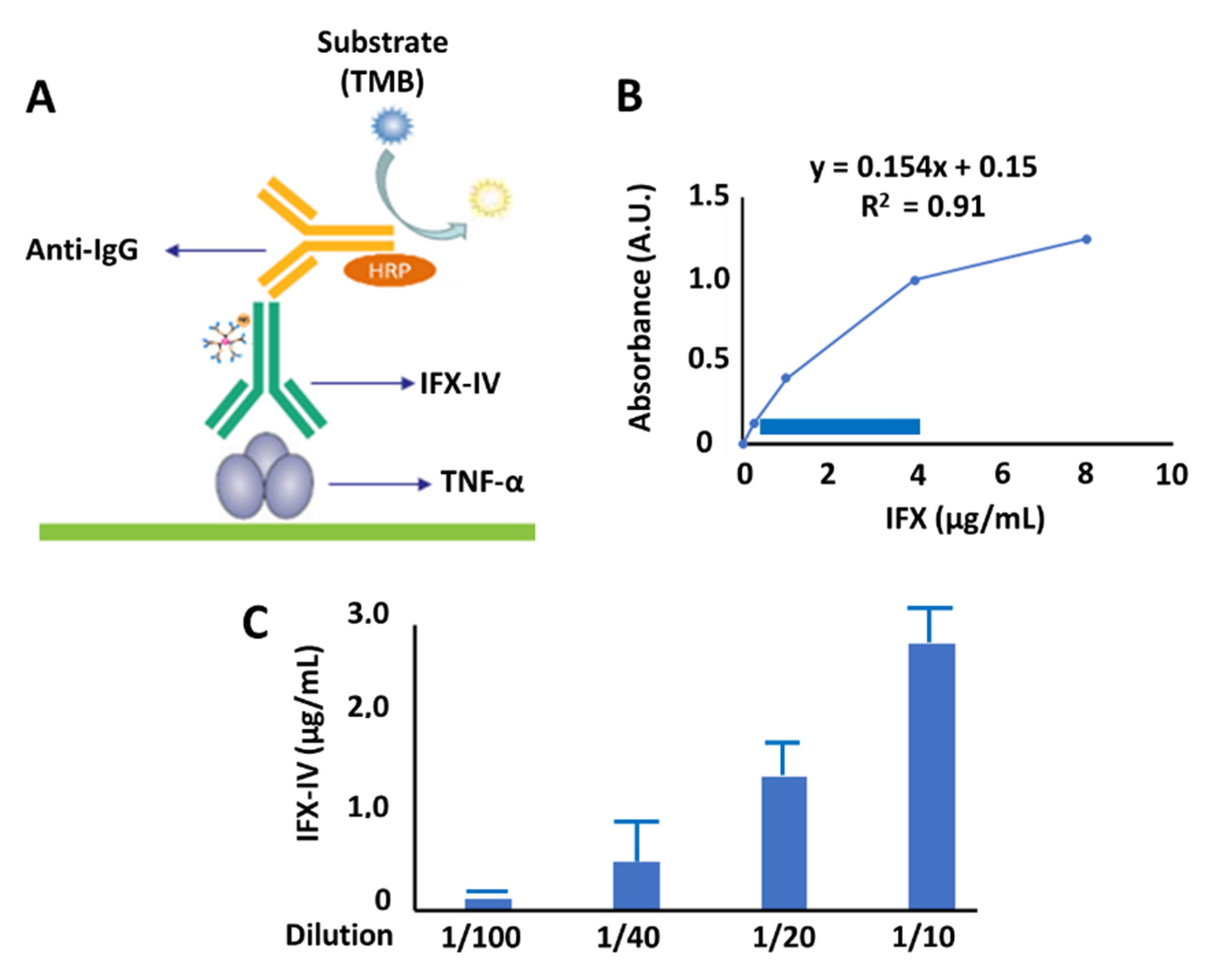
2.4. Evaluation of Functionality of Compound IV-IFX through ELISA
3. Discussion
4. Materials and Methods
4.1. Synthesis of G3Si(Allyl)31(SC2H4NH2HCl) (I)
4.2. Synthesis of G3Si(SC2H4NH2)(SCH2CO2CH3)31 (II)
4.3. Synthesis of G3Si(SC2H4NH2)(SCH2CO2Na)31 (III)
4.4. Synthesis of G3Si(SC2H4NHFITC)(SCH2CO2Na)31 (IV)
4.5. SDS-PAGE
4.6. Formation of Dendriplex Infliximab-IV (IFX-IV)
4.7. Characterization of the Dendriplex by SE-HPLC, SDS-PAGE and Inmunoblot
4.8. Functional Characterization of Dendriplex IFX-IV by ELISA
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Van der Woude, D.; van der Helm-van Mil, A. Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best practice research. Clin. Rheumatol. 2018, 32, 174–187. [Google Scholar] [CrossRef]
- Deane, K.D.; Demoruelle, M.K.; Kelmenson, L.B.; Kuhn, K.A.; Norris, J.M.; Holers, V.M. Genetic and environmental risk factors for rheumatoid arthritis. Best practice research. Clin. Rheumatol. 2017, 31, 3–18. [Google Scholar] [CrossRef]
- Dougados, M. Comorbidities in rheumatoid arthritis. Curr. Opin. Rheumatol. 2016, 28, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Van Onna, M.; Boonen, A. The challenging interplay between rheumatoid arthritis, ageing and comorbidities. BMC Musculoskelet. Disord. 2016, 17, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janeway, C.A.; Travers, P.; Walport, M.; Capra, D. Inmunología, el Sistema Inmunitario en Condiciones de Salud y Enfermedad; MASSON S.A.: Barcelona, Spain, 2000; p. 644. [Google Scholar]
- Stanfield, R.L.; Wilson, I.A. Antibody Structure. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef]
- Wootla, B.; Denic, A.; Rodriguez, M. Polyclonal and monoclonal antibodies in clinic. Methods Mol. Biol. 2014, 1060, 79–110. [Google Scholar] [CrossRef]
- Elgundi, Z.; Reslan, M.; Cruz, E.; Sifniotis, V.; Kayser, V. The state-of-play and future of antibody therapeutics. Adv. Drug Deliv. Rev. 2017, 122, 2–19. [Google Scholar] [CrossRef]
- Ecker, D.M.; Jones, S.D.; Levine, H.L. The therapeutic monoclonal antibody market. mAbs 2015, 7, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Grilo, A.L.; Mantalaris, A. The Increasingly Human and Profitable Monoclonal Antibody Market. Trends Biotechnol. 2019, 37, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Thomas, A.; Teicher, B.A.; Hassan, R. Antibody-drug conjugates for cancer therapy. Lancet Oncol. 2016, 17, e254–e262. [Google Scholar] [CrossRef]
- Carter, P.J.; Lazar, G.A. Next generation antibody drugs: Pursuit of the ‘high-hanging fruit’. Nature reviews. Drug Discov. 2018, 17, 197–223. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Byrne, H.; O’Kennedy, R.J. Antibodies and antibody-derived analytical biosensors. Essays Biochem. 2016, 60, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Gao, Y.; He, X.; Zhang, Y. Theranostic Applications of Antibody-Based Systems in Human Diseases. J. Biomed. Nanotechnol. 2018, 14, 405–429. [Google Scholar] [CrossRef] [PubMed]
- Masters, J.C.; Nickens, D.J.; Xuan, D.; Shazer, R.L.; Amantea, M. Clinical toxicity of antibody drug conjugates: A meta-analysis of payloads. Investig. New Drugs 2018, 36, 121–135. [Google Scholar] [CrossRef]
- Zimmermann, M.; Rind, D.; Chapman, R.; Kumar, V.; Kahn, S.; Carlson, J. Economic Evaluation of Dupilumab for Moderate-to-Severe Atopic Dermatitis: A Cost-Utility Analysis. J. Drugs Dermatol. 2018, 17, 750–756. [Google Scholar]
- Kaminskas, L.M.; Boyd, B.J.; Porter, C.J.H. Dendrimer pharmacokinetics: The effect of size, structure and surface characteristics on ADME properties. Nanomedicine 2011, 6, 1063–1084. [Google Scholar] [CrossRef]
- Martinho, N.; Florindo, H.; Silva, L.; Brocchini, S.; Zloh, M.; Barata, T. Molecular modeling to study dendrimers for biomedical applications. Molecules 2014, 19, 20424–20467. [Google Scholar] [CrossRef] [Green Version]
- Fuentes-Paniagua, E.; Sánchez-Nieves, J.; Hernández-Ros, J.M.; Fernández-Ezequiel, A.; Soliveri, J.; Copa-Patino, J.L.; Gómez, R.; De la Mata, J.F. Structure-activity relationship study of cationic carbosilane dendritic systems as antibacterial agents. RDC Adv. 2016, 6, 7022–7033. [Google Scholar] [CrossRef]
- Sepúlveda-Crespo, D.; Gómez, R.; De La Mata, J.M.; Jiménez, J.L.; Muñoz-Fernández, M.Á. Polyanionic carbosilane dendrimer-conjugated antiviral drugs as efficient microbicides: Recent trends and developments in HIV treatment/therapy. Nanomedicine 2015, 11, 1481–1498. [Google Scholar]
- Ortega, M.A.; Guzmán Merino, A.; Fraile-Martínez, O.; Recio-Ruiz, J.; Pekarek, L.G.; Guijarro, L.; García-Honduvilla, N.; Álvarez-Mon, M.; Buján, J.; García-Gallego, S. Dendrimers and Dendritic Materials: From Laboratory to Medical Practice in Infectious Diseases. Pharmaceutics 2020, 12, 874. [Google Scholar] [CrossRef]
- Caminade, A.M.; Majoral, J.P. Which Dendrimer to Attain the Desired Properties? Focus on Phosphorhydrazone Dendrimers. Molecules 2018, 23, 622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, C.; Cai, Z.; Reilly, R.M. Trastuzumab labeled to high apecific activity with 111In by conjugation to G4 PAMAM dendrimers derivatized with multiple DTPA chelators exhibits increased cytotoxic potency on HER2-Positive breast cancer cells. Pharm. Res. 2013, 30, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Patri, A.K.; Myc, A.; Beals, J.; Thomas, T.P.; Bander, N.H.; Baker, J.R. Synthesis and in vitro testing of J591 antibody-dendrimer conjugates for targeted prostate cancer therapy. Bioconjug. Chem. 2004, 15, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, E.; Colescott, R.L.; Bossiriger, C.D.; Cook, P.I. Color test for detection of free terminal amino groups in the solid-phase synthesis os peptides. Anal. Biochem. 1970, 34, 595–598. [Google Scholar] [CrossRef]
- Pabari, R.M.; Mattu, C.; Partheeban, S.; Almarhoon, A.; Boffito, M.; Ciardelli, G.; Ramtoola, Z. Novel polyurethane-based nanoparticles of infliximab to reduce inflammation in an in-vitro intestinal epithelial barrier model. Int. J. Pharm. 2019, 565, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, A.M. Diagnosis and management of rheumatoid arthritis. Am. Fam. Physician 2011, 84, 1245–1252. [Google Scholar]
- Fiehn, C.; Krüger, K. Management der rheumatoiden Arthritis [Management of rheumatoid arthritis]. Der Internist 2016, 57, 1042–1051. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Thakur, K.K.; Rana, V.; Bora, B.; Banik, K.; Khatoon, E.; Sailo, B.L.; Shabnam, B.; Girisa, S.; Gupta, S.C.; et al. Upside and Downside of Tumor Necrosis Factor Blockers for Treatment of Immune/Inflammatory Diseases. Crit. Rev. Immunol. 2019, 39, 439–479. [Google Scholar] [CrossRef]
- Costa, J.; Lemos, L.L.; Machado, M.A.; Almeida, A.M.; Kakehasi, A.M.; Araújo, V.; Cherchiglia, M.L.; Andrade, E.I.; Acurcio, F. Infliximab, methotrexate and their combination for the treatment of rheumatoid arthritis: A systematic review and meta-analysis. Rev. Bras. Reumatol. 2015, 55, 146–158. [Google Scholar] [CrossRef] [Green Version]
- Quistrebert, J.; Hässler, S.; Bachelet, D.; Mbogning, C.; Musters, A.; Tak, P.P.; Wijbrandts, C.A.; Herenius, M.; Bergstra, S.A.; Akdemir, G.; et al. Incidence and risk factors for adalimumab and infliximab anti-drug antibodies in rheumatoid arthritis: A European retrospective multicohort analysis. Semin. Arthritis Rheum. 2019, 48, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Siljehult, F.; Ärlestig, L.; Eriksson, C.; Rantapää-Dahlqvist, S. Concentrations of infliximab and anti-drug antibodies in relation to clinical response in patients with rheumatoid arthritis. Scand. J. Rheumatol. 2018, 47, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Feng, X.; Ding, J.; Chang, F.; Chen, X. Nanotherapeutics relieve rheumatoid arthritis. J. Control. Release 2017, 252, 108–124. [Google Scholar] [CrossRef]
- Patel, P.; Meghani, N.; Kansara, K.; Kumar, A. Nanotherapeutics for the Treatment of Cancer and Arthritis. Curr. Drug Metab. 2019, 20, 430–445. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.P.; da Silva Santos, S.; da Silva, J.V.; Parise-Filho, R.; Igne Ferreira, E.; Seoud, O.E.; Giarolla, J. Dendrimers in the context of nanomedicine. Int. J. Pharm. 2020, 573, 118814. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.P.; Goonewardena, S.N.; Majoros, I.J.; Kotlyar, A.; Cao, Z.; Leroueil, P.R.; Baker, J.R. Folate-targeted nanoparticles show efficacy in the treatment of inflammatory arthritis. Arthritis Rheum. 2011, 63, 2671–2680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobrovolskaia, M.A. Dendrimers Effects on the Immune System: Insights into Toxicity and Therapeutic Utility. Curr. Pharm. Des. 2017, 23, 3134–3141. [Google Scholar] [CrossRef]
- Wängler, C.; Moldenhauer, G.; Eisenhut, M.; Haberkorn, U.; Mier, W. Antibody-dendrimer conjugates: The number, not the size of the dendrimers, determines the immunoreactivity. Bioconjug. Chem. 2008, 19, 813–820. [Google Scholar] [CrossRef]
- Marcinkowska, M.; Stańczyk, M.; Klajnert-Maculewicz, B. Przeciwciało monoklonalne trastuzumab i dendrymery w terapii celowanej raka piersi [Trastuzumab—A monoclonal antibody and dendrimers in a targeted therapy for breast cancer]. Postepy Hig. Med. Dosw. 2015, 69, 1313–1324. [Google Scholar] [CrossRef]
- Silvestre, A.; Oshiro-Júnior, J.A.; Garcia, C.; Turco, B.O.; da Silva Leite, J.M.; de Lima Damasceno, B.; Soares, J.; Chorilli, M. Monoclonal antibodies carried in drug delivery nanosystems as a strategy for cancer treatment. Curr. Med. Chem. 2020. [Google Scholar] [CrossRef]
- Thomas, T.P.; Patri, A.K.; Myc, A.; Myaing, M.T.; Ye, J.Y.; Norris, T.B.; Baker, J.R., Jr. In vitro targeting of synthesized antibody-conjugated dendrimer nanoparticles. Biomacromolecules 2004, 5, 2269–2274. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Thomas, T.P.; Peters, J.L.; Desai, A.M.; Kukowska-Latallo, J.; Patri, A.K.; Kotlyar, A.; Baker, J.R. HER2 specific tumor targeting with dendrimer conjugated anti-HER2 mAb. Bioconjug. Chem. 2006, 17, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Barth, R.F.; Yang, W.; Kawabata, S.; Zhang, L.; Green-Church, K. Targeted delivery of methotrexate to epidermal growth factor receptor-positive brain tumors by means of cetuximab (IMC-C225) dendrimer bioconjugates. Mol. Cancer Ther. 2006, 5, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef] [Green Version]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcinkowska, M.; Sobierajska, E.; Stanczyk, M.; Janaszewska, A.; Chworos, A.; Klajnert-Maculewicz, B. Conjugate of PAMAM Dendrimer, Doxorubicin and Monoclonal Antibody-Trastuzumab: The New Approach of a Well-Known Strategy. Polymers 2018, 10, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, P.; Zhang, X.; Ni, L.; Li, J.; Zhang, F.; Wang, Z.; Lian, S.; Sun, K. Targeted delivery of polyamidoamine-paclitaxel conjugate functionalized with anti-human epidermal growth factor receptor 2 trastuzumab. Int. J. Nanomed. 2015, 10, 2173–2190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atreya, R.; Neumann, H.; Neufert, C.; Waldner, M.J.; Billmeier, U.; Zopf, Y.; Willma, M.; App, C.; Münster, T.; Kessler, H.; et al. In vivo imaging using fluorescent antibodies to tumor necrosis factor predicts therapeutic response in Crohn’s disease. Nat. Med. 2014, 20, 313–318. [Google Scholar] [CrossRef] [Green Version]
- Galan, M.; Fuentes-Paniagua, E.; De la Mata, F.J.; Gómez, R. Heterofunctionalized carbosilane dendritic systems: Bifunctionalized dendrons as building blocks versus statistically decorated dendrimers. Organometallics 2014, 33, 3977–3989. [Google Scholar] [CrossRef]
- Schlenk, C.; Frey, H. Carbosilane dendrimers-synthesis, functionalization, application. In Silicon Chemistry; Springer: Vienna, Austria, 1999; pp. 3–14. [Google Scholar]
- Hernández-Breijo, B.; Chaparro, M.; Cano-Martínez, D.; Guerra, I.; Iborra, M.; Cabriada, J.L.; Bujanda, L.; Taxonera, C.; García-Sánchez, V.; Marín-Jiménez, I.; et al. Standardization of the homogeneous mobility shift assay protocol for evaluation of anti-infliximab antibodies. Application of the method to Crohn’s disease patients treated with infliximab. Biochem. Pharmacol. 2016, 122, 33–41. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Prieto, T.; Hernández-Breijo, B.; Ortega, M.A.; Gómez, R.; Sánchez-Nieves, J.; Guijarro, L.G. Dendritic Nanotheranostic for the Delivery of Infliximab: A Potential Carrier in Rheumatoid Arthritis Therapy. Int. J. Mol. Sci. 2020, 21, 9101. https://doi.org/10.3390/ijms21239101
Rodríguez-Prieto T, Hernández-Breijo B, Ortega MA, Gómez R, Sánchez-Nieves J, Guijarro LG. Dendritic Nanotheranostic for the Delivery of Infliximab: A Potential Carrier in Rheumatoid Arthritis Therapy. International Journal of Molecular Sciences. 2020; 21(23):9101. https://doi.org/10.3390/ijms21239101
Chicago/Turabian StyleRodríguez-Prieto, Tamara, Borja Hernández-Breijo, Miguel A. Ortega, Rafael Gómez, Javier Sánchez-Nieves, and Luis G. Guijarro. 2020. "Dendritic Nanotheranostic for the Delivery of Infliximab: A Potential Carrier in Rheumatoid Arthritis Therapy" International Journal of Molecular Sciences 21, no. 23: 9101. https://doi.org/10.3390/ijms21239101







