Hedging against Neuropathic Pain: Role of Hedgehog Signaling in Pathological Nerve Healing
Abstract
:1. Introduction
2. Hedgehog Signaling in Peripheral Nerve Morphogenesis
3. Shhaping Peripheral Nerve Healing and Homeostasis
3.1. The Multifaceted Role of Hedgehog Signaling during Peripheral Nerve Healing
3.1.1. Promotion of Axonal Survival
3.1.2. Clearance of Axonal and Myelin Debris
3.1.3. Formation of Regeneration Tracks
3.1.4. Neurotrophic Effect
3.1.5. Neurite Formation and Pruning
3.1.6. Axonal Guidance
3.1.7. Promotion of Remyelination
3.2. Hedgehog Signaling and Peripheral Nerve Homeostasis
3.2.1. Hedgehog Morphogens and the Concept of Peripheral Nerve Homeostasis
3.2.2. Prevention of Myelin Degradation
3.2.3. Homeostasis of the Blood–Nerve Barrier
3.3. Hedgehog Signaling in Peripheral Nerve Healing and Homeostasis
3.4. Localization and Function of Hedgehog Pathway Effectors in Peripheral Nerve Healing
4. Nerve Healing over the hedge: Hedgehog Pathway Disruption and Neuropathic Pain Installation
4.1. Exploring Hedgehog Signaling Modifications in Different Peripheral Nerve Injury Models
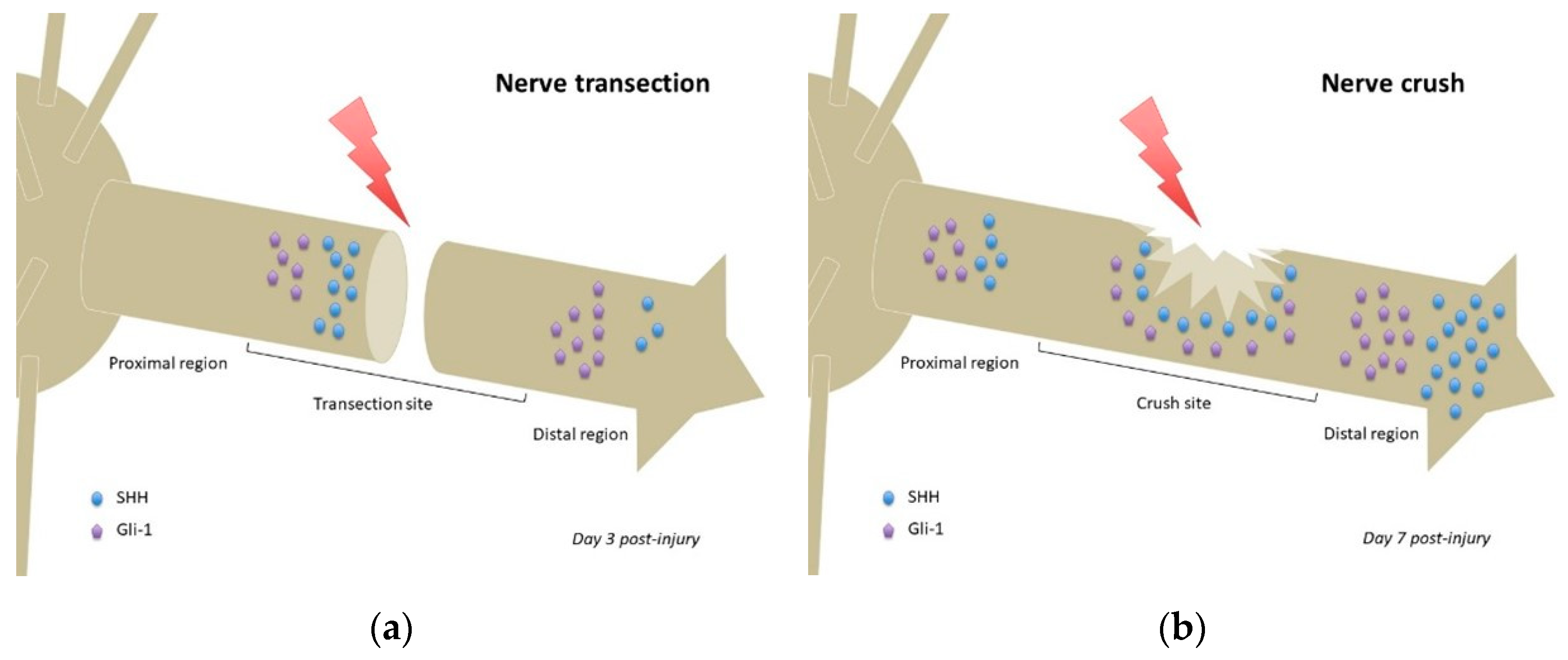
4.1.1. Nerve Transection
4.1.2. Crush Injury
4.1.3. Comparing the Regeneration Response in Acute Injury Models
4.1.4. Chronic Nerve Injuries: Compressions and Constrictions
4.1.5. Summarizing Hedgehog Signaling Modifications in Peripheral Nerve Injury Models
4.2. The Critical Issue of Timing in Nerve Regeneration
4.3. Dysfunctional Hedgehog Signaling as a New Culprit of Post-Traumatic Peripheral Neuropathic pain Development
4.3.1. Understanding Dysfunctional Nerve Regeneration
4.3.2. Abnormal Sprouting and Neuroma Formation
4.3.3. Neuropathic Pain Development Following Post-Traumatic Blood–Nerve Barrier Disruption
4.3.4. Could SHH Be a Treatment for Neuropathic Pain?
5. Future Research and Clinical Perspectives
5.1. Could Hyperactive Nerve Regeneration Drive Neuropathic Pain Development?
5.2. Understanding Hedgehog Signaling Inhibition in the Chronic Constriction Injury (CCI) Model
5.3. Investigating and Developing New Nerve Regeneration Strategies
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BNB | Blood–nerve barrier |
| CCI | Chronic constriction injury |
| DHH | Desert Hedgehog |
| DRG | Dorsal root ganglia |
| GDNF | Glial-derived neurotrophic factor |
| IAN | Inferior alveolar nerve |
| IoN | Infra-orbital nerve |
| IHH | Indian Hedgehog |
| NOS | Not otherwise specified |
| PG | Pelvic ganglia |
| PNI | Peripheral nerve injury |
| SHH | Sonic Hedgehog |
| TG | Trigeminal ganglia |
| VEGF | Vascular endothelial growth factor |
References
- Richner, M.; Ulrichsen, M.; Elmegaard, S.L.; Dieu, R.; Pallesen, L.T.; Vaegter, C.B. Peripheral nerve injury modulates neurotrophin signaling in the peripheral and central nervous system. Mol. Neurobiol. 2014, 50, 945–970. [Google Scholar] [CrossRef]
- Nocera, G.; Jacob, C. Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cell. Mol. Life Sci. 2020, 77, 3977–3989. [Google Scholar] [CrossRef] [Green Version]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, K.H.; Hung, H.A.; Svaren, J. Epigenomic regulation of Schwann cell reprogramming in peripheral nerve injury. J. Neurosci. 2016, 36, 9135–9147. [Google Scholar] [CrossRef] [PubMed]
- Arthur-Farraj, P.J.; Latouche, M.; Wilton, D.K.; Quintes, S.; Chabrol, E.; Banerjee, A.; Woodhoo, A.; Jenkins, B.; Rahman, M.; Turmaine, M.; et al. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 2012, 75, 633–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.P.; Oksuz, I.; Hurley, E.; Wrabetz, L.; Awatramani, R. Microprocessor complex subunit DiGeorge syndrome critical region gene 8 (Dgcr8) is required for Schwann cell myelination and myelin maintenance. J. Biol. Chem. 2015, 290, 24294–24307. [Google Scholar] [CrossRef] [Green Version]
- Charrier, J.B.; Lapointe, F.; Le Douarin, N.M.; Teillet, M.A. Anti-apoptotic role of Sonic Hedgehog protein at the early stages of nervous system organogenesis. Development 2001, 128, 4011–4020. [Google Scholar]
- Yam, P.T.; Langlois, S.D.; Morin, S.; Charron, F. Sonic Hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron 2009, 62, 349–362. [Google Scholar] [CrossRef] [Green Version]
- Petrova, R.; Joyner, A.L. Roles of Hedgehog signaling in adult organ homeostasis and repair. Development 2014, 141, 3445–3457. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Fabre, P.J.; Dolique, T.; Swikert, S.M.; Kermasson, L.; Shimogori, T.; Charron, F. Sonic Hedgehog is a remotely produced cue that controls axon guidance trans-axonally at a midline choice point. Neuron 2018, 97, 326–340.e4. [Google Scholar] [CrossRef] [Green Version]
- Parmantier, E.; Lynn, B.; Lawson, D.; Turmaine, M.; Namini, S.S.; Chakrabarti, L.; McMahon, A.P.; Jessen, K.R.; Mirsky, R. Schwann cell-derived Desert hedgehog controls the development of peripheral nerve sheaths. Neuron 1999, 23, 713–724. [Google Scholar] [CrossRef] [Green Version]
- Vergara, M.N.; Arsenijevic, Y.; Del Rio-Tsonis, K. CNS regeneration: A morphogen’s tale. J. Neurobiol. 2005, 64, 491–507. [Google Scholar] [CrossRef]
- Patel, S.S.; Tomar, S.; Sharma, D.; Mahindroo, N.; Udayabanu, M. Targeting Sonic Hedgehog signaling in neurological disorders. Neurosci Biobehav Rev. 2017, 74 Pt A, 76–97. [Google Scholar] [CrossRef]
- Akazawa, C.; Tsuzuki, H.; Nakamura, Y.; Sasaki, Y.; Ohsaki, K.; Nakamura, S.; Arakawa, Y.; Kohsaka, S. The upregulated expression of Sonic Hedgehog in motor neurons after rat facial nerve axotomy. J. Neurosci. 2004, 24, 7923–7930. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, M.; Ishii, K.; Nakamura, Y.; Watabe, K.; Kohsaka, S.; Akazawa, C. Neuroprotective effect of Sonic Hedgehog up-regulated in Schwann cells following sciatic nerve injury. J. Neurochem. 2008, 107, 918–927. [Google Scholar] [CrossRef]
- Martinez, J.A.; Kobayashi, M.; Krishnan, A.; Webber, C.; Christie, K.; Guo, G.; Singh, V.; Zochodne, D.W. Intrinsic facilitation of adult peripheral nerve regeneration by the Sonic Hedgehog morphogen. Exp. Neurol. 2015, 271, 493–505. [Google Scholar] [CrossRef]
- Yamada, Y.; Ohazama, A.; Maeda, T.; Seo, K. The Sonic Hedgehog signaling pathway regulates inferior alveolar nerve regeneration. Neurosci. Lett. 2018, 671, 114–119. [Google Scholar] [CrossRef]
- Moreau, N.; Mauborgne, A.; Bourgoin, S.; Couraud, P.O.; Romero, I.A.; Weksler, B.B.; Villanueva, L.; Pohl, M.; Boucher, Y. Early alterations of Hedgehog signaling pathway in vascular endothelial cells after peripheral nerve injury elicit blood-nerve barrier disruption, nerve inflammation, and neuropathic pain development. Pain 2016, 157, 827–839. [Google Scholar] [CrossRef]
- Moreau, N.; Dieb, W.; Mauborgne, A.; Bourgoin, S.; Villanueva, L.; Pohl, M.; Boucher, Y. Hedgehog pathway-mediated vascular alterations following trigeminal nerve injury. J. Dent. Res. 2017, 96, 450–457. [Google Scholar] [CrossRef]
- Moreau, N.; Mauborgne, A.; Couraud, P.O.; Romero, I.A.; Weksler, B.B.; Villanueva, L.; Pohl, M.; Boucher, Y. Could an endoneurial endothelial crosstalk between Wnt/β-catenin and Sonic Hedgehog pathways underlie the early disruption of the infra-orbital blood-nerve barrier following chronic constriction injury? Mol. Pain. 2017, 13, 1744806917727625. [Google Scholar] [CrossRef]
- Fledrich, R.; Kungl, T.; Nave, K.A.; Stassart, R.M. Axo-glial interdependence in peripheral nerve development. Development 2019, 146, dev151704. [Google Scholar] [CrossRef] [PubMed]
- Richard, L.; Védrenne, N.; Vallat, J.M.; Funalot, B. Characterization of endoneurial fibroblast-like cells from human and rat peripheral nerves. J. Histochem. Cytochem. 2014, 62, 424–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessen, K.R.; Mirsky, R. The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 2005, 6, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Umehara, F.; Tate, G.; Itoh, K.; Yamaguchi, N.; Douchi, T.; Mitsuya, T.; Osame, M. A novel mutation of Desert Hedgehog in a patient with 46,XY partial gonadal dysgenesis accompanied by minifascicular neuropathy. Am. J. Hum. Genet. 2000, 67, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanchez, J.A.; Carty, L.; Iruarrizaga-Lejarreta, M.; Palomo-Irigoyen, M.; Varela-Rey, M.; Griffith, M.; Hantke, J.; Macias-Camara, N.; Azkargorta, M.; Aurrekoetxea, I.; et al. Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J. Cell Biol. 2015, 210, 153–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, N.; Nishihara, T.; Yorozuya, T.; Tanaka, J. Microglia and macrophages in the pathological central and peripheral nervous systems. Cells 2020, 9, 2132. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. Negative regulation of myelination: Relevance for development, injury, and demyelinating disease. Glia 2008, 56, 1552–1565. [Google Scholar] [CrossRef]
- Mirsky, R.; Woodhoo, A.; Parkinson, D.B.; Arthur-Farraj, P.; Bhaskaran, A.; Jessen, K.R. Novel signals controlling embryonic Schwann cell development, myelination and dedifferentiation. J. Periph Nerve Syst. 2008, 13, 122–135. [Google Scholar] [CrossRef]
- Ma, K.H.; Duong, P.; Moran, J.J.; Junaidi, N.; Svaren, J. Polycomb repression regulates Schwann cell proliferation and axon regeneration after nerve injury. Glia 2018, 66, 2487–2502. [Google Scholar] [CrossRef]
- Ma, K.H.; Hung, H.A.; Srinivasan, R.; Xie, H.; Orkin, S.H.; Svaren, J. Regulation of peripheral nerve myelin maintenance by gene repression through Polycomb Repressive Complex 2. J. Neurosci. 2015, 35, 8640–8652. [Google Scholar] [CrossRef] [Green Version]
- Sekiguchi, H.; Ii, M.; Jujo, K.; Renault, M.A.; Thorne, T.; Clarke, T.; Ito, A.; Tanaka, T.; Klyachko, E.; Tabata, Y.; et al. Estradiol triggers Sonic Hedgehog-induced angiogenesis during peripheral nerve regeneration by downregulating hedgehog-interacting protein. Lab. Invest. 2012, 92, 532–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angeloni, N.L.; Bond, C.W.; Tang, Y.; Harrington, D.A.; Zhang, S.; Stupp, S.I.; McKenna, K.E.; Podlasek, C.A. Regeneration of the cavernous nerve by Sonic Hedgehog using aligned peptide amphiphile nanofibers. Biomaterials 2011, 32, 1091–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brosuis Lutz, A.; Chung, W.S.; Sloan, S.A.; Carson, G.A.; Zhou, L.; Lovelett, E.; Posada, S.; Zuchero, J.B.; Barres, B.A. Schwann cells use TAM receptor-mediated phagocytosis in addition to autophagy to clear myelin in a mouse model of nerve injury. Proc. Natl. Acad. Sci. USA 2017, 114, E8072–E8080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cattin, A.L.; Burden, J.J.; Van Emmenis, L.; Mackenzie, F.E.; Hoving, J.J.; Garcia Calvia, N.; Guo, Y.; McLaughlin, M.; Rosenberg, L.H.; Querada, V.; et al. Macrophage-induced blood vessels guide Schwann cell-mediated regeneration of peripheral nerves. Cell 2015, 162, 1127–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geoffroy, C.G.; Zheng, B. Myelin-associated inhibitors in axonal growth after CNS injury. Curr. Opin. Neurobiol. 2014, 27, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, M.A.; Donnelly, J.M.; Engevik, A.C.; Xiao, C.; Yang, L.; Kenny, S.; Varro, A.; Hollande, F.; Samuelson, L.C.; Zavros, Y. Gastric Sonic Hedgehog acts as a macrophage chemoattractant during the immune response to Helicobacter pylori. Gastroenterology 2012, 142, 1150–1159.e6. [Google Scholar] [CrossRef] [Green Version]
- Rauhauser, A.A.; Ren, C.; Lu, D.; Li, B.; Zhu, J.; McEnery, K.; Vadnagara, K.; Zepeda-Orozco, D.; Zhou, X.J.; Lin, F.; et al. Hedgehog signaling indirectly affects tubular cell survival after obstructive kidney injury. Am. J. Physiol. Renal Physiol. 2015, 309. [Google Scholar] [CrossRef] [Green Version]
- De Valverde, L.F.; de Pereira, T.A.; Dias, R.B.; Guimaraes, V.S.; Ramos, E.A.; Santos, J.N.; Gurgel Rocha, C.A. Macrophages and endothelial cells orchestrate tumor-associated angiogenesis in oral cancer via Hedgehog pathway activation. Tumour Biol. 2016, 37, 9233–9241. [Google Scholar] [CrossRef]
- Bobarnac Dogaru, G.L.; Juneja, S.C.; Shokrani, A.; Hui, R.Y.; Chai, Y.; Pepper, J.P. The role of Hedgehog-responsive fibroblasts in facial nerve regeneration. Exp. Neurol. 2018, 303, 72–79. [Google Scholar] [CrossRef]
- Cattin, A.L.; Lloyd, A.C. The multicellular complexity of peripheral nerve regeneration. Curr. Opin. Neurobiol. 2016, 39, 38–46. [Google Scholar] [CrossRef]
- Gomez-Sanchez, J.A.; Pilch, K.S.; van der Lans, M.; Fazal, S.V.; Benito, C.; Wagstaff, L.J.; Mirsky, R.; Jessen, K.R. After nerve injury, lineage tracing shows that myelin and Remak Schwann cells elongate extensively and branch to form repair Schwann cells, which shorten radically on remyelination. J. Neurosci. 2017, 37, 9086–9099. [Google Scholar] [CrossRef]
- Kusano, K.F.; Allendoerfer, K.L.; Munger, W.; Pola, R.; Bosch-Marce, M.; Kirchmair, R.; Yoon, Y.S.; Curry, C.; Silver, M.; Kearney, M.; et al. Sonic Hedgehog induced arteriogenesis in diabetic vasa nervorum and restores function in diabetic neuropathy. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2102–2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angeloni, N.; Bond, C.W.; Harrington, D.; Stupp, S.; Podlasek, C.A. Sonic Hedgehog is neuroprotective in the cavernous nerve with crush injury. J. Sex. Med. 2013, 10, 1240–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bond, C.W.; Angeloni, N.; Harrington, D.; Stupp, S.; Podlasek, C.A. Sonic Hedgehog regulates brain-derived neurotrophic factor in normal and regenerating cavernous nerves. J. Sex. Med. 2013, 10, 730–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omura, T.; Sano, M.; Omura, K.; Hasegawa, T.; Doi, M.; Sawada, T.; Nagano, A. Different expressions of BDNF, NT3, and NT4 in muscle and nerve after various types of peripheral nerve injuries. J. Periph. Nerv. Syst. 2005, 10, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Watabe, K.; Hayashi, Y.; Kawazoe, Y. Peripheral nerve avulsion injuries as experimental models for adult motoneuron degeneration. Neuropathology 2005, 25, 371–380. [Google Scholar] [CrossRef]
- So, P.L.; Yip, P.K.; Bunting, S.; Wong, L.F.; Mazarakis, N.D.; Hall, S.; McMahon, S.; Maden, M.; Corcoran, J.P. Interactions between retinoid acid, nerve growth factor and Sonic Hedgehog signalling pathways in neurite outgrowth. Dev. Biol. 2006, 298, 167–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrette, B.; Calvo, E.; Vallières, N.; Lacroix, S. Transcriptional profiling of the injured sciatic nerve of mice carrying the Wld(S) mutant gene: Identification of genes involved in neuroprotection, Neuroinflammation, and nerve regeneration. Brain Behav. Immun. 2010, 24, 1254–1267. [Google Scholar] [CrossRef]
- Dobbs, R.; Choe, S.; Kalmanek, E.; Harrington, D.A.; Stupp, S.I.; McVary, K.T.; Podlasek, C.A. Peptide amphiphile delivery of Sonic Hedgehog protein promotes neurite formation in penile projecting neurons. Nanomedicine 2018, 14, 2087–2094. [Google Scholar] [CrossRef]
- Dobbs, R.; Kalmanek, E.; Choe, S.; Harrington, D.A.; Stupp, S.I.; McVary, K.T.; Podlasek, C.A. Sonic Hedgehog regulation of cavernous nerve regeneration and neurite formation in aged pelvic plexus. Exp. Neurol. 2019, 312, 10–19. [Google Scholar] [CrossRef]
- Watts, R.J.; Hoopfer, E.D.; Luo, L. Axon pruning during Drosophila metamorphosis: Evidence for local degeneration and requirement of the ubiquitin-proteasome system. Neuron 2003, 38, 871–885. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.G.; Midha, R.; Martinez, J.A.; Guo, G.F.; Zochodne, D.W. Facilitated sprouting in a peripheral nerve injury. Neuroscience 2008, 152, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Kolpak, A.; Zhang, J.; Bao, Z.Z. Sonic Hedgehog has a dual effect on the growth of retinal ganglion axons depending on its concentration. J. Neurosci. 2005, 25, 3432–3441. [Google Scholar] [CrossRef] [PubMed]
- Bajestan, S.N.; Umehara, F.; Shirahama, Y.; Itoh, K.; Sharghi-Namini, S.; Jessen, K.R.; Mirsky, R.; Osame, M. Desert Hedgehog-Patched 2 expression in peripheral nerves during Wallerian degeneration and regeneration. J. Neurobiol. 2006, 66, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Ng, B.K.; Chen, L.; Mandemakers, W.; Cosgaya, J.M.; Chan, J.R. Anterograde transport and secretion of brain-derived neurotrophic factor along sensory axons promote Schwann cell myelination. J. Neurosci. 2007, 27, 7597–7603. [Google Scholar] [CrossRef] [Green Version]
- Podlasek, C.A.; Meroz, C.L.; Tang, Y.; McKenna, K.E.; McVary, K.T. Regulation of cavernous nerve injury-induced apoptosis by Sonic Hedgehog. Biol. Reprod. 2007, 76, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Miura, H.; Kusakabe, Y.; Sugiyama, C.; Kawamatsu, M.; Ninomiya, Y.; Motoyama, J.; Hino, A. Shh and Ptc are associated with taste bud maintenance in the adult mouse. Mech. Dev. 2001, 106, 143–145. [Google Scholar] [CrossRef]
- Zhao, H.; Feng, J.; Seidel, K.; Shi, S.; Klein, O.; Sharpe, P.; Chai, Y. Secretion of Shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem. Cell 2018, 23, 147. [Google Scholar] [CrossRef]
- Jung, J.; Frump, D.; Su, J.; Wang, W.; Mozaffar, T.; Gupta, R. Desert Hedgehog is a mediator of demyelination in compression neuropathies. Exp. Neurol. 2015, 271, 84–94. [Google Scholar] [CrossRef]
- Yamada, Y.; Trakanant, S.; Nihara, J.; Kudo, T.; Seo, K.; Saeki, M.; Kurose, M.; Matsumaru, D.; Maeda, T.; Ohazama, A. Gli3 is a key factor in the Schwann cells from both intact and injured peripheral nerves. Neuroscience 2020, 432, 229–239. [Google Scholar] [CrossRef]
- Choe, S.; Bond, C.W.; Harrington, D.A.; Stupp, S.I.; McVary, K.T.; Podlasek, C.A. Peptide amphiphile nanofiber hydrogel delivery of Sonic Hedgehog protein to the cavernous nerve to promote regeneration and prevent erectile dysfunction. Nanomedicine 2017, 13, 95–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimura, K.; Takeda, S. Hedgehog signaling regulates myelination in the peripheral nervous system through primary cilia. Differentiation 2012, 83, S78–S85. [Google Scholar] [CrossRef] [PubMed]
- Sharghi-Namini, S.; Turmaine, M.; Meier, C.; Sahni, V.; Umehara, F.; Jessen, K.R.; Mirsky, R. The structural and functional integrity of peripheral nerves depends on the glial-derived signal Desert Hedgehog. J. Neurosci. 2006, 26, 6364–6376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizisin, A.P.; Weerasuriya, A. Homeostatic regulation of the endoneurial microenvironment during development, aging and in response to trauma, disease and toxic insult. Acta Neuropathol. 2011, 121, 291–312. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, J.I.; Dodelet-Devillers, A.; Kebir, H.; Ifergan, I.; Fabre, P.J.; Terouz, S.; Sabbagh, M.; Wosik, K.; Bourbonnière, L.; Bernard, M.; et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 2011, 334, 1727–1731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ubogu, E.E. Biology of the human blood-nerve barrier in health and disease. Exp. Neurol. 2020, 328, 113272. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Popovich, P.G.; Ramer, M.S. Wallerian degeneration: Gaining perspective on inflammatory events after peripheral nerve injury. J. Neuroinflammation 2011, 8, 110. [Google Scholar] [CrossRef] [Green Version]
- Nicotra, L.; Loram, L.C.; Watkins, L.R.; Hutchinson, M.R. Toll-like receptors in chronic pain. Exp. Neurol. 2012, 234, 316–329. [Google Scholar] [CrossRef] [Green Version]
- Calcutt, N.A.; Allendoerfer, K.L.; Mizisin, A.P.; Middlemas, A.; Freshwater, J.D.; Burgers, M.; Ranciato, R.; Delcroix, J.D.; Taylor, F.R.; Shapiro, R.; et al. Therapeutic efficacy of Sonic Hedgehog protein in experimental diabetic neuropathy. J. Clin. Investig. 2003, 111, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.L.; Yu, W.M.; Strickland, S. Peripheral regeneration. Annu. Rev. Neurosci. 2007, 30, 209–233. [Google Scholar] [CrossRef]
- Zochodne, D.W. The challenges and beauty of peripheral nerve regrowth. J. Periph. Nerv. Syst. 2012, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Krishnan, A.; Micu, I.; Koshy, K.; Singh, V.; Martinez, J.A.; Koshy, D.; Xu, F.; Chandrasekhar, A.; Dalton, C.; et al. Peripheral neuron plasticity is enhanced by brief electrical stimulation and overrides attenuated regrowth in experimental diabetes. Neurobiol. Dis. 2015, 83, 134–151. [Google Scholar] [CrossRef]
- Pham, K.; Gupta, R. Understanding the mechanisms of entrapment neuropathies. Neurosurg. Focus 2009, 26, E7. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Nassiri, N.; Hazel, A.; Bathen, M.; Mozaffar, T. Chronic nerve compression alters Schwann cell myelin architecture in a murine model. Muscle Nerve 2012, 45, 231–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, Y.; Chen, D.; Jiang, Y.; Qiu, D.; Li, W.; Li, H. The regenerative potential of facial nerve motoneurons following chronic axotomy in rats. Neural. Plast. 2020, 2020, 8884511. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, H.Y.; Kim, S.Y.; Lee, S.H.; Lee, W.K.; Yang, D.Y. Spontaneous recovery of cavernous nerve crush injury. Korean J. Urol. 2011, 52, 560–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, G.J.; Xie, Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Vos, B.P.; Strassman, A.M.; Maciewicz, R.J. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat’s infraorbital nerve. J. Neurosci. 1994, 14 Pt 1, 2708–2723. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Li, H.; Lim, G.; McCabe, M.F.; Zhao, W.; Yang, Y.; Ma, W.; Li, N. Different effects of dexmedetomidine and midazolam on the expression of NR2B and GABAA-α1 following peripheral nerve injury in rats. IUBMB Life 2018, 70, 143–152. [Google Scholar] [CrossRef]
- Höke, A. Neuroprotection in the peripheral nervous system: Rationale for more effective therapies. Arch. Neurol. 2006, 63, 1681–1685. [Google Scholar] [CrossRef]
- Benito, C.; Davis, C.M.; Gomez-Sanchez, J.A.; Turmaine, M.; Meijer, D.; Poli, V.; Mirsky, R.; Jessen, K.R. STAT3 controls the long-term survival and phenotype of repair Schwann cells during nerve regeneration. J. Neurosci. 2017, 37, 4255–4269. [Google Scholar] [CrossRef]
- Ronchi, G.; Cillino, M.; Gambarotta, G.; Fornasari, B.E.; Raimondo, S.; Pugliese, P.; Tos, P.; Cordova, A.; Moschella, F.; Geuna, S. Irreversible changes occurring in long-term denervated Schwann cells affect delayed nerve repair. J. Neurosurg. 2017, 127, 843–856. [Google Scholar] [CrossRef] [Green Version]
- Fontana, X.; Hristova, M.; Da Costa, C.; Patodia, S.; Thei, L.; Makwana, M.; Spencer-Dene, B.; Latouche, M.; Mirsky, R.; Jessen, K.R.; et al. c-Jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling. J. Cell Biol. 2012, 198, 127–141. [Google Scholar] [CrossRef] [Green Version]
- Jonsson, S.; Wiberg, R.; McGrath, A.M.; Novikov, L.N.; Wiberg, M.; Novikova, L.N.; Kingham, P.J. Effect of delayed peripheral nerve repair on nerve regeneration, Schwann cell function and target muscle recovery. PLoS ONE 2013, 8, e56484. [Google Scholar] [CrossRef] [Green Version]
- Vaughan, D.W. Effects of advancing age on peripheral nerve regeneration. J. Comp. Neurol. 1992, 323, 219–237. [Google Scholar] [CrossRef]
- Painter, M.W.; Brosuis Lutz, A.; Cheng, Y.C.; Latremoliere, A.; Duong, K.; Miller, C.M.; Posada, S.; Cobos, E.J.; Zhang, A.X.; Wagers, A.J.; et al. Diminished Schwann cell repair responses underlie age-associated impaired axonal regeneration. Neuron 2014, 83, 331–343. [Google Scholar] [CrossRef] [Green Version]
- Van Vliet, A.C.; Tannemaat, M.R.; van Duinen, S.G.; Verhaagen, J.; Malessy, M.J.A.; De Winter, F. Human neuroma-in-continuity contains focal deficits in myelination. J. Neuropathol Exp. Neurol. 2015, 74, 901–911. [Google Scholar] [CrossRef] [Green Version]
- Tal, M.; Devor, M. Ectopic discharge in injured nerves: Comparison of trigeminal and somatic afferents. Brain Res. 1992, 579, 148–151. [Google Scholar] [CrossRef]
- Poppler, L.H.; Parikh, R.P.; Bichanich, M.J.; Rebehn, K.; Bettlach, C.R.; Mackinnon, S.E.; Moore, A.M. Surgical interventions for the treatment of painful neuroma: A comparative meta-analysis. Pain 2018, 159, 214–223. [Google Scholar] [CrossRef]
- Lim, T.K.Y.; Shi, X.Q.; Johnson, J.M.; Rone, M.B.; Antel, J.P.; David, S.; Zhang, J. Peripheral nerve injury induces persistent vascular dysfunction and endoneurial hypoxia, contributing to the genesis of neuropathic pain. J. Neurosci. 2015, 35, 3346–3359. [Google Scholar] [CrossRef] [Green Version]
- Chapouly, C.; Yao, Q.; Vandierdonck, S.; Larrieu-Lahargue, F.; Mariani, J.N.; Gadeau, A.P.; Renault, M.A. Impaired Hedgehog signalling-induced endothelial dysfunction is sufficient to induce neuropathy: Implication in diabetes. Cardiovasc. Res. 2016, 109, 217–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinhold, A.K.; Schwabe, J.; Lux, T.J.; Salvador, E.; Rittner, H.L. Quantitative and microstructural changes of the blood-nerve barrier in peripheral neuropathy. Front. Neurosci. 2018, 12, 936. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Hahm, S.C.; Choi, K.A.; Park, S.H.; Jeong, H.; Yea, J.H.; Kim, J.; Hong, S. Intrathecal transplantation of embryonic stem cell-derived spinal GABAergic neural precursor cells attenuates neuropathic pain in a spinal cord injury rat model. Cell Transplant. 2016, 25, 593–607. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Strong, J.A.; Zhang, J.M. Active nerve regeneration with failed target reinnervation drives persistent neuropathic pain. eNeuro 2017, 4, e0008-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bautista, J.; Chandrasekhar, A.; Komirishetty, P.K.; Duraikannu, A.; Zochodne, D.W. Regenerative plasticity of intact human skin axons. J. Neurol Sci. 2020, 417, 117058. [Google Scholar] [CrossRef]
- Sasai, N.; Briscoe, J. Primary cilia and graded Sonic Hedgehog signaling. Wiley Interdiscip Rev. Dev. Biol. 2012, 1, 753–772. [Google Scholar] [CrossRef]
- Pan, J.; Seeger-Nukpezah, T.; Golemis, E.A. The role of the cilium in normal and abnormal cell cycles: Emphasis on renal cystic pathologies. Cell Mol. Life Sci. 2013, 70, 1849–1874. [Google Scholar] [CrossRef] [Green Version]
- Das, R.M.; Storey, K.G. Apical abscission alters cell polarity and dismantles the primary cilium during neurogenesis. Science 2014, 343, 200–204. [Google Scholar] [CrossRef] [Green Version]
- Gazea, M.; Tasouri, E.; Tolve, M.; Bosch, V.; Kabanova, A.; Gojak, C.; Kurtulmus, B.; Novikov, O.; Spatz, J.; Pereira, G.; et al. Primary cilia are critical for Sonic Hedgehog-mediated dopaminergic neurogenesis in the embryonic midbrain. Dev. Biol. 2016, 409, 55–71. [Google Scholar] [CrossRef] [Green Version]
- Huangfu, D.; Anderson, K.V. Cilia and Hedgehog responsiveness in the mouse. Proc. Natl. Acad. Sci. USA 2005, 102, 11325–11330. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Yu, Y.; Deng, C. Protein and mRNA expression of Shh, Smo and Gli1 and inhibition by cyclopamine in hepatocytes of rats with chronic fluorosis. Toxicol. Lett. 2014, 225, 318+24. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Amirjani, N.; Edwards, D.C.; Chan, K.M. Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients. Exp. Neurol. 2010, 223, 192–202. [Google Scholar] [CrossRef] [PubMed]



| Hedgehog Pathway Effectors | Localization | Functions | References | |
|---|---|---|---|---|
| Hedgehog proteins | SHH | Schwann cells (injured nerve) | Nerve regeneration following PNI | [6,32,60] |
| Injured nerve | Nerve regeneration following PNI Promotion of neuronal survival | [14,60] | ||
| Neuronal cell bodies (DRG, TG, PG) | Promotion of neurite outgrowth Neuroprotection | [16,43,47,58] | ||
| Regrowing axons | Nerve regeneration following PNI | [16,17] | ||
| Glial cells (PG) | Neuro-glial interactions following PNI | [43] | ||
| DHH | Schwann cells | Blood–nerve barrier homeostasis | [11,54,63] | |
| Healthy (sciatic) nerve (NOS) | Blood–nerve barrier homeostasis; prevention of myelin degradation | [54,59,60,69] | ||
| Crushed sciatic nerve | Nerve regeneration following PNI | [59] | ||
| Transmembrane receptors | Patched-1 | Endoneurium and perineurium of healthy (sciatic) nerve | Peripheral nerve homeostasis | [60] |
| Schwann cells (healthy nerve) | Peripheral nerve homeostasis | [60] | ||
| Schwann cells (injured nerve) | Nerve regeneration following PNI | [60] | ||
| Glial cells (PG) | Neuro-glial interactions following PNI | [43] | ||
| Neurons (PG) | Neurite outgrowth | [50] | ||
| Endoneurial endothelial cells (sciatic nerve) | Blood–nerve barrier homeostasis | [18] | ||
| Patched-2 | Healthy sciatic nerve Schwann cells | DHH-mediated signaling | [54] | |
| Injured sciatic nerve Schwann cells | DHH-mediated signaling | [54] | ||
| Nerve-derived fibroblasts | DHH-mediated signaling | [54] | ||
| Smoothened | Neurons (PG) | Neurite outgrowth | [43,50] | |
| Neurons (facial motor nerve) | Promotion of neuronal survival | [14] | ||
| Sciatic nerve (NOS) | Physiological hedgehog signaling (including blood–nerve barrier homeostasis) | [54] and present article | ||
| Transcription factors | Gli-1 | Perineurium (strong signal) and endoneurium (weak signal) of healthy (sciatic) nerve | Peripheral nerve homeostasis | [60] |
| Injured (sciatic) nerve | Nerve regeneration following PNI | [60] | ||
| Endoneurial endothelial cells (sciatic nerve) | Blood–nerve barrier homeostasis | [18] | ||
| Endoneurial fibroblasts (facial nerve) | Nerve regeneration following PNI | [39] | ||
| Gli-3 | Schwann cells (healthy nerve) | Hedgehog signaling repression under physiological conditions | [60] | |
| Proximal region of injured nerve | Hedgehog signaling repression during peripheral nerve healing | [60] | ||
| Peripheral Nerve Injury Model | Localization | Modifications of Hedgehog Signaling | References |
|---|---|---|---|
| Sciatic nerve crush | Schwann cells | Upregulation of Shh mRNA | [15] |
| Upregulation of Shh mRNA 6 h post-injury Upregulation of Gli-1 and Patched-1 24–72 h post-injury | [31] | ||
| Neuronal cells bodies | Induced Shh mRNA at day 3 post-injury | [47] | |
| Sciatic nerve (NOS) | Shh mRNA detected at day 1 post-injury in areas proximal and distal to the crush but not detected in noncrushed sciatic nerve Gli-1 mRNA upregulated after injury, more importantly in the distal segment | [15] | |
| Axonal end bulbs | Increased production of SHH at day 7 post-injury Important increase in Shh mRNA at 24 h post-injury | [52] | |
| Sciatic nerve transection | Proximal stump and distal region of injured nerve (NOS) | Increased expression of SHH from day 1 to day 7 and decreased expression of DHH from day 1 to day 7 (=ligand switching) Reduced expression of SHH at 14 days and increased expression of DHH at 14 days Decreased Gli-3 from day 1 to day 7 | [60] |
| Regrowing axons | SHH labeling at 7 days in regrowing axons | [16] | |
| DRG neurons | SHH labeling | [16] | |
| Cavernous nerve crush | Schwann cells | SHH protein localization on either side of crush | [32] |
| Cavernous nerve (NOS) | SHH protein decrease during the first week (in aged rats) | [43] | |
| Inferior alveolar nerve transection | Inferior alveolar nerve (NOS) | Strong SHH/Gli1 expression at day 3 post-injury in the proximal stump Weak expression in distal transected IAN but not the proximal region of transected IAN Both signals reduced at day 7 post-injury | [17] |
| Facial nerve axotomy | Facial nucleus motoneurons | Shh transcripts increase at 6 h post-injury with a peak at 24 h, maintained for 4 weeks Smoothened receptor mRNA upregulated at 24 h post-axotomy (but not Patched-1) | [14] |
| SHH increase up to 36 weeks and decrease following that timepoint | [75] | ||
| Sciatic nerve chronic constriction injury | L4-L5 dorsal horn | Increased SHH protein | [79] |
| Endoneurial endothelial cells of sciatic nerve | Transient increase in Shh mRNA (from 3 to 24 h) Profound downregulation of Patched-1 and Gli-1 mRNA lasting for 30 and 60 days, respectively | [18] | |
| Sciatic nerve (NOS) | Smoothened mRNA downregulation between 6 h and 15 days post-injury | Present article | |
| Infra-orbital nerve chronic constriction injury | Endoneurial endothelial cells of infra-orbital nerve | Profound downregulation of Gli-1 and Patched-1 mRNAs from 3 to 48 h and 24 h, respectively | [19] |
| Chronic nerve compression | Sciatic nerve (NOS) | Decreased dhh mRNA and protein at 2 weeks post-injury | [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreau, N.; Boucher, Y. Hedging against Neuropathic Pain: Role of Hedgehog Signaling in Pathological Nerve Healing. Int. J. Mol. Sci. 2020, 21, 9115. https://doi.org/10.3390/ijms21239115
Moreau N, Boucher Y. Hedging against Neuropathic Pain: Role of Hedgehog Signaling in Pathological Nerve Healing. International Journal of Molecular Sciences. 2020; 21(23):9115. https://doi.org/10.3390/ijms21239115
Chicago/Turabian StyleMoreau, Nathan, and Yves Boucher. 2020. "Hedging against Neuropathic Pain: Role of Hedgehog Signaling in Pathological Nerve Healing" International Journal of Molecular Sciences 21, no. 23: 9115. https://doi.org/10.3390/ijms21239115




