The Role of Copper (II) on Kininogen Binding to Tropomyosin in the Presence of a Histidine–Proline-Rich Peptide
Abstract
1. Introduction
2. Results
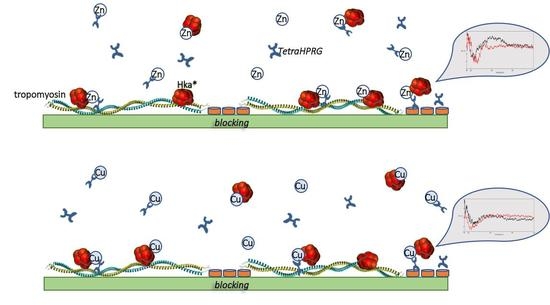
2.1. Tropomyosin/HKa Binding: In Presence of TetraHPRG, Cu2+ or Zn2+
2.2. pH Effect
2.3. TetraHPRG Effect in “Pre-Treatment”
3. Discussion
4. Materials and Methods
4.1. Proteins, Peptides, and Materials
4.2. ELISA Assay
4.3. CD Spectroscopy
4.4. Potentiometric Titrations
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lijnen, H.R.; Hoylaerts, M.; Collen, D. Heparin binding properties of human histidine-rich glycoprotein. Mechanism and role in the neutralization of heparin in plasma. J. Biol. Chem. 1983, 258, 3803–3808. [Google Scholar]
- Poon, I.K.H.; Patel, K.K.; Davis, D.S.; Parish, C.R.; Hulett, M.D. Histidine-rich glycoprotein: The Swiss Army knife of mammalian plasma. Blood 2011, 117, 2093–2101. [Google Scholar] [CrossRef]
- Lijnen, H.R.; Hoylaerts, M.; Collen, D. Isolation and characterization of a human plasma protein with affinity for the lysine binding sites in plasminogen. Role in the regulation of fibrinolysis and identification as histidine-rich glycoprotein. J. Biol. Chem. 1980, 255, 10214–10222. [Google Scholar]
- Leung, L.L.K. Interaction of histidine-rich glycoprotein with fibrinogen and fibrin. J. Clin. Investig. 1986, 77, 1305–1311. [Google Scholar] [CrossRef]
- Gorgani, N.N.; Parish, C.R.; Altin, J.G. Differential binding of histidine-rich glycoprotein (HRG) to human IgG subclasses and IgG molecules containing κ and λ light chains. J. Biol. Chem. 1999, 274, 29633–29640. [Google Scholar] [CrossRef] [PubMed]
- Morgan, W.T. Human serum histidine-rich glycoprotein. I. Interactions with heme, metal ions and organic ligands. BBA Protein Struct. 1978, 535, 319–333. [Google Scholar] [CrossRef]
- Ranieri-Raggi, M.; Moir, A.J.G.; Raggi, A. The role of histidine-proline-rich glycoprotein as zinc chaperone for skeletal muscle AMP deaminase. Biomolecules 2014, 4, 474–497. [Google Scholar] [CrossRef]
- Ronca, F.; Raggi, A. Structure-function relationships in mammalian histidine-proline-rich glycoprotein. Biochimie 2015, 118, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Gunning, P.; O’Neill, G.; Hardeman, E. Tropomyosin-based regulation of the actin cytoskeleton in time and space. Physiol. Rev. 2008, 88, 1–35. [Google Scholar] [CrossRef]
- Jones, A.L.; Hulett, M.D.; Parish, C.R. Histidine-rich glycoprotein: A novel adaptor protein in plasma that modulates the immune, vascular and coagulation systems. Immunol. Cell Biol. 2005, 83, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Doñate, F.; Juarez, J.C.; Guan, X.; Shipulina, N.V.; Plunkett, M.L.; Tel-Tsur, Z.; Shaw, D.E.; Morgan, W.T.; Mazar, A.P. Peptides derived from the histidine-proline domain of the histidine-proline-rich glycoprotein bind to tropomyosin and have antiangiogenic and antitumor activities. Cancer Res. 2004, 64, 5812–5817. [Google Scholar] [CrossRef] [PubMed]
- Dantas, E.; Erra Díaz, F.; Pereyra Gerber, P.; Varese, A.; Jerusalinsky, D.A.; Epstein, A.L.; García Rivello, H.J.; del Valle Jaén, A.; Pandolfi, J.B.; Ceballos, A.; et al. Histidine-Rich Glycoprotein Inhibits HIV-1 Infection in a pH-Dependent Manner. J. Virol. 2018, 93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.C.; Doñate, F.; Qi, X.; Ziats, N.P.; Juarez, J.C.; Mazar, A.P.; Pang, Y.P.; McCrae, K.R. The antiangiogenic activity of cleaved high molecular weight kininogen is mediated through binding to endothelial cell tropomyosin. Proc. Natl. Acad. Sci. USA 2002, 99, 12224–12229. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Juarez, J.C.; Qi, X.; Shipulina, N.V.; Shaw, D.E.; Morgan, W.T.; McCrae, K.R.; Mazar, A.P.; Doñate, F. Histidine-Proline Rich Glycoprotein (HPRG) binds and transduces anti-angiogenic signals through cell surface tropomyosin on endothelial cells. Thromb. Haemost. 2004, 92, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Gorodetsky, R.; Mou, X.; Blankenfeld, A.; Marx, G. Platelet multielemental composition, lability, and subcellular localization. Am. J. Hematol. 1993, 42, 278–283. [Google Scholar] [CrossRef]
- Ishida, T. Anti-Cancer Effects of Zinc (II) Ion in Tumor Formation and Growth, Proliferation, Metastasis and DNA Damage. In Degenerative Intellectual & Developmental Disabilities; Crimson Publishers, LLC: New York, NY, USA, 2017; pp. 1–8. [Google Scholar]
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting copper in cancer therapy: “Copper That Cancer”. Metallomics 2015, 7, 1459–1476. [Google Scholar] [CrossRef]
- La Mendola, D.; Giacomelli, C.; Rizzarelli, E. Intracellular Bioinorganic Chemistry and Cross Talk Among Different -Omics. Curr. Top. Med. Chem. 2016, 16, 3103–3130. [Google Scholar] [CrossRef]
- Schwartz, M.K. Role of Trace Elements in Cancer. Cancer Res. 1975, 35, 3481–3487. [Google Scholar]
- Gupte, A.; Mumper, R.J. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat. Rev. 2009, 35, 32–46. [Google Scholar] [CrossRef]
- Kuo, H.W.; Chen, S.F.; Wu, C.C.; Chen, D.R.; Lee, J.H. Serum and Tissue Trace Elements in Patients with Breast Cancer in Taiwan. Biol. Trace Element Res. 2002, 89, 1–11. [Google Scholar] [CrossRef]
- Sharma, K.; Mittal, D.K.; Kesarwani, R.C.; Kamboj, V.P. Chowdhery Diagnostic and prognostic significance of serum and tissue trace elements in breast malignancy. Indian J. Med. Sci. 1994, 48, 227–232. [Google Scholar] [PubMed]
- Zowczak, A.; Iskra, M.; Torlin, L.; Cofta, S. Analysis of Serum Copper and Zinc Concentrations in Cancer Patients. Biol. Trace Elem. Res. 2001, 82, 1–8. [Google Scholar] [CrossRef]
- Gupta, S.K.; Shukla, V.K.; Vaidya, M.P.; Roy, S.K.; Gupta, S. Serum and tissue trace elements in colorectal cancer. J. Surg. Oncol. 1993, 52, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Finney, L.; Mandava, S.; Ursos, L.; Zhang, W.; Rodi, D.; Vogt, S.; Legnini, D.; Maser, J.; Ikpatt, F.; Olopade, O.I.; et al. X-ray flourescence microscopy reveals large-scale relocalization and extracellular translocation of cellular copper during angiogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 2247–2252. [Google Scholar] [CrossRef]
- Finney, L.; Vogt, S.; Fukai, T.; Glesne, D. Copper and angiogenesis: Unravelling a relationship key to cancer progression. Clin. Exp. Pharmacol. Physiol. 2009, 36, 88–94. [Google Scholar] [CrossRef]
- Magrì, A.; Grasso, G.; Corti, F.; Finetti, F.; Greco, V.; Santoro, A.M.; Sciuto, S.; La Mendola, D.; Morbidelli, L.; Rizzarelli, E. Peptides derived from the histidine–proline rich glycoprotein bind copper ions and exhibit anti-angiogenic properties. Dalt. Trans. 2018, 47, 9492–9503. [Google Scholar] [CrossRef]
- Herwald, H.; Mörgelin, M.; Svensson, H.G.; Sjöbring, U. Zinc-dependent conformational changes in domain D5 of high molecular mass kininogen modulate contact activation. Eur. J. Biochem. 2001, 268, 396–404. [Google Scholar] [CrossRef]
- Johnson, W.C., Jr. Methods of Biochemical Analysis; Wiley-Interscience: Hoboken, NJ, USA, 1985; Volume 31, ISBN 0471821772. [Google Scholar]
- Woody, R.W. Circular Dichroism. Methods Enzymol. 1995, 246, 34–71. [Google Scholar] [CrossRef]
- Woody, R.W. Theory of Circular Dichroism of Proteins. In Circular Dichroism and the Conformational Analysis of Biomolecules; Springer: New York, NY, USA, 1996; pp. 25–67. [Google Scholar]
- Corrêa, D.; Henrique, C.; Ramos, I.; Corrêa, D.H.A.; Ramos, C.H.I. The use of circular dichroism spectroscopy to study protein folding, form and function Protein stability, folding and misfolding View project Structure and function of molecular chaperones View project The use of circular dichroism spectroscopy to study protein folding, form and function. Afr. J. Biochem. Res. 2009, 3, 164–173. [Google Scholar]
- La Mendola, D.; Bonomo, R.P.; Caminati, S.; Di Natale, G.; Emmi, S.S.; Hansson, Ö.; Maccarrone, G.; Pappalardo, G.; Pietropaolo, A.; Rizzarelli, E. Copper(II) complexes with an avian prion N-terminal region and their potential SOD-like activity. J. Inorg. Biochem. 2009, 103, 195–204. [Google Scholar] [CrossRef]
- Woody, R.W. Circular dichroism spectrum of peptides in the poly(Pro)II conformation. J. Am. Chem. Soc. 2009, 131, 8234–8245. [Google Scholar] [CrossRef] [PubMed]
- Borza, D.B.; Tatum, F.M.; Morgan, W.T. Domain structure and conformation of histidine—Proline-rich glycoprotein. Biochemistry 1996, 35, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- La Mendola, D.; Magrì, A.; Campagna, T.; Campitiello, M.A.; Raiola, L.; Isernia, C.; Hansson, Ö.; Bonomo, R.P.; Rizzarelli, E. A Doppel α-Helix Peptide Fragment Mimics the Copper(II) Interactions with the Whole Protein. Chem. A Eur. J. 2010, 16, 6212–6223. [Google Scholar] [CrossRef] [PubMed]
- Kállay, C.; Ősz, K.; Dávid, A.; Valastyán, Z.; Malandrinos, G.; Hadjiliadis, N.; Sóvágó, I. Zinc(ii) binding ability of tri-, tetra- and penta-peptides containing two or three histidyl residues. Dalton Trans. 2007, 36, 4040–4047. [Google Scholar] [CrossRef]
- La Mendola, D.; Magrì, A.; Santoro, A.M.; Nicoletti, V.G.; Rizzarelli, E. Copper(II) interaction with peptide fragments of histidine–proline-rich glycoprotein: Speciation, stability and binding details. J. Inorg. Biochem. 2012, 111, 59–69. [Google Scholar] [CrossRef]
- Hecel, A.; Wątły, J.; Rowińska-Żyrek, M.; Świątek-Kozłowska, J.; Kozłowski, H. Histidine tracts in human transcription factors: Insight into metal ion coordination ability. J. Biol. Inorg. Chem. 2018, 23, 81–90. [Google Scholar] [CrossRef]
- Valensin, D.; Szyrwiel, Ł.; Camponeschi, F.; Rowińska-Żyrek, M.; Molteni, E.; Jankowska, E.; Szymańska, A.; Gaggelli, E.; Valensin, G.; Kozlowski, H. Heteronuclear and homonuclear Cu2+ and Zn2+ complexes with multihistidine peptides based on zebrafish prion-like protein. Inorg. Chem. 2009, 48, 7330–7340. [Google Scholar] [CrossRef]
- Pushie, M.J.; Nienaber, K.H.; McDonald, A.; Millhauser, G.L.; George, G.N. Combined EXAFS and DFT Structure Calculations Provide Structural Insights into the 1:1 Multi-Histidine Complexes of CuII, CuI, and ZnII with the Tandem Octarepeats of the Mammalian Prion Protein. Chem. A Eur. J. 2014, 20, 9770–9783. [Google Scholar] [CrossRef]
- Jakab, N.I.; Jancsó, A.; Gajda, T.; Gyurcsik, B.; Rockenbauer, A. Copper(II), nickel(II) and zinc(II) complexes of N-acetyl-His-Pro-His-His-NH2: Equilibria, solution structure and enzyme mimicking. J. Inorg. Biochem. 2008, 102, 1438–1448. [Google Scholar] [CrossRef]
- Travaglia, A.; La Mendola, D.; Magrì, A.; Pietropaolo, A.; Nicoletti, V.G.; Grasso, G.; Malgieri, G.; Fattorusso, R.; Isernia, C.; Rizzarelli, E. Zinc(II) interactions with brain-derived neurotrophic factor N-terminal peptide fragments: Inorganic features and biological perspectives. Inorg. Chem. 2013, 52, 11075–11083. [Google Scholar] [CrossRef]
- Pietropaolo, A.; Magrì, A.; Greco, V.; Losasso, V.; La Mendola, D.; Sciuto, S.; Carloni, P.; Rizzarelli, E. Binding of Zn(II) to Tropomyosin Receptor Kinase A in Complex with Its Cognate Nerve Growth Factor: Insights from Molecular Simulation and in Vitro Essays. ACS Chem. Neurosci. 2018, 9, 1095–1103. [Google Scholar] [CrossRef]
- Grasso, G.; Magrì, A.; Bellia, F.; Pietropaolo, A.; La Mendola, D.; Rizzarelli, E. The copper(II) and zinc(II) coordination mode of HExxH and HxxEH motif in small peptides: The role of carboxylate location and hydrogen bonding network. J. Inorg. Biochem. 2014, 130, 92–102. [Google Scholar] [CrossRef]
- Juarez, J.C.; Guan, X.; Shipulina, N.V.; Plunkett, M.L.; Parry, G.C.; Shaw, D.E.; Zhang, J.-C.; Rabbani, S.A.; McCrae, K.R.; Mazar, A.P.; et al. Histidine-Proline-rich Glycoprotein Has Potent Antiangiogenic Activity Mediated through the Histidine-Proline-rich Domain. Cancer Res. 2002, 62, 62. [Google Scholar]
- Harrison, J.S.; Higgins, C.D.; O’Meara, M.J.; Koellhoffer, J.F.; Kuhlman, B.A.; Lai, J.R. Role of electrostatic repulsion in controlling pH-dependent conformational changes of viral fusion proteins. Structure 2013, 21, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Driller, R.; Ballaschk, M.; Schmieder, P.; Uchanska-Ziegler, B.; Ziegler, A.; Loll, X.B. Metal-triggered conformational reorientation of a self-peptide bound to a disease-associated HLA-B*27 subtype. JBC 2019, 294, 13269–13279. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Seuthe, A.B.; Ehrler, O.T.; Zhang, X.; Wyttenbach, T.; Hsu, J.F.; Bowers, M.T. Oxytocin-receptor binding: Why divalent metals are essential. J. Am. Chem. Soc. 2005, 127, 2024–2025. [Google Scholar] [CrossRef]
- La Mendola, D.; Magrì, A.; Hansson, O.; Bonomo, R.P.; Rizzarelli, E. Copper(II) complexes with peptide fragments encompassing the sequence 122-130 of human doppel protein. J. Inorg. Biochem. 2009, 103, 758–765. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Alderighi, L.; Gans, P.; Ienco, A.; Peters, D.; Sabatini, A.; Vacca, A. Hyperquad simulation and speciation (HySS): A utility program for the investigation of equilibria involving soluble and partially soluble species. Coord. Chem. Rev. 1999, 184, 311–318. [Google Scholar] [CrossRef]









| Species | Log Beta |
|---|---|
| 1 1 10 | 71.65 (4) |
| 1 1 6 | 51.08 (6) |
| 1 1 4 | 39.98 (4) |
| 1 1 2 | 23.44 (5) |
| 1 1 0 | 4.45 (6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoro, A.M.; Zimbone, S.; Magrì, A.; La Mendola, D.; Grasso, G. The Role of Copper (II) on Kininogen Binding to Tropomyosin in the Presence of a Histidine–Proline-Rich Peptide. Int. J. Mol. Sci. 2020, 21, 9343. https://doi.org/10.3390/ijms21249343
Santoro AM, Zimbone S, Magrì A, La Mendola D, Grasso G. The Role of Copper (II) on Kininogen Binding to Tropomyosin in the Presence of a Histidine–Proline-Rich Peptide. International Journal of Molecular Sciences. 2020; 21(24):9343. https://doi.org/10.3390/ijms21249343
Chicago/Turabian StyleSantoro, Anna Maria, Stefania Zimbone, Antonio Magrì, Diego La Mendola, and Giulia Grasso. 2020. "The Role of Copper (II) on Kininogen Binding to Tropomyosin in the Presence of a Histidine–Proline-Rich Peptide" International Journal of Molecular Sciences 21, no. 24: 9343. https://doi.org/10.3390/ijms21249343
APA StyleSantoro, A. M., Zimbone, S., Magrì, A., La Mendola, D., & Grasso, G. (2020). The Role of Copper (II) on Kininogen Binding to Tropomyosin in the Presence of a Histidine–Proline-Rich Peptide. International Journal of Molecular Sciences, 21(24), 9343. https://doi.org/10.3390/ijms21249343