A Rational Insight into the Effect of Dimethyl Sulfoxide on TNF-α Activity
Abstract
1. Introduction
2. Results
2.1. DMSO Inhibits TNF-α-Induced Cytokine Secretion and Cell Proliferation
2.2. DMSO Inhibits TNF-α-Induced Cell Death
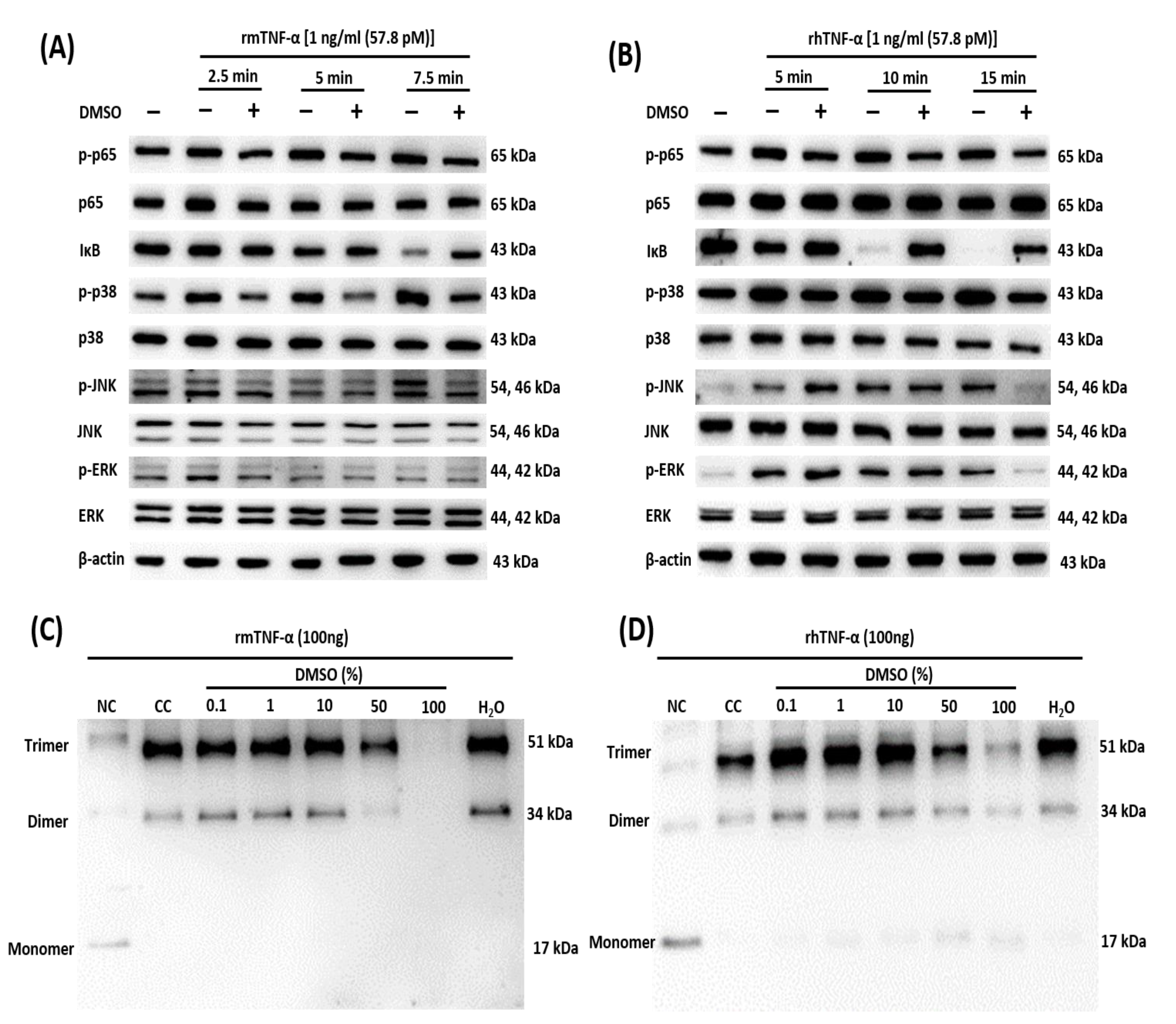
2.3. DMSO Suppresses Multiple TNF-α-Mediated Signaling Pathways
2.4. DMSO Prevents Oligomerization of TNF-α
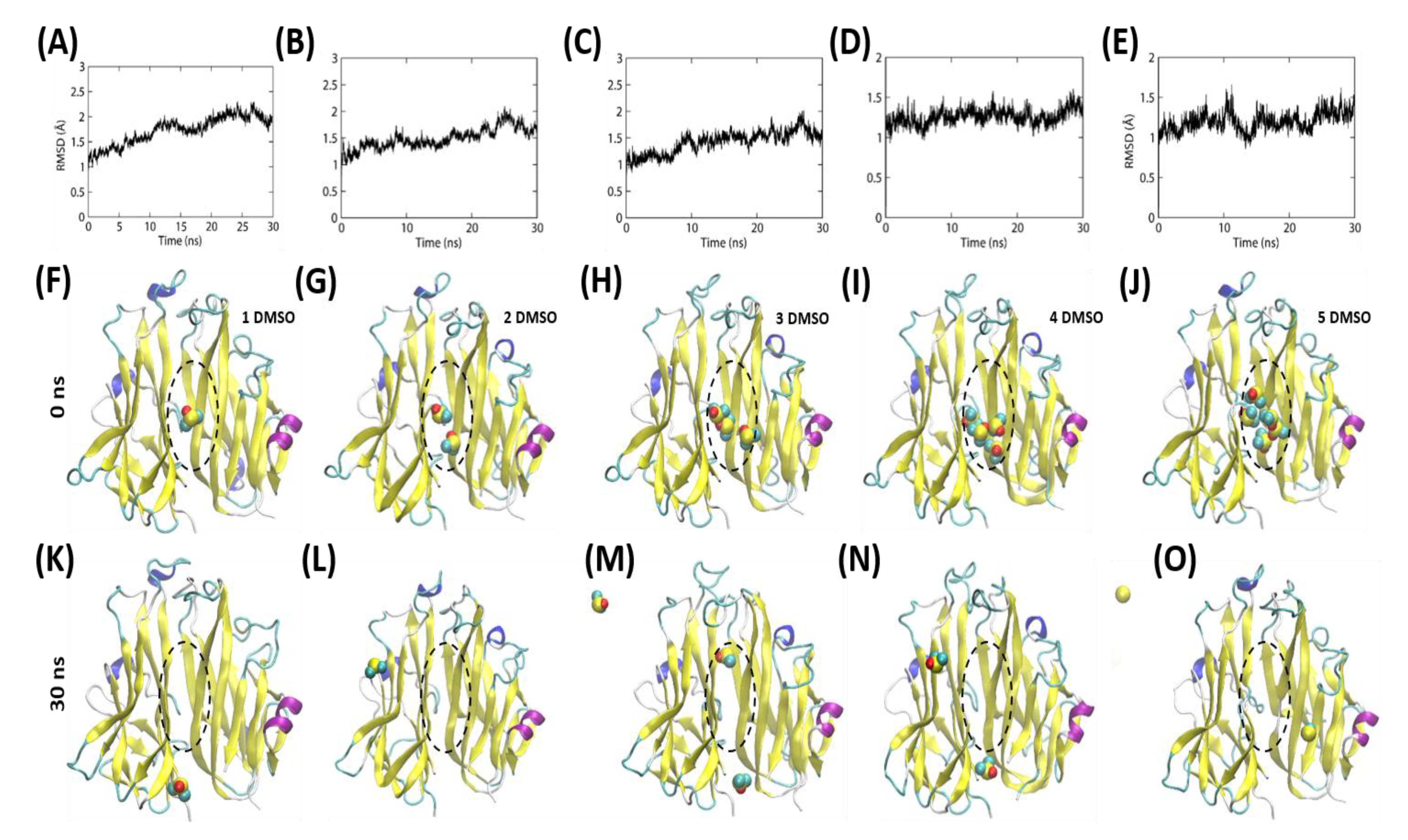
2.5. The DMSO Molecules Engage in a Transient Interaction within the Hydrophobic Cavity of the TNF Homodimer
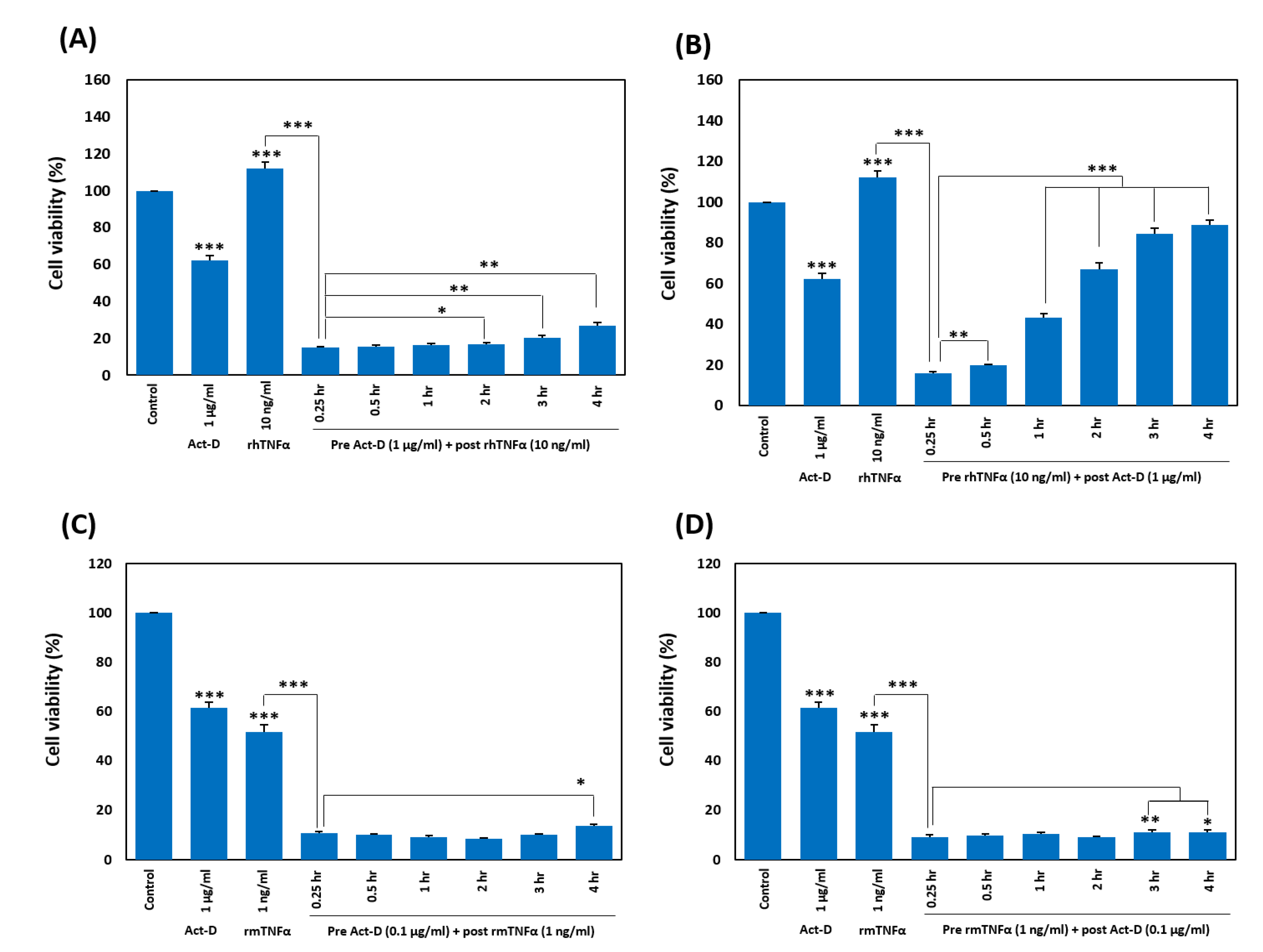
2.6. Pretreatment with TNF-α Weakens the Sensitization by Act-D in a Timing-Dependent Manner
3. Discussion
4. Methods
4.1. Cell Lines and Reagents
4.2. The Cell Viability Assay
4.3. The Death Recovery Assay
cotreatment value)) ×100)
4.4. Western Blot Analyses
4.5. Cytokine Detection Assays
4.6. Dissociation of a TNF-α Oligomer
4.7. MD Simulations
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| act-D | actinomycin D |
| DMSO | dimethyl sulfoxide |
| HDFs | human dermal fibroblasts |
| hIL-6 | human interleukin 6 |
| hIL-8 | human interleukin 8 |
| MAPK | mitogen-activated protein kinase |
| MD | molecular dynamics |
| MTT | 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan |
| p- | phospho- |
| rhTNF-α | recombinant human TNF-α |
| rmTNF-α | recombinant murine TNF-α |
| TNF | tumor necrosis factor |
References
- Aggarwal, B.B.; Gupta, S.C.; Kim, J.H. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood 2012, 119, 651–665. [Google Scholar] [CrossRef]
- Beutler, B.; Milsark, I.W.; Cerami, A.C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science 1985, 229, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Victor, F.C.; Gottlieb, A.B. TNF-alpha and apoptosis: Implications for the pathogenesis and treatment of psoriasis. J. Drugs Derm. 2002, 1, 264–275. [Google Scholar]
- Brynskov, J.; Foegh, P.; Pedersen, G.; Ellervik, C.; Kirkegaard, T.; Bingham, A.; Saermark, T. Tumour necrosis factor alpha converting enzyme (TACE) activity in the colonic mucosa of patients with inflammatory bowel disease. Gut 2002, 51, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Locksley, R.M.; Killeen, N.; Lenardo, M.J. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell 2001, 104, 487–501. [Google Scholar] [CrossRef]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctot, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Swardfager, W.; Lanctot, K.; Rothenburg, L.; Wong, A.; Cappell, J.; Herrmann, N. A meta-analysis of cytokines in Alzheimer’s disease. Biol. Psychiatry 2010, 68, 930–941. [Google Scholar] [CrossRef]
- Monaco, C.; Nanchahal, J.; Taylor, P.; Feldmann, M. Anti-TNF therapy: Past, present and future. Int. Immunol. 2015, 27, 55–62. [Google Scholar] [CrossRef]
- Eck, M.J.; Sprang, S.R. The structure of tumor necrosis factor-alpha at 2.6 A resolution. Implications for receptor binding. J. Biol. Chem. 1989, 264, 17595–17605. [Google Scholar]
- Marušič, J.; Podlipnik, Č.; Jevševar, S.; Kuzman, D.; Vesnaver, G.; Lah, J. Recognition of Human Tumor Necrosis Factor α (TNF-α) by Therapeutic Antibody Fragment Energetics and Structural Features. J. Biol. Chem. 2012, 287, 8613–8620. [Google Scholar] [CrossRef]
- Pennica, D.; Lam, V.T.; Weber, R.F.; Kohr, W.J.; Basa, L.J.; Spellman, M.W.; Ashkenazi, A.; Shire, S.J.; Goeddel, D.V. Biochemical characterization of the extracellular domain of the 75-kilodalton tumor necrosis factor receptor. Biochemistry 1993, 32, 3131–3138. [Google Scholar] [CrossRef] [PubMed]
- Loetscher, H.; Gentz, R.; Zulauf, M.; Lustig, A.; Tabuchi, H.; Schlaeger, E.; Brockhaus, M.; Gallati, H.; Manneberg, M.; Lesslauer, W. Recombinant 55-kDa tumor necrosis factor (TNF) receptor. Stoichiometry of binding to TNF alpha and TNF beta and inhibition of TNF activity. J. Biol. Chem. 1991, 266, 18324–18329. [Google Scholar] [PubMed]
- Roy, U. Structural modeling of tumor necrosis factor: A protein of immunological importance. Biotechnol. Appl. Biochem. 2017, 64, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Roy, U. 3D Modeling of Tumor Necrosis Factor Receptor and Tumor Necrosis Factor-bound Receptor Systems. Mol. Inform. 2019, 38, 1800011. [Google Scholar] [CrossRef]
- Narhi, L.O.; Arakawa, T. Dissociation of recombinant tumor necrosis factor-α studied by gel permeation chromatography. Biochem. Biophys. Res. Commun. 1987, 147, 740–746. [Google Scholar] [CrossRef]
- Corti, A.; Fassina, G.; Marcucci, F.; Barbanti, E.; Cassani, G. Oligomeric tumour necrosis factor α slowly converts into inactive forms at bioactive levels. Biochem. J. 1992, 284, 905–910. [Google Scholar] [CrossRef]
- Ameloot, P.; Declercq, W.; Fiers, W.; Vandenabeele, P.; Brouckaert, P. Heterotrimers formed by tumor necrosis factors of different species or muteins. J. Biol. Chem. 2001, 276, 27098–27103. [Google Scholar] [CrossRef]
- Kunitani, M.G.; Cunico, R.L.; Staats, S.J. Reversible subunit dissociation of tumor necrosis factor during hydrophobic interaction chromatography. J. Chromatogr. A 1988, 443, 205–220. [Google Scholar] [CrossRef]
- Beil, E.J.; Heavner, G.A.; Wu, S.J.; Nemeth, J.F. Probing the solution structure of tumor necrosis factor-α homotrimer and heterotrimer after complex perturbation using electrospray ionization mass spectrometry. J. Mol. Recognit. 2012, 25, 174–183. [Google Scholar] [CrossRef]
- Verwer, P.; Leusen, F.J. Computer simulation to predict possible crystal polymorphs. Rev. Comput. Chem. 1998, 12, 327–366. [Google Scholar]
- Davies, D.T. Aromatic Heterocyclic Chemistry; Oxford University Press: Oxford, UK; New York, NY, USA, 1992. [Google Scholar]
- Marcus, Y. The Properties of Solvents; Wiley: Hoboken, NJ, USA, 1998. [Google Scholar]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Kennedy, T. Managing the drug discovery/development interface. Drug Discov. Today 1997, 2, 436–444. [Google Scholar] [CrossRef]
- Alsenz, J.; Kansy, M. High throughput solubility measurement in drug discovery and development. Adv. Drug Deliv. Rev. 2007, 59, 546–567. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Fish, P.V.; Mano, T. Bridging solubility between drug discovery and development. Drug Discov. Today 2012, 17, 486–495. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, W.S. The chemical and physical properties of DMSO. Ann. N. Y. Acad. Sci. 1967, 141, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Brown, J. A double-blind clinical study—DMSO for acute injuries and inflammations compared to accepted standard therapy. Curr. Ther. Res. Clin. Exp. 1971, 13, 536. [Google Scholar]
- Régnier, J.; Richard, J. Lack of developmental toxicity in rats treated with dimethylsulfoxide (DMSO). Toxicologist 1998, 42, 256–257. [Google Scholar]
- O’Connell, J.; Porter, J.; Kroeplien, B.; Norman, T.; Rapecki, S.; Davis, R.; McMillan, D.; Arakaki, T.; Burgin, A.; Fox Iii, D. Small molecules that inhibit TNF signalling by stabilising an asymmetric form of the trimer. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Niwayama, S.; Turk, B.E.; Liu, J.O. Potent inhibition of tumor necrosis factor-α production by tetrafluorothalidomide and tetrafluorophthalimides. J. Med. Chem. 1996, 39, 3044–3045. [Google Scholar] [CrossRef]
- Sampaio, E.P.; Sarno, E.N.; Galilly, R.; Cohn, Z.A.; Kaplan, G. Thalidomide selectively inhibits tumor necrosis factor alpha production by stimulated human monocytes. J. Exp. Med. 1991, 173, 699–703. [Google Scholar] [CrossRef]
- Jackson, R.W.; Gelinas, R.; Baughman, T.A.; Cox, T.; Howbert, J.J.; Kucera, K.A.; Latham, J.A.; Ramsdell, F.; Singh, D.; Darwish, I.S. Benzobicyclooctanes as novel inhibitors of TNF-α signaling. Bioorganic Med. Chem. Lett. 2002, 12, 1093–1097. [Google Scholar] [CrossRef]
- Luzi, S.; Kondo, Y.; Bernard, E.; Stadler, L.K.; Vaysburd, M.; Winter, G.; Holliger, P. Subunit disassembly and inhibition of TNFα by a semi-synthetic bicyclic peptide. Protein Eng. Des. Sel. 2015, 28, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Papaneophytou, C.; Alexiou, P.; Papakyriakou, A.; Ntougkos, E.; Tsiliouka, K.; Maranti, A.; Liepouri, F.; Strongilos, A.; Mettou, A.; Couladouros, E. Synthesis and biological evaluation of potential small molecule inhibitors of tumor necrosis factor. MedChemComm 2015, 6, 1196–1209. [Google Scholar] [CrossRef]
- Hu, Z.; Qin, J.; Zhang, H.; Wang, D.; Hua, Y.; Ding, J.; Shan, L.; Jin, H.; Zhang, J.; Zhang, W. Japonicone A antagonizes the activity of TNF-α by directly targeting this cytokine and selectively disrupting its interaction with TNF receptor-1. Biochem. Pharmacol. 2012, 84, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- He, M.M.; Smith, A.S.; Oslob, J.D.; Flanagan, W.M.; Braisted, A.C.; Whitty, A.; Cancilla, M.T.; Wang, J.; Lugovskoy, A.A.; Yoburn, J.C.; et al. Small-molecule inhibition of TNF-alpha. Science 2005, 310, 1022–1025. [Google Scholar] [CrossRef] [PubMed]
- Douni, E.; Kollias, G. A critical role of the p75 tumor necrosis factor receptor (p75TNF-R) in organ inflammation independent of TNF, lymphotoxin alpha, or the p55TNF-R. J. Exp. Med. 1998, 188, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Ting, A.T.; Bertrand, M.J.M. More to Life than NF-kappaB in TNFR1 Signaling. Trends Immunol. 2016, 37, 535–545. [Google Scholar] [CrossRef]
- Annibaldi, A.; Meier, P. Checkpoints in TNF-Induced Cell Death: Implications in Inflammation and Cancer. Trends Mol. Med. 2018, 24, 49–65. [Google Scholar] [CrossRef]
- Wajant, H.; Pfizenmaier, K.; Scheurich, P. Tumor necrosis factor signaling. Cell Death Differ. 2003, 10, 45–65. [Google Scholar] [CrossRef]
- De Abreu Costa, L.; Henrique Fernandes Ottoni, M.; Dos Santos, M.G.; Meireles, A.B.; Gomes de Almeida, V.; de Fátima Pereira, W.; Alves de Avelar-Freitas, B.; Eustáquio Alvim Brito-Melo, G. Dimethyl sulfoxide (DMSO) decreases cell proliferation and TNF-α, IFN-γ, and IL-2 cytokines production in cultures of peripheral blood lymphocytes. Molecules 2017, 22, 1789. [Google Scholar] [CrossRef]
- Da Violante, G.; Zerrouk, N.; Richard, I.; Provot, G.; Chaumeil, J.C.; Arnaud, P. Evaluation of the cytotoxicity effect of dimethyl sulfoxide (DMSO) on Caco2/TC7 colon tumor cell cultures. Biol. Pharm. Bull. 2002, 25, 1600–1603. [Google Scholar] [CrossRef]
- Ahn, H.; Kim, J.; Jeung, E.-B.; Lee, G.-S. Dimethyl sulfoxide inhibits NLRP3 inflammasome activation. Immunobiology 2014, 219, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Tselepis, C.; Perry, I.; Dawson, C.; Hardy, R.; Darnton, S.J.; McConkey, C.; Stuart, R.C.; Wright, N.; Harrison, R.; Jankowski, J.A.Z. Tumour necrosis factor-α in Barrett’s oesophagus: A potential novel mechanism of action. Oncogene 2002, 21, 6071–6081. [Google Scholar] [CrossRef]
- Humblet, C.; Greimers, R.; Delvenne, P.; Deman, J.; Boniver, J.; Defresne, M.P. Prevention of murine radiogenic thymic lymphomas by tumor necrosis factor or by marrow grafting. JNCI J. Natl. Cancer Inst. 1996, 88, 824–831. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.; Blaser, H.; Mak, T.W. Regulation of tumour necrosis factor signalling: Live or let die. Nat. Rev. Immunol. 2015, 15, 362–374. [Google Scholar] [CrossRef]
- Blevitt, J.M.; Hack, M.D.; Herman, K.L.; Jackson, P.F.; Krawczuk, P.J.; Lebsack, A.D.; Liu, A.X.; Mirzadegan, T.; Nelen, M.I.; Patrick, A.N.; et al. Structural Basis of Small-Molecule Aggregate Induced Inhibition of a Protein-Protein Interaction. J. Med. Chem. 2017, 60, 3511–3517. [Google Scholar] [CrossRef]
- Chen, S.; Feng, Z.; Wang, Y.; Ma, S.; Hu, Z.; Yang, P.; Chai, Y.; Xie, X. Discovery of novel ligands for TNF-α and TNF receptor-1 through structure-based virtual screening and biological assay. J. Chem. Inf. Model. 2017, 57, 1101–1111. [Google Scholar] [CrossRef]
- Szmant, H.H. Physical properties of dimethyl sulfoxide and its function in biological systems. Ann. N. Y. Acad. Sci. 1975, 243, 20–23. [Google Scholar] [CrossRef]
- Sankpal, U.T.; Abdelrahim, M.; Connelly, S.F.; Lee, C.M.; Madero-Visbal, R.; Colon, J.; Smith, J.; Safe, S.; Maliakal, P.; Basha, R. Small molecule tolfenamic acid inhibits PC-3 cell proliferation and invasion in vitro, and tumor growth in orthotopic mouse model for prostate cancer. Prostate 2012, 72, 1648–1658. [Google Scholar] [CrossRef]
- Modesitt, S.C.; Parsons, S.J. In vitro and in vivo histone deacetylase inhibitor therapy with vorinostat and paclitaxel in ovarian cancer models: Does timing matter? Gynecol. Oncol. 2010, 119, 351–357. [Google Scholar] [CrossRef]
- Li, S.-Y.; Lo, A.C. Lutein protects RGC-5 cells against hypoxia and oxidative stress. Int. J. Mol. Sci. 2010, 11, 2109–2117. [Google Scholar] [CrossRef] [PubMed]
- Julien, C.; Marcouiller, F.; Bretteville, A.; El Khoury, N.B.; Baillargeon, J.; Hébert, S.S.; Planel, E. Dimethyl sulfoxide induces both direct and indirect tau hyperphosphorylation. PLoS ONE 2012, 7, e40020. [Google Scholar] [CrossRef] [PubMed]
- Hanslick, J.L.; Lau, K.; Noguchi, K.K.; Olney, J.W.; Zorumski, C.F.; Mennerick, S.; Farber, N.B. Dimethyl sulfoxide (DMSO) produces widespread apoptosis in the developing central nervous system. Neurobiol. Dis. 2009, 34, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, M.; Maini, R.N. Anti-TNFα therapy of rheumatoid arthritis: What have we learned? Ann. Rev. Immunol. 2001, 19, 163–196. [Google Scholar] [CrossRef]
- Bradley, J. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Pennica, D.; Nedwin, G.E.; Hayflick, J.S.; Seeburg, P.H.; Derynck, R.; Palladino, M.A.; Kohr, W.J.; Aggarwal, B.B.; Goeddel, D.V. Human tumour necrosis factor: Precursor structure, expression and homology to lymphotoxin. Nature 1984, 312, 724–729. [Google Scholar] [CrossRef]
- Kriegler, M.; Perez, C.; DeFay, K.; Albert, I.; Lu, S. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: Ramifications for the complex physiology of TNF. Cell 1988, 53, 45–53. [Google Scholar] [CrossRef]
- Luettig, B.; Decker, T.; Lohmann-Matthes, M. Evidence for the existence of two forms of membrane tumor necrosis factor: An integral protein and a molecule attached to its receptor. J. Immunol. 1989, 143, 4034–4038. [Google Scholar]
- Moss, M.L.; Jin, S.-L.C.; Milla, M.E.; Burkhart, W.; Carter, H.L.; Chen, W.-J.; Clay, W.C.; Didsbury, J.R.; Hassler, D.; Hoffman, C.R. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-α. Nature 1997, 385, 733–736. [Google Scholar] [CrossRef]
- Vandenabeele, P.; Declercq, W.; Beyaert, R.; Fiers, W. Two tumour necrosis factor receptors: Structure and function. Trends Cell Biol. 1995, 5, 392–399. [Google Scholar] [CrossRef]
- Tang, P.; Hung, M.-C.; Klostergaard, J. Human pro-tumor necrosis factor is a homotrimer. Biochemistry 1996, 35, 8216–8225. [Google Scholar] [CrossRef] [PubMed]
- Papaneophytou, C.P.; Mettou, A.K.; Rinotas, V.; Douni, E.; Kontopidis, G.A. Solvent selection for insoluble ligands, a challenge for biological assay development: A TNF-α/SPD304 study. ACS Med. Chem. Lett. 2013, 4, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Micheau, O.; Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 2003, 114, 181–190. [Google Scholar] [CrossRef]
- Beg, A.A.; Baltimore, D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science 1996, 274, 782–784. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Mayo, M.W.; Baldwin, A.S. TNF-and cancer therapy-induced apoptosis: Potentiation by inhibition of NF-κB. Science 1996, 274, 784–787. [Google Scholar] [CrossRef]
- Martin, S.; Lennon, S.; Bonham, A.; Cotter, T. Induction of apoptosis (programmed cell death) in human leukemic HL-60 cells by inhibition of RNA or protein synthesis. J. Immunol. 1990, 145, 1859–1867. [Google Scholar]
- Fulda, S.; Meyer, E.; Debatin, K.-M. Metabolic inhibitors sensitize for CD95 (APO-1/Fas)-induced apoptosis by down-regulating Fas-associated death domain-like interleukin 1-converting enzyme inhibitory protein expression. Cancer Res. 2000, 60, 3947–3956. [Google Scholar]
- Gozzelino, R.; Sole, C.; Llecha, N.; Segura, M.F.; Moubarak, R.S.; Iglesias-Guimarais, V.; Perez-Garcia, M.J.; Reix, S.; Zhang, J.; Badiola, N. BCL-X L regulates TNF-α-mediated cell death independently of NF-κB, FLIP and IAPs. Cell Res. 2008, 18, 1020–1036. [Google Scholar] [CrossRef]
- Leist, M.; Gantner, F.; Bohlinger, I.; Germann, P.G.; Tiegs, G.; Wendel, A. Murine hepatocyte apoptosis induced in vitro and in vivo by TNF-alpha requires transcriptional arrest. J. Immunol. 1994, 153, 1778–1788. [Google Scholar]
- Ma, L.; Gong, H.; Zhu, H.; Ji, Q.; Su, P.; Liu, P.; Cao, S.; Yao, J.; Jiang, L.; Han, M. A novel small-molecule tumor necrosis factor α inhibitor attenuates inflammation in a hepatitis mouse model. J. Biol. Chem. 2014, 289, 12457–12466. [Google Scholar] [CrossRef]
- Schuttelkopf, A.W.; van Aalten, D.M. PRODRG: A tool for high-throughput crystallography of protein-ligand complexes. Acta Cryst. D Biol. Cryst. 2004, 60, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- MOE. Molecular Operating Environment (MOE), 2013.08; Chemical Computing Group Inc.: Montreal, QC, Canada, 2017. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javaid, N.; Patra, M.C.; Seo, H.; Yasmeen, F.; Choi, S. A Rational Insight into the Effect of Dimethyl Sulfoxide on TNF-α Activity. Int. J. Mol. Sci. 2020, 21, 9450. https://doi.org/10.3390/ijms21249450
Javaid N, Patra MC, Seo H, Yasmeen F, Choi S. A Rational Insight into the Effect of Dimethyl Sulfoxide on TNF-α Activity. International Journal of Molecular Sciences. 2020; 21(24):9450. https://doi.org/10.3390/ijms21249450
Chicago/Turabian StyleJavaid, Nasir, Mahesh Chandra Patra, Hana Seo, Farzana Yasmeen, and Sangdun Choi. 2020. "A Rational Insight into the Effect of Dimethyl Sulfoxide on TNF-α Activity" International Journal of Molecular Sciences 21, no. 24: 9450. https://doi.org/10.3390/ijms21249450
APA StyleJavaid, N., Patra, M. C., Seo, H., Yasmeen, F., & Choi, S. (2020). A Rational Insight into the Effect of Dimethyl Sulfoxide on TNF-α Activity. International Journal of Molecular Sciences, 21(24), 9450. https://doi.org/10.3390/ijms21249450






