Non-Thermal Plasma—A New Green Priming Agent for Plants?
Abstract
:1. Introduction
2. Characterization of Non-Thermal Plasma
3. Interaction of Non-Thermal Plasma with Biological Material
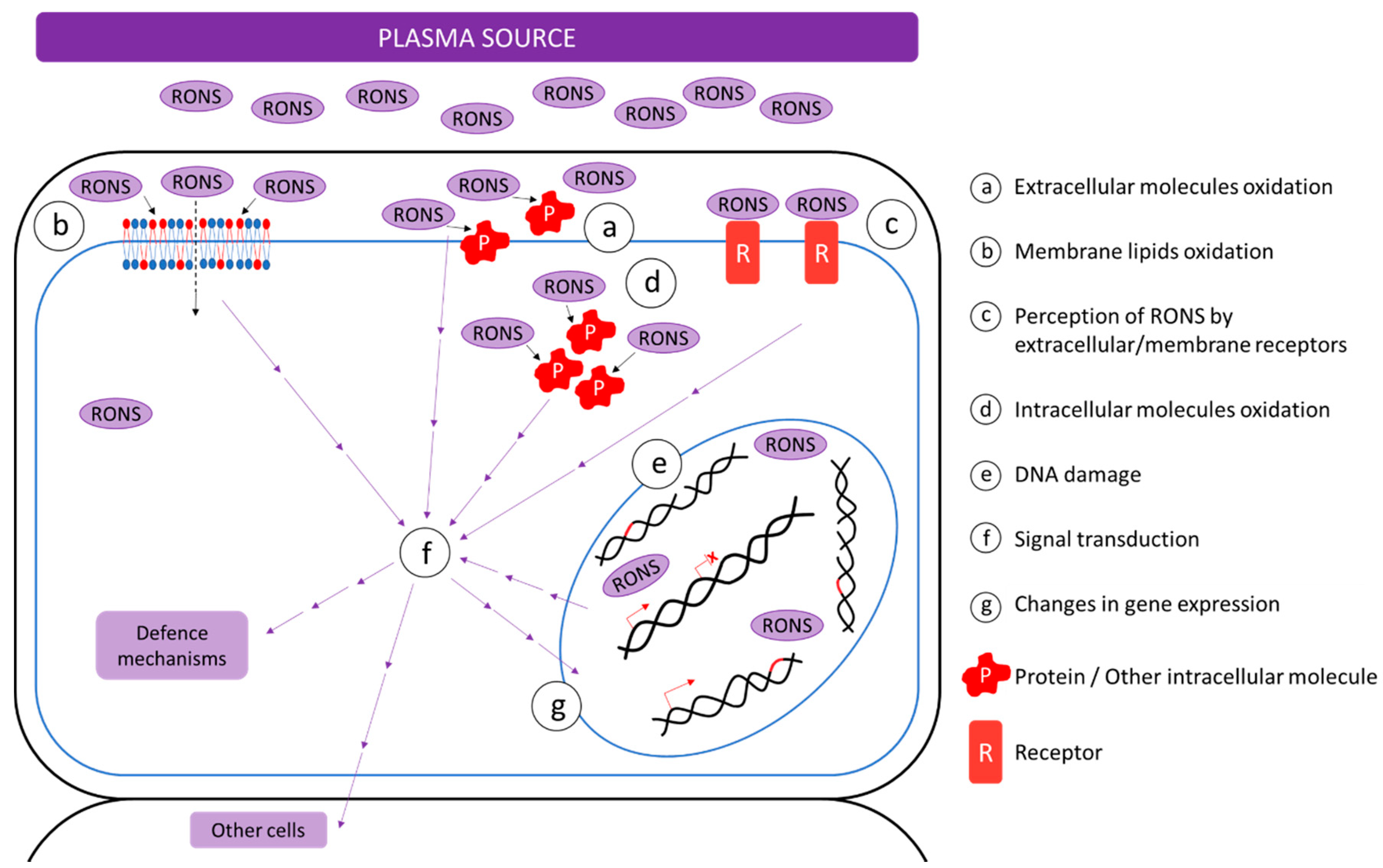
3.1. Effect of NTP on Cells
3.2. Effect of NTP on Plant Physiological and Biochemical Parameters
3.3. Changes of Plant Antioxidant Capacity after NTP-Treatment
3.4. Changes in Plant Gene Expression after the NTP-Treatment
3.5. Genotoxic Effects of NTP in Plants
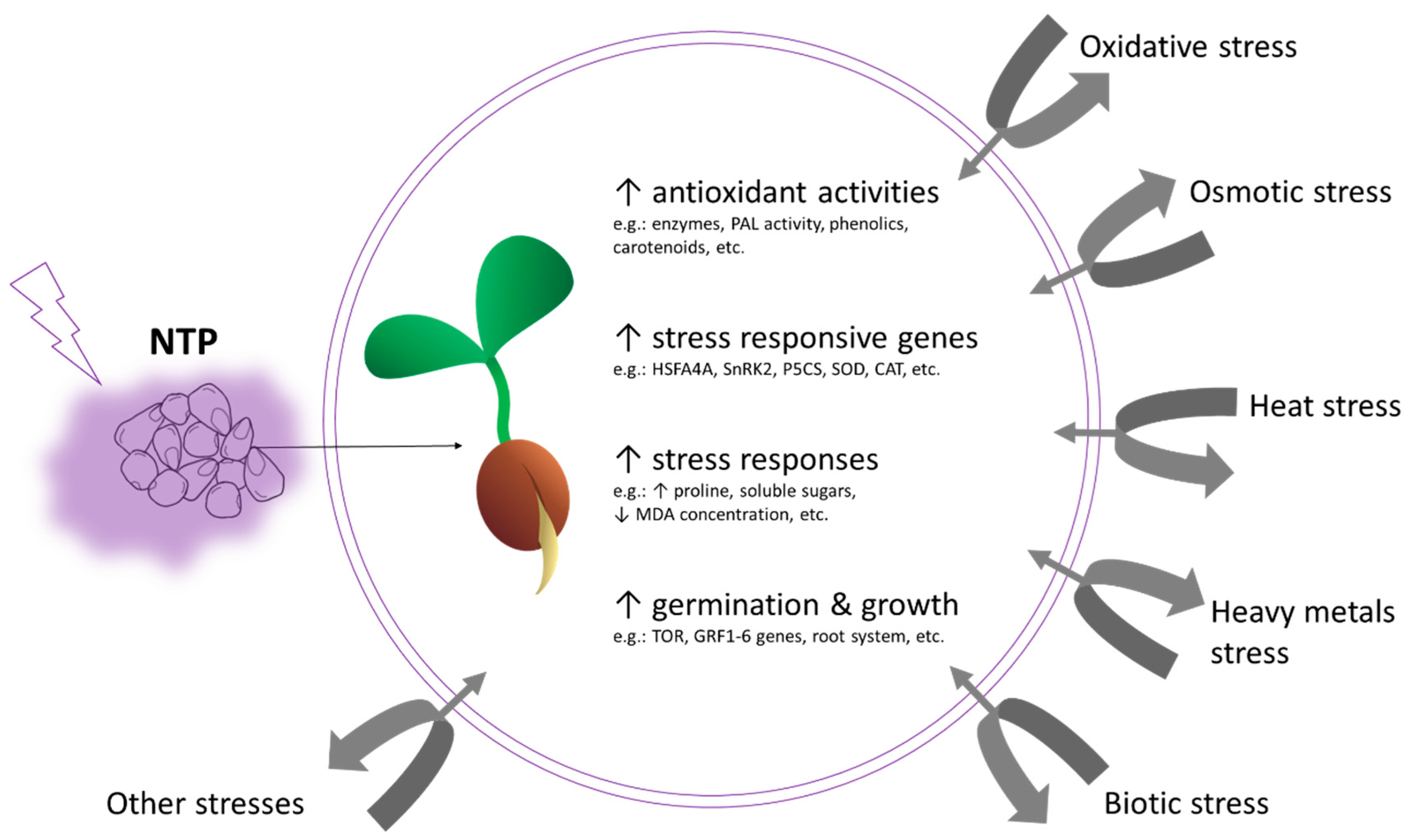
4. Non-Thermal Plasma as an Inductor of Adaptive Response to Abiotic Stress
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chukhlantsev, K.I. Development of Plasma-Generating Devices and History of Plasma Utilization. In Proceedings of the International Student Scientific Conference Poster—22/2018, Prague, Czech Republic, 10 May 2018; Libor Husník; Czech Technical University in Prague: Prague, Czech Republic, 2018; HS01. [Google Scholar]
- Isbary, G.; Zimmermann, J.L.; Shimizu, T.; Li, Y.-F.; Morfill, G.E.; Thomas, H.M.; Steffes, B.; Heinlin, J.; Karrer, S.; Stolz, W. Non-Thermal Plasma—More than Five Years of Clinical Experience. Clin. Plasma Med. 2013, 1, 19–23. [Google Scholar] [CrossRef]
- Štěpánová, V.; Slavíček, P.; Kelar, J.; Prášil, J.; Smékal, M.; Stupavská, M.; Jurmanová, J.; Černák, M. Atmospheric Pressure Plasma Treatment of Agricultural Seeds of Cucumber (Cucumis Sativus L.) and Pepper (Capsicum Annuum L.) with Effect on Reduction of Diseases and Germination Improvement. Plasma Process. Polym. 2018, 15, 1700076. [Google Scholar] [CrossRef]
- Sera, B.; Gajdova, I.; Cernak, M.; Gavril, B.; Hnatiuc, E.; Kovacik, D.; Kriha, V.; Slama, J.; Sery, M.; Spatenka, P. How various plasma sources may affect seed germination and growth. In Proceedings of the 2012 13th International Conference on Optimization of Electrical and Electronic Equipment (OPTIM), Brasov, Romania, 24–26 May 2012; IEEE: Brasov, Romania, 2012; pp. 1365–1370. [Google Scholar] [CrossRef]
- Kim, K.C.; Piao, M.J.; Hewage, S.R.K.M.; Han, X.; Kang, K.A.; Jo, J.O.; Mok, Y.S.; Shin, J.H.; Park, Y.; Yoo, S.J.; et al. Non-Thermal Dielectric-Barrier Discharge Plasma Damages Human Keratinocytes by Inducing Oxidative Stress. Int. J. Mol. Med. 2016, 37, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastor, V.; Luna, E.; Mauch-Mani, B.; Ton, J.; Flors, V. Primed Plants Do Not Forget. Environ. Exp. Bot. 2013, 94, 46–56. [Google Scholar] [CrossRef]
- Dhanya Thomas, T.T.; Puthur, J.T. UV Radiation Priming: A Means of Amplifying the Inherent Potential for Abiotic Stress Tolerance in Crop Plants. Environ. Exp. Bot. 2017, 138, 57–66. [Google Scholar] [CrossRef]
- Piel, A. Plasma Physics, 2nd ed.; Graduate Texts in Physics; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Whitehead, J.C. The Chemistry of Cold Plasma. In Cold Plasma in Food and Agriculture; Academic Press, Ltd.: San Diego, CA, USA, 2016; pp. 53–81. [Google Scholar] [CrossRef]
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive Species in Non-Equilibrium Atmospheric-Pressure Plasmas: Generation, Transport, and Biological Effects. Phys. Rep. 2016, 630, 1–84. [Google Scholar] [CrossRef] [Green Version]
- Misra, N.N.; Yepez, X.; Xu, L.; Keener, K. In-Package Cold Plasma Technologies. J. Food Eng. 2019, 244, 21–31. [Google Scholar] [CrossRef]
- Bárdos, L.; Baránková, H. Plasma Processes at Atmospheric and Low Pressures. Vacuum 2008, 83, 522–527. [Google Scholar] [CrossRef]
- López, M.; Calvo, T.; Prieto, M.; Múgica-Vidal, R.; Muro-Fraguas, I.; Alba-Elías, F.; Alvarez-Ordóñez, A. A Review on Non-Thermal Atmospheric Plasma for Food Preservation: Mode of Action, Determinants of Effectiveness, and Applications. Front. Microbiol. 2019, 10, 622. [Google Scholar] [CrossRef]
- Reuter, S.; von Woedtke, T.; Weltmann, K.-D. The KINPen—A Review on Physics and Chemistry of the Atmospheric Pressure Plasma Jet and Its Applications. J. Phys. Appl. Phys. 2018, 51, 233001. [Google Scholar] [CrossRef] [Green Version]
- Arjunan, K.; Sharma, V.; Ptasinska, S. Effects of Atmospheric Pressure Plasmas on Isolated and Cellular DNA—A Review. Int. J. Mol. Sci. 2015, 16, 2971–3016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric Pressure Plasmas: A Review. Spectrochim. Acta Part B At. Spectrosc. 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Deynse, A.V.; Morent, R.; Geyter, N.D. Surface modification of polymers using atmospheric pressure cold plasma technology. In Polymer Science: Research Advances, Practical Applications and Educational Aspects; Formatex Research Center: Badajoz, Spain, 2016; pp. 506–516. [Google Scholar]
- Puač, N.; Gherardi, M.; Shiratani, M. Plasma Agriculture: A Rapidly Emerging Field. Plasma Process. Polym. 2018, 15, 1700174. [Google Scholar] [CrossRef]
- Surowsky, B.; Schlüter, O.; Knorr, D. Interactions of Non-Thermal Atmospheric Pressure Plasma with Solid and Liquid Food Systems: A Review. Food Eng. Rev. 2015, 7, 82–108. [Google Scholar] [CrossRef]
- Dojčinović, B.P.; Manojlović, D.; Roglić, G.M.; Obradović, B.M.; Kuraica, M.M.; Purić, J. Plasma Assisted Degradation of Phenol Solutions. Vacuum 2008, 83, 234–237. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, H.; Zhang, Q.; Huang, Q. Degradation of Norfloxacin in Aqueous Solution by Atmospheric-Pressure Non-Thermal Plasma: Mechanism and Degradation Pathways. Chemosphere 2018, 210, 433–439. [Google Scholar] [CrossRef]
- von Woedtke, T.; Reuter, S.; Masur, K.; Weltmann, K.-D. Plasmas for Medicine. Phys. Rep. 2013, 530, 291–320. [Google Scholar] [CrossRef]
- Graves, D.B. Oxy-Nitroso Shielding Burst Model of Cold Atmospheric Plasma Therapeutics. Clin. Plasma Med. 2014, 2, 38–49. [Google Scholar] [CrossRef] [Green Version]
- Szili, E.J.; Hong, S.-H.; Oh, J.-S.; Gaur, N.; Short, R.D. Tracking the Penetration of Plasma Reactive Species in Tissue Models. Trends Biotechnol. 2018, 36, 594–602. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, B.C.; Chang, C.J. Chemistry and Biology of Reactive Oxygen Species in Signaling or Stress Responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.-H.; Szili, E.J.; Jenkins, A.T.A.; Short, R.D. Ionized Gas (Plasma) Delivery of Reactive Oxygen Species (ROS) into Artificial Cells. J. Phys. Appl. Phys. 2014, 47, 362001. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Chi, Y.; Jiang, Z.; Xu, Y.; Xie, L.; Huang, F.; Wan, D.; Ni, J.; Yuan, F.; Wu, X.; et al. Hydrogen Peroxide Sensor HPCA1 Is an LRR Receptor Kinase in Arabidopsis. Nature 2020, 578, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Naviaux, R.K. Oxidative Shielding or Oxidative Stress? J. Pharmacol. Exp. Ther. 2012, 342, 608–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tero, R.; Yamashita, R.; Hashizume, H.; Suda, Y.; Takikawa, H.; Hori, M.; Ito, M. Nanopore Formation Process in Artificial Cell Membrane Induced by Plasma-Generated Reactive Oxygen Species. Arch. Biochem. Biophys. 2016, 605, 26–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Dios Alché, J. A Concise Appraisal of Lipid Oxidation and Lipoxidation in Higher Plants. Redox Biol. 2019, 23, 101136. [Google Scholar] [CrossRef]
- Ki, S.H.; Park, J.K.; Sung, C.; Lee, C.B.; Uhm, H.; Choi, E.H.; Baik, K.Y. Artificial Vesicles as an Animal Cell Model for the Study of Biological Application of Non-Thermal Plasma. J. Phys. Appl. Phys. 2016, 49, 085401. [Google Scholar] [CrossRef]
- Wende, K.; Landsberg, K.; Lindequist, U.; Weltmann, K.-D.; von Woedtke, T. Distinctive Activity of a Nonthermal Atmospheric-Pressure Plasma Jet on Eukaryotic and Prokaryotic Cells in a Cocultivation Approach of Keratinocytes and Microorganisms. IEEE Trans. Plasma Sci. 2010, 38, 2479–2485. [Google Scholar] [CrossRef]
- Morrison, K.A.; Akintayo, R.; Jin, J.; Kaymakcalan, O.; Weinreb, R.; Dong, X.; Westblade, L.F.; Golkowski, C.; Spector, J.A. Abstract: Non-Thermal Plasma Treatment Safely and Rapidly Eradicates MRSA from Infected Wounds. Plast. Reconstr. Surg.-Glob. Open 2016, 4, 52–53. [Google Scholar] [CrossRef]
- Lunov, O.; Zablotskii, V.; Churpita, O.; Jäger, A.; Polívka, L.; Syková, E.; Dejneka, A.; Kubinová, Š. The Interplay between Biological and Physical Scenarios of Bacterial Death Induced by Non-Thermal Plasma. Biomaterials 2016, 82, 71–83. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A.B. Gram Positive and Gram Negative Bacteria Differ in Their Sensitivity to Cold Plasma. Sci. Rep. 2016, 6, 38610. [Google Scholar] [CrossRef] [Green Version]
- Zahoranová, A.; Hoppanová, L.; Šimončicová, J.; Tučeková, Z.; Medvecká, V.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Černák, M. Effect of Cold Atmospheric Pressure Plasma on Maize Seeds: Enhancement of Seedlings Growth and Surface Microorganisms Inactivation. Plasma Chem. Plasma Process. 2018, 38, 969–988. [Google Scholar] [CrossRef]
- Zahoranová, A.; Henselová, M.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Medvecká, V.; Černák, M. Effect of Cold Atmospheric Pressure Plasma on the Wheat Seedlings Vigor and on the Inactivation of Microorganisms on the Seeds Surface. Plasma Chem. Plasma Process. 2016, 36, 397–414. [Google Scholar] [CrossRef]
- Šerá, B.; Zahoranová, A.; Bujdáková, H.; Šerý, M. Disinfection from Pine Seeds Contaminated with Fusarium Circinatum Nirenberg & O’Donell Using Non-Thermal Plasma Treatment. Rom. Rep. Phys. 2019, 71, 1–12. [Google Scholar]
- Zhang, X.; Liu, D.; Zhou, R.; Song, Y.; Sun, Y.; Zhang, Q.; Niu, J.; Fan, H.; Yang, S. Atmospheric Cold Plasma Jet for Plant Disease Treatment. Appl. Phys. Lett. 2014, 104, 043702. [Google Scholar] [CrossRef]
- Babajani, A.; Iranbakhsh, A.; Oraghi Ardebili, Z.; Eslami, B. Seed Priming with Non-Thermal Plasma Modified Plant Reactions to Selenium or Zinc Oxide Nanoparticles: Cold Plasma as a Novel Emerging Tool for Plant Science. Plasma Chem. Plasma Process. 2019, 39, 21–34. [Google Scholar] [CrossRef]
- Seddighinia, F.S.; Iranbakhsh, A.; Oraghi Ardebili, Z.; Nejad Satari, T.; Soleimanpour, S. Seed Priming with Cold Plasma and Multi-Walled Carbon Nanotubes Modified Growth, Tissue Differentiation, Anatomy, and Yield in Bitter Melon (Momordica charantia). J. Plant Growth Regul. 2020, 39, 87–98. [Google Scholar] [CrossRef]
- Abedi, S.; Iranbakhsh, A.; Oraghi Ardebili, Z.; Ebadi, M. Seed Priming with Cold Plasma Improved Early Growth, Flowering, and Protection of Cichorium intybus against Selenium Nanoparticle. J. Theor. Appl. Phys. 2020, 14, 113–119. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Zhang, H.; Qu, G.; Wang, T.; Sun, Q.; Liang, D. Alleviation of Adverse Effects of Drought Stress on Wheat Seed Germination Using Atmospheric Dielectric Barrier Discharge Plasma Treatment. Sci. Rep. 2017, 7, 16680. [Google Scholar] [CrossRef] [Green Version]
- Iranbakhsh, A.; Ardebili, N.O.; Ardebili, Z.O.; Shafaati, M.; Ghoranneviss, M. Non-Thermal Plasma Induced Expression of Heat Shock Factor A4A and Improved Wheat (Triticum aestivum L.) Growth and Resistance against Salt Stress. Plasma Chem. Plasma Process. 2018, 38, 29–44. [Google Scholar] [CrossRef]
- Kabir, A.H.; Rahman, M.M.; Das, U.; Sarkar, U.; Roy, N.C.; Reza, M.A.; Talukder, M.R.; Uddin, M.A. Reduction of Cadmium Toxicity in Wheat through Plasma Technology. PLoS ONE 2019, 14, e0214509. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Qu, G.; Wang, T.; Sun, Q.; Liang, D.; Hu, S. Enhancement of Germination and Seedling Growth of Wheat Seed Using Dielectric Barrier Discharge Plasma with Various Gas Sources. Plasma Chem. Plasma Process. 2017, 37, 1105–1119. [Google Scholar] [CrossRef]
- Iranbakhsh, A.; Oraghi Ardebili, Z.; Oraghi Ardebili, N.; Ghoranneviss, M.; Safari, N. Cold Plasma Relieved Toxicity Signs of Nano Zinc Oxide in Capsicum Annuum Cayenne via Modifying Growth, Differentiation, and Physiology. Acta Physiol. Plant. 2018, 40, 154. [Google Scholar] [CrossRef]
- Kyzek, S.; Holubová, Ľ.; Medvecká, V.; Tomeková, J.; Gálová, E.; Zahoranová, A. Cold Atmospheric Pressure Plasma Can Induce Adaptive Response in Pea Seeds. Plasma Chem. Plasma Process. 2019, 39, 475–486. [Google Scholar] [CrossRef]
- Švubová, R.; Kyzek, S.; Medvecká, V.; Slováková, Ľ.; Gálová, E.; Zahoranová, A. Novel Insight at the Effect of Cold Atmospheric Pressure Plasma on the Activity of Enzymes Essential for the Germination of Pea (Pisum sativum L. Cv. Prophet) Seeds. Plasma Chem. Plasma Process. 2020, 40, 1221–1240. [Google Scholar] [CrossRef]
- Sera, B.; Sery, M.; Gavril, B.; Gajdova, I. Seed Germination and Early Growth Responses to Seed Pre-Treatment by Non-Thermal Plasma in Hemp Cultivars (Cannabis sativa L.). Plasma Chem. Plasma Process. 2017, 37, 207–221. [Google Scholar] [CrossRef]
- Ling, L.; Jiangang, L.; Minchong, S.; Chunlei, Z.; Yuanhua, D. Cold Plasma Treatment Enhances Oilseed Rape Seed Germination under Drought Stress. Sci. Rep. 2015, 5, 13033. [Google Scholar] [CrossRef]
- Bormashenko, E.; Shapira, Y.; Grynyov, R.; Whyman, G.; Bormashenko, Y.; Drori, E. Interaction of Cold Radiofrequency Plasma with Seeds of Beans (Phaseolus vulgaris). J. Exp. Bot. 2015, 66, 4013–4021. [Google Scholar] [CrossRef] [Green Version]
- Alves Junior, C.; de Oliveira Vitoriano, J.; da Silva, D.L.S.; de Lima Farias, M.; de Lima Dantas, N.B. Water Uptake Mechanism and Germination of Erythrina velutina Seeds Treated with Atmospheric Plasma. Sci. Rep. 2016, 6, 33722. [Google Scholar] [CrossRef]
- Bormashenko, E.; Grynyov, R.; Bormashenko, Y.; Drori, E. Cold Radiofrequency Plasma Treatment Modifies Wettability and Germination Speed of Plant Seeds. Sci. Rep. 2012, 2, 741. [Google Scholar] [CrossRef]
- de Groot, G.J.J.B.; Hundt, A.; Murphy, A.B.; Bange, M.P.; Mai-Prochnow, A. Cold Plasma Treatment for Cotton Seed Germination Improvement. Sci. Rep. 2018, 8, 14372. [Google Scholar] [CrossRef] [Green Version]
- Khamsen, N.; Onwimol, D.; Teerakawanich, N.; Dechanupaprittha, S.; Kanokbannakorn, W.; Hongesombut, K.; Srisonphan, S. Rice (Oryza sativa L.) Seed Sterilization and Germination Enhancement via Atmospheric Hybrid Nonthermal Discharge Plasma. ACS Appl. Mater. Interfaces 2016, 8, 19268–19275. [Google Scholar] [CrossRef] [PubMed]
- Sadhu, S.; Thirumdas, R.; Deshmukh, R.R.; Annapure, U.S. Influence of Cold Plasma on the Enzymatic Activity in Germinating Mung Beans (Vigna radiate). LWT 2017, 78, 97–104. [Google Scholar] [CrossRef]
- Dobrynin, D.; Fridman, G.; Friedman, G.; Fridman, A. Physical and Biological Mechanisms of Direct Plasma Interaction with Living Tissue. New J. Phys. 2009, 11, 115020. [Google Scholar] [CrossRef]
- Li, H.-P.; Wang, L.-Y.; Li, G.; Jin, L.-H.; Le, P.-S.; Zhao, H.-X.; Xing, X.-H.; Bao, C.-Y. Manipulation of Lipase Activity by the Helium Radio-Frequency, Atmospheric-Pressure Glow Discharge Plasma Jet. Plasma Process. Polym. 2011, 8, 224–229. [Google Scholar] [CrossRef]
- Ling, L.; Jiafeng, J.; Jiangang, L.; Minchong, S.; Xin, H.; Hanliang, S.; Yuanhua, D. Effects of Cold Plasma Treatment on Seed Germination and Seedling Growth of Soybean. Sci. Rep. 2014, 4, 5859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.J.; Jo, J.O.; Huynh, D.L.; Mongre, R.K.; Ghosh, M.; Singh, A.K.; Lee, S.B.; Mok, Y.S.; Hyuk, P.; Jeong, D.K. Growth-Inducing Effects of Argon Plasma on Soybean Sprouts via the Regulation of Demethylation Levels of Energy Metabolism-Related Genes. Sci. Rep. 2017, 7, 41917. [Google Scholar] [CrossRef] [PubMed]
- Shapira, Y.; Bormashenko, E.; Drori, E. Pre-Germination Plasma Treatment of Seeds Does Not Alter Cotyledon DNA Structure, nor Phenotype and Phenology of Tomato and Pepper Plants. Biochem. Biophys. Res. Commun. 2019, 519, 512–517. [Google Scholar] [CrossRef]
- Meiqiang, Y.; Mingjing, H.; Buzhou, M.; Tengcai, M. Stimulating Effects of Seed Treatment by Magnetized Plasma on Tomato Growth and Yield. Plasma Sci. Technol. 2005, 7, 3143–3147. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Shen, M.; Hou, J.; Shao, H.; Dong, Y.; Jiang, J. Improving Seed Germination and Peanut Yields by Cold Plasma Treatment. Plasma Sci. Technol. 2016, 18, 1027–1033. [Google Scholar] [CrossRef] [Green Version]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as Key Players in Plant Stress Signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Noormohammadi, Z.; Mohammadzadeh-Shahir, M.; Fahmi, D.; Atyabi, S.M.; Farahani, F. Induced Genetic and Morphological Changes in Catharanthus roseus L. by Cold Atmospheric Plasma. Nova Biol. Reper. 2019, 6, 302–310. [Google Scholar] [CrossRef] [Green Version]
- Henselová, M.; Slováková, Ľ.; Martinka, M.; Zahoranová, A. Growth, Anatomy and Enzyme Activity Changes in Maize Roots Induced by Treatment of Seeds with Low-Temperature Plasma. Biologia 2012, 67. [Google Scholar] [CrossRef]
- Tong, J.; He, R.; Zhang, X.; Zhan, R.; Chen, W.; Yang, S. Effects of Atmospheric Pressure Air Plasma Pretreatment on the Seed Germination and Early Growth of Andrographis paniculata. Plasma Sci. Technol. 2014, 16, 260–266. [Google Scholar] [CrossRef] [Green Version]
- Wada, K.C.; Mizuuchi, K.; Koshio, A.; Kaneko, K.; Mitsui, T.; Takeno, K. Stress Enhances the Gene Expression and Enzyme Activity of Phenylalanine Ammonia-Lyase and the Endogenous Content of Salicylic Acid to Induce Flowering in Pharbitis. J. Plant Physiol. 2014, 171, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A Review of Recent Studies on Malondialdehyde as Toxic Molecule and Biological Marker of Oxidative Stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.; Chételat, A.; Reymond, P.; Farmer, E.E. Selective and Powerful Stress Gene Expression in Arabidopsis in Response to Malondialdehyde. Plant J. 2004, 37, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Mildaziene, V.; Pauzaite, G.; Naucienė, Z.; Malakauskiene, A.; Zukiene, R.; Januskaitiene, I.; Jakstas, V.; Ivanauskas, L.; Filatova, I.; Lyushkevich, V. Pre-Sowing Seed Treatment with Cold Plasma and Electromagnetic Field Increases Secondary Metabolite Content in Purple Coneflower (Echinacea purpurea) Leaves. Plasma Process. Polym. 2018, 15, 1700059. [Google Scholar] [CrossRef]
- Al-Whaibi, M.H. Plant Heat-Shock Proteins: A Mini Review. J. King Saud Univ.-Sci. 2011, 23, 139–150. [Google Scholar] [CrossRef] [Green Version]
- Scharf, K.-D.; Berberich, T.; Ebersberger, I.; Nover, L. The Plant Heat Stress Transcription Factor (Hsf) Family: Structure, Function and Evolution. Biochim. Biophys. Acta BBA-Gene Regul. Mech. 2012, 1819, 104–119. [Google Scholar] [CrossRef]
- Pérez-Salamó, I.; Papdi, C.; Rigó, G.; Zsigmond, L.; Vilela, B.; Lumbreras, V.; Nagy, I.; Horváth, B.; Domoki, M.; Darula, Z.; et al. The Heat Shock Factor A4A Confers Salt Tolerance and Is Regulated by Oxidative Stress and the Mitogen-Activated Protein Kinases MPK3 and MPK6. Plant Physiol. 2014, 165, 319–334. [Google Scholar] [CrossRef] [Green Version]
- Kulik, A.; Wawer, I.; Krzywińska, E.; Bucholc, M.; Dobrowolska, G. SnRK2 Protein Kinases—Key Regulators of Plant Response to Abiotic Stresses. OMICS J. Integr. Biol. 2011, 15, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Tomeková, J.; Kyzek, S.; Medvecká, V.; Gálová, E.; Zahoranová, A. Influence of Cold Atmospheric Pressure Plasma on Pea Seeds: DNA Damage of Seedlings and Optical Diagnostics of Plasma. Plasma Chem. Plasma Process. 2020. [Google Scholar] [CrossRef]
- Zhu, J.-K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittler, R. Abiotic Stress, the Field Environment and Stress Combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Hilker, M.; Schwachtje, J.; Baier, M.; Balazadeh, S.; Bäurle, I.; Geiselhardt, S.; Hincha, D.K.; Kunze, R.; Mueller-Roeber, B.; Rillig, M.C.; et al. Priming and Memory of Stress Responses in Organisms Lacking a Nervous System: Priming and Memory of Stress Responses. Biol. Rev. 2016, 91, 1118–1133. [Google Scholar] [CrossRef]
- Esposito, G.; Campa, A.; Pinto, M.; Simone, G.; Tabocchini, M.A.; Belli, M. Adaptive Response: Modelling and Experimental Studies. Radiat. Prot. Dosim. 2011, 143, 320–324. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Hormesis: A Compelling Platform for Sophisticated Plant Science. Trends Plant Sci. 2019, 24, 318–327. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.M.; Langenbach, C.J.G.; Jaskiewicz, M.R. Priming for Enhanced Defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef]
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.J.; Pozo, M.J.; Ton, J.; van Dam, N.M.; Conrath, U. Recognizing Plant Defense Priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef] [Green Version]
- Whittle, C.A.; Otto, S.P.; Johnston, M.O.; Krochko, J.E. Adaptive Epigenetic Memory of Ancestral Temperature Regime in Arabidopsis Thaliana This Paper Is One of a Selection of Papers Published in a Special Issue from the National Research Council of Canada—Plant Biotechnology Institute. Botany 2009, 87, 650–657. [Google Scholar] [CrossRef] [Green Version]
- Nisa, M.-U.; Huang, Y.; Benhamed, M.; Raynaud, C. The Plant DNA Damage Response: Signaling Pathways Leading to Growth Inhibition and Putative Role in Response to Stress Conditions. Front. Plant Sci. 2019, 10, 653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Adhikari, B.C.; Park, G.; Choi, E.H. Cold Plasma Seed Priming Modulates Growth, Redox Homeostasis and Stress Response by Inducing Reactive Species in Tomato (Solanum lycopersicum). Free Radic. Biol. Med. 2020, 156, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Gierczik, K.; Vukušić, T.; Kovács, L.; Székely, A.; Szalai, G.; Milošević, S.; Kocsy, G.; Kutasi, K.; Galiba, G. Plasma-activated Water to Improve the Stress Tolerance of Barley. Plasma Process. Polym. 2020, 17, 1900123. [Google Scholar] [CrossRef]
- Bafoil, M.; Le Ru, A.; Merbahi, N.; Eichwald, O.; Dunand, C.; Yousfi, M. New Insights of Low-Temperature Plasma Effects on Germination of Three Genotypes of Arabidopsis Thaliana Seeds under Osmotic and Saline Stresses. Sci. Rep. 2019, 9, 8649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holubová, Ľ.; Kyzek, S.; Ďurovcová, I.; Fabová, J.; Horváthová, E.; Ševčovičová, A.; Gálová, E. Non-Thermal Plasma—A New Green Priming Agent for Plants? Int. J. Mol. Sci. 2020, 21, 9466. https://doi.org/10.3390/ijms21249466
Holubová Ľ, Kyzek S, Ďurovcová I, Fabová J, Horváthová E, Ševčovičová A, Gálová E. Non-Thermal Plasma—A New Green Priming Agent for Plants? International Journal of Molecular Sciences. 2020; 21(24):9466. https://doi.org/10.3390/ijms21249466
Chicago/Turabian StyleHolubová, Ľudmila, Stanislav Kyzek, Ivana Ďurovcová, Jana Fabová, Eva Horváthová, Andrea Ševčovičová, and Eliška Gálová. 2020. "Non-Thermal Plasma—A New Green Priming Agent for Plants?" International Journal of Molecular Sciences 21, no. 24: 9466. https://doi.org/10.3390/ijms21249466




