Heme Degradation in Pathophysiology of and Countermeasures to Inflammation-Associated Disease
Abstract
1. Heme Structure, Function and Chemistry
2. Tissue Deposition and Degradation of Heme
3. Representative Aberrant Hemoglobin and Heme Degradation Pathway
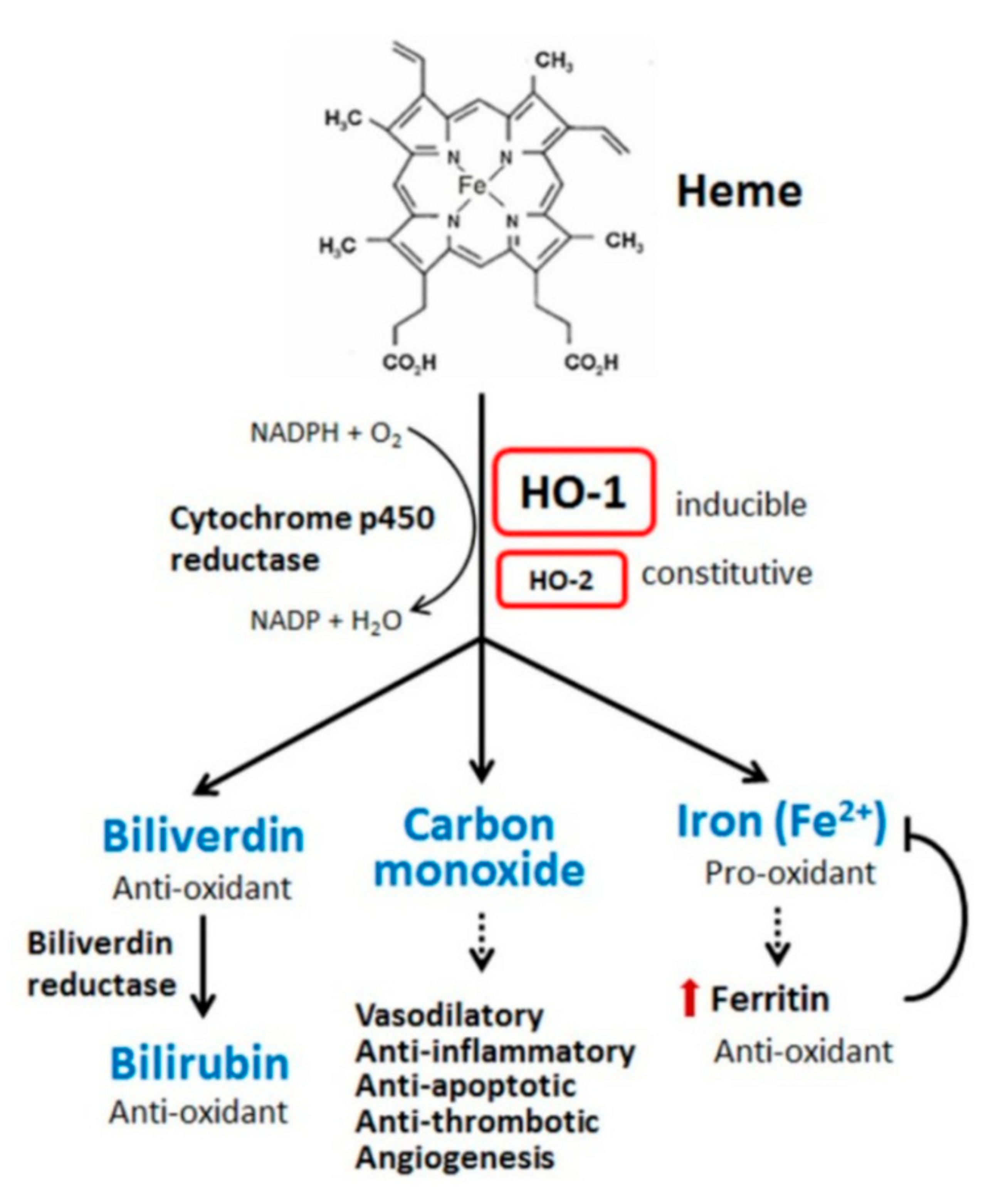
4. Heme Oxygenase, Cytoprotection and Therapeutic Potential
5. Haptoglobin and Hemopexin: Extracellular Countermeasures to Free Hemoglobin and Heme
6. Heme Degradation Products: Impact on Normal Physiologic Function, Disease Risk and Pathogenesis
7. Biliverdin and Bilirubin
8. Phycocyanobilin: An Algal Bilirubin Analog
9. CO, Iron and Ferritin
10. Heme Degradation Deficiency-Associated Tissue Damage and Major Disease Risks
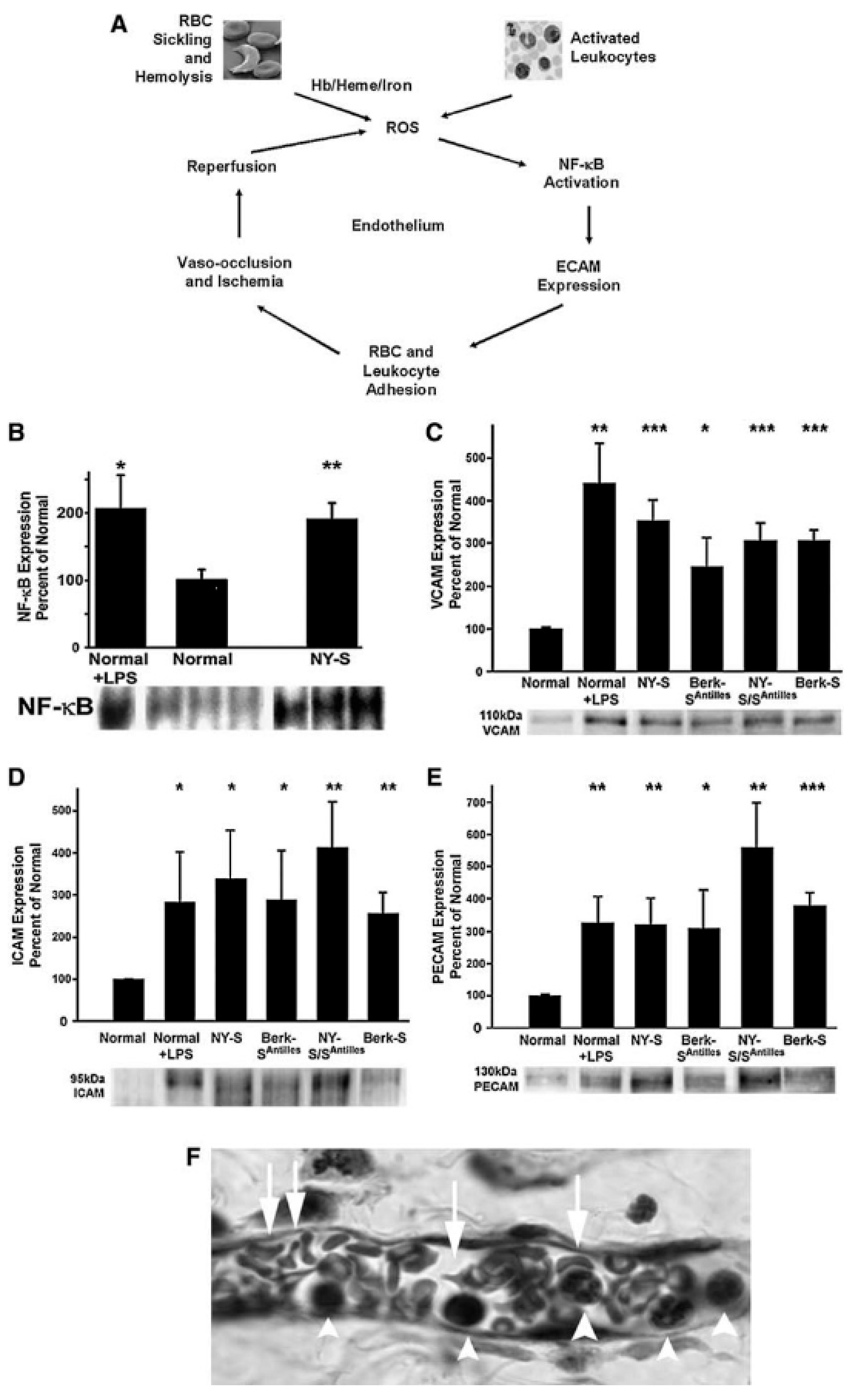
11. Heme Degradation Deficiency and Vascular Tissue Injury: The Sickling Disease Paradigm
12. Augmentation of Heme Degradation Mechanisms and Cardiovascular Health
13. HO-1, CO, Arrhythmias and Sudden Cardiac Death
14. Role of the Kidney in Heme Clearance and Relationship of Renal Tissue to Hemolytic Disease
15. Pulmonary Function and Heme-Related Pathologies
16. Neurological Function and Heme-Related Pathologies
17. Phytochemical Enhancement of Heme Degradation in Prevention of and Therapy for Inflammatory Pathologies: Evolving Clinical Strategies
18. Ginkgo Biloba, “Ginkgoflavonoids” and HO-1
19. Limitations
20. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Ni, M.; Jirt, J.; Koata, B.; Jenkins, A.; McNaught, A. IUPAC Compendium of Chemical Terminology; IUPAC: Piedmont, NC, USA, 2009. [Google Scholar]
- David, L.N.; Michael, M.C. Lehninger, Principles of Biochemistry, 3rd ed.; Worth Publishers: New York, NY, USA, 2000. [Google Scholar]
- Paoli, M.; Marles-Wright, J.; Smith, A. Structure–Function Relationships in Heme-Proteins. DNA Cell Biol. 2002, 21, 271–280. [Google Scholar] [CrossRef]
- Milani, M.; Pesce, A.; Nardini, M.; Ouellet, H.; Ouellet, Y.; Dewilde, S.; Bocedi, A.; Ascenzi, P.; Guertin, M.; Moens, L. Structural bases for heme binding and diatomic ligand recognition in truncated hemoglobins. J. Inorg. Biochem. 2005, 99, 97–109. [Google Scholar] [CrossRef]
- Bohr, C.; Hasselbalch, K.; Krogh, A. Concerning a biologically important relationship—The influence of the carbon dioxide content of blood on its oxygen binding. Skand. Arch. Physiol. 1904, 401–412. [Google Scholar]
- Thom, C.S.; Dickson, C.F.; Gell, D.A.; Weiss, M.J. Hemoglobin Variants: Biochemical Properties and Clinical Correlates. Cold Spring Harb. Perspect. Med. 2013, 3, a011858. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.-K.; Chen, S.-E.; Chang, L.-C. A Dual Role of Heme Oxygenase-1 in Cancer Cells. Int. J. Mol. Sci. 2018, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Belcher, J.D.; Beckman, J.D.; Balla, G.; Balla, J.; Vercellotti, G.M. Heme Degradation and Vascular Injury. Antioxid. Redox Signal. 2010, 12, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bandyopadhyay, U. Free heme toxicity and its detoxification systems in human. Toxicol. Lett. 2005, 157, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Barton, S.; Rampton, D.S.; Winrow, V.; Domizio, P.; Feakins, R. Expression of heat shock protein 32 (hemoxygenase-1) in the normal and inflamed human stomach and colon: An immunohistochemical study. Cell Stress Chaperon 2003, 8, 329–334. [Google Scholar] [CrossRef]
- Haines, D.D.; Tosaki, A. Emerging Clinical Applications of Heme Oxygenase. Curr. Pharm. Des. 2018, 24, 2227–2228. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxidative Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef]
- Tosaki, A.; Das, D.K. The role of heme oxygenase signaling in various disorders. Mol. Cell. Biochem. 2002, 232, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Csepanyi, E.; Czompa, A.; Haines, D.; Lekli, I.; Bakondi, E.; Balla, G.; Tosaki, A.; Bak, I. Cardiovascular effects of low versus high-dose beta-carotene in a rat model. Pharmacol. Res. 2015, 100, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.D.; Lekli, I.; Teissier, P.; Bak, I.; Tosaki, A. Role of haeme oxygenase-1 in resolution of oxidative stress-related pathologies: Focus on cardiovascular, lung, neurological and kidney disorders. Acta Physiol. 2012, 204, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Lanone, S.; Bloc, S.; Foresti, R.; Almolki, A.; Taillé, C.; Callebert, J.; Conti, M.; Goven, D.; Aubier, M.; Dureuil, B.; et al. Bilirubin decreases NOS2 expression via inhibition of NAD(P)H oxidase: Implications for protection against endotoxic shock in rats. FASEB J. 2005, 19, 1890–1892. [Google Scholar] [CrossRef]
- Kim, H.P.; Ryter, S.W.; Choi, A.M. CO as a Cellular Signaling Molecule. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 411–449. [Google Scholar] [CrossRef]
- Panahian, N.; Yoshiura, M.; Maines, M.D. Overexpression of Heme Oxygenase-1 Is Neuroprotective in a Model of Permanent Middle Cerebral Artery Occlusion in Transgenic Mice. J. Neurochem. 2008, 72, 1187–1203. [Google Scholar] [CrossRef]
- Hopper, C.P.; Meinel, L.; Steiger, C.; Otterbein, L.E. Where is the Clinical Breakthrough of Heme Oxygenase-1/Carbon Monoxide Therapeutics? Curr. Pharm. Des. 2018, 24, 2264–2282. [Google Scholar] [CrossRef]
- Rossi, M.; Delbauve, S.; Roumeguère, T.; Wespes, E.; Leo, O.; Flamand, V.; Le Moine, A.; Hougardy, J.-M. HO-1 mitigates acute kidney injury and subsequent kidney-lung cross-talk. Free. Radic. Res. 2019, 53, 1035–1043. [Google Scholar] [CrossRef]
- Schipper, H.M.; Song, W.; Tavitian, A.; Cressatti, M. The sinister face of heme oxygenase-1 in brain aging and disease. Prog. Neurobiol. 2019, 172, 40–70. [Google Scholar] [CrossRef]
- Tien, M.; Svingen, B.A.; Aust, S.D. An investigation into the role of hydroxyl radical in xanthine oxidase-dependent lipid peroxidation. Arch. Biochem. Biophys. 1982, 216, 142–151. [Google Scholar] [CrossRef]
- Balla, J.; Jacob, H.S.; Balla, G.; Nath, K.; Vercellotti, G.M. Endothelial cell heme oxygenase and ferritin induction by heme proteins: A possible mechanism limiting shock damage. Trans. Assoc. Am. Physicians 1992, 105, 1–6. [Google Scholar] [PubMed]
- Andersson, J.A.; Egesten, A.; Cardell, L.O. Hemin, a heme oxygenase substrate analog, inhibits the cell surface expression of CD11b and CD66b on human neutrophils. Allergy 2002, 57, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Mayer, R.D.; Wang, X.; Maines, M.D. Nitric oxide inhibitor N omega -nitro-l-arginine methyl ester potentiates induction of heme oxygenase-1 in kidney ischemia/reperfusion model: A novel mechanism for regulation of the oxygenase. J. Pharm. Exp. Ther. 2003, 306, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Nath, K.A.; Grande, J.P.; Belcher, J.D.; Garovic, V.D.; Croatt, A.J.; Hillestad, M.L.; Barry, M.A.; Nath, M.C.; Regan, R.F.; Vercellotti, G.M. Antithrombotic effects of heme-degrading and heme-binding proteins. Am. J. Physiol. Circ. Physiol. 2020, 318, H671–H681. [Google Scholar] [CrossRef]
- Schaer, D.J.; Vinchi, F.; Ingoglia, G.; Tolosano, E.; Buehler, P.W. Haptoglobin, hemopexin, and related defense pathways-basic science, clinical perspectives, and drug development. Front. Physiol. 2014, 5, 415. [Google Scholar] [CrossRef]
- Kristiansen, M.; Graversen, J.H.; Jacobsen, C.; Sonne, O.; Hoffman, H.-J.; Law, S.A.; Moestrup, S.K. Identification of the haemoglobin scavenger receptor. Nat. Cell Biol. 2001, 409, 198–201. [Google Scholar] [CrossRef]
- Schaer, C.A.; Schoedon, G.; Imhof, A.; Kurrer, M.O.; Schaer, D.J. Constitutive Endocytosis of CD163 Mediates Hemoglobin-Heme Uptake and Determines the Noninflammatory and Protective Transcriptional Response of Macrophages to Hemoglobin. Circ. Res. 2006, 99, 943–950. [Google Scholar] [CrossRef]
- Tolosano, E.; Altruda, F. Hemopexin: Structure, Function, and Regulation. DNA Cell Biol. 2002, 21, 297–306. [Google Scholar] [CrossRef]
- Hvidberg, V.; Maniecki, M.B.; Jacobsen, C.; Højrup, P.; Møller, H.J.; Moestrup, S.K. Identification of the receptor scavenging hemopexin-heme complexes. Blood 2005, 106, 2572–2579. [Google Scholar] [CrossRef]
- Davies, D.; Smith, A.; Muller-Eberhard, U.; Morgan, W.T. Hepatic subcellular metabolism of heme from heme-hemopexin: Incorporation of iron into ferritin. Biochem. Biophys. Res. Commun. 1979, 91, 1504–1511. [Google Scholar] [CrossRef]
- Nyakundi, B.B.; Erdei, J.; Tóth, A.; Balogh, E.; Nagy, A.; Nagy, B.; Novák, L.; Bognár, L.; Paragh, G.; Kappelmayer, J.; et al. Formation and Detection of Highly Oxidized Hemoglobin Forms in Biological Fluids during Hemolytic Conditions. Oxidative Med. Cell. Longev. 2020, 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fasano, M.; Mattu, M.; Coletta, M.; Ascenzi, P. The heme-iron geometry of ferrous nitrosylated heme-serum lipoproteins, hemopexin, and albumin: A comparative EPR study. J. Inorg. Biochem. 2002, 91, 487–490. [Google Scholar] [CrossRef]
- Balla, G.; Jacob, H.S.; Eaton, J.W.; Belcher, J.D.; Vercellotti, G.M. Hemin: A possible physiological mediator of low density lipoprotein oxidation and endothelial injury. Arter. Thromb. A J. Vasc. Biol. 1991, 11, 1700–1711. [Google Scholar] [CrossRef] [PubMed]
- Juckett, M.B.; Balla, J.; Balla, G.; Jessurun, J.; Jacob, H.S.; Vercellotti, G.M. Ferritin protects endothelial cells from oxidized low density lipoprotein in vitro. Am. J. Pathol. 1995, 147, 782–789. [Google Scholar] [PubMed]
- Allhorn, M.; Berggård, T.; Nordberg, J.; Olsson, M.L.; Åkerström, B. Processing of the lipocalin α1-microglobulin by hemoglobin induces heme-binding and heme-degradation properties. Blood 2002, 99, 1894–1901. [Google Scholar] [CrossRef]
- Allhorn, M.; Klapyta, A.; Åkerström, B. Redox properties of the lipocalin α1-microglobulin: Reduction of cytochrome c, hemoglobin, and free iron. Free. Radic. Biol. Med. 2005, 38, 557–567. [Google Scholar] [CrossRef]
- Stocker, R.; McDonagh, A.F.; Glazer, A.N.; Ames, B.N. Antioxidant activities of bile pigments: Biliverdin and bilirubin. Methods Enzymol. 1990, 186, 301–309. [Google Scholar] [CrossRef]
- Wegiel, B.; Nemeth, Z.; Correa-Costa, M.; Bulmer, A.C.; Otterbein, L.E. Heme Oxygenase-1: A Metabolic Nike. Antioxidants Redox Signal. 2014, 20, 1709–1722. [Google Scholar] [CrossRef]
- Canesin, G.; Hejazi, S.M.; Swanson, K.D.; Wegiel, B. Heme-Derived Metabolic Signals Dictate Immune Responses. Front. Immunol. 2020, 11, 66. [Google Scholar] [CrossRef]
- Maines, M.D. Biliverdin Reductase: PKC Interaction at the Cross-Talk of MAPK and PI3K Signaling Pathways. Antioxid. Redox Signal. 2007, 9, 2187–2196. [Google Scholar] [CrossRef]
- Sedlak, T.W.; Snyder, S.H. Bilirubin Benefits: Cellular Protection by a Biliverdin Reductase Antioxidant Cycle. Pediatrics 2004, 113, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Ishikawa, K.; Itabe, H.; Maruyama, Y. Carbon monoxide and bilirubin from heme oxygenase-1 suppresses reactive oxygen species generation and plasminogen activator inhibitor-1 induction. Mol. Cell. Biochem. 2006, 291, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.; Ames, B.N. Potential role of conjugated bilirubin and copper in the metabolism of lipid peroxides in bile. Proc. Natl. Acad. Sci. USA 1987, 84, 8130–8134. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.; Yamamoto, Y.; McDonagh, A.F.; Glazer, A.N.; Ames, B.N. Bilirubin is an antioxidant of possible physiological importance. Science 1987, 235, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.J.B.; Macedo-Ribeiro, S.; Párraga, A.; Pérez-Luque, R.; Cunningham, O.; Darcy, K.; Mantle, T.J.; Coll, M. Structure of human biliverdin IXbeta reductase, an early fetal bilirubin IXbeta producing enzyme. Nat. Genet. 2001, 8, 215–220. [Google Scholar] [CrossRef][Green Version]
- Cunningham, O.; Gore, M.G.; Mantle, T.J. Initial-rate kinetics of the flavin reductase reaction catalysed by human biliverdin-IXbeta reductase (BVR-B). Biochem. J. 2000, 345, 393–399. [Google Scholar] [CrossRef]
- Aziz, S.; Kotal, P.; Leroy, P.; Servaes, R.; Eggermont, E.; Fevery, J. Bilirubin-IXalpha and -IXbeta pigments, coproporphyrins and bile acids in meconium and stools from full-term and preterm neonates during the first month of life. Acta Paediatr 2001, 90, 81–87. [Google Scholar] [CrossRef]
- Chen, W.; Maghzal, G.J.; Ayer, A.; Suarna, C.; Dunn, L.L.; Stocker, R. Absence of the biliverdin reductase-a gene is associated with increased endogenous oxidative stress. Free Radic. Biol. Med. 2018, 115, 156–165. [Google Scholar] [CrossRef]
- Vasavda, C.; Kothari, R.; Malla, A.P.; Tokhunts, R.; Lin, A.; Ji, M.; Ricco, C.; Xu, R.; Saavedra, H.G.; Sbodio, J.I.; et al. Bilirubin Links Heme Metabolism to Neuroprotection by Scavenging Superoxide. Cell Chem. Biol. 2019, 26, 1450–1460. [Google Scholar] [CrossRef]
- Adin, C.A.; VanGundy, Z.C.; Papenfuss, T.L.; Xu, F.; Ghanem, M.; Lakey, J.; Hadley, G.A. Physiologic Doses of Bilirubin Contribute to Tolerance of Islet Transplants by Suppressing the Innate Immune Response. Cell Transplant. 2017, 26, 11–21. [Google Scholar] [CrossRef]
- Sundararaghavan, V.L.; Binepal, S.; Stec, D.E.; Sindhwani, P.; Hinds, T.D. Bilirubin, a new therapeutic for kidney transplant? Transplant. Rev. 2018, 32, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Mccarty, M.F.; Aaron, L. Nutraceuticals Targeting Generation and Oxidant Activity of Peroxynitrite May Aid Prevention and Control of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 3624. [Google Scholar] [CrossRef] [PubMed]
- Pentón-Rol, G.; Marín-Prida, J.; Falcón, V. C-Phycocyanin and Phycocyanobilin as Remyelination Therapies for Enhancing Recovery in Multiple Sclerosis and Ischemic Stroke: A Preclinical Perspective. Behav. Sci. 2018, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Pentón-Rol, G.; Cervantes-Llanos, M. Report on the Symposium “Molecular Mechanisms Involved in Neurodegeneration”. Behav. Sci. 2018, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Mccarty, M.F.; Iloki-Assanga, S. Co-administration of Phycocyanobilin and/or Phase 2-Inducer Nutraceuticals for Prevention of Opiate Tolerance. Curr. Pharm. Des. 2018, 24, 2250–2254. [Google Scholar] [CrossRef] [PubMed]
- Iloki-Assanga, S.; Mccarty, M.F. Nutraceutical Targeting of Placental Synthesis of Soluble Fms-Like Tyrosine Kinase- 1 (sFlt-1) as Strategy for Preventing and Controlling Pre-eclampsia. Curr. Pharm. Des. 2018, 24, 2255–2263. [Google Scholar] [CrossRef]
- Csiki, Z.; Papp-Bata, A.; Czompa, A.; Nagy, A.; Bak, I.; Lekli, I.; Javor, A.; Haines, D.D.; Balla, G.; Tosaki, A. Orally Delivered Sour Cherry Seed Extract (SCSE) Affects Cardiovascular and Hematological Parameters in Humans. Phytother. Res. 2015, 29, 444–449. [Google Scholar] [CrossRef]
- Balla, J.; Jacob, H.S.; Balla, G.; Nath, K.; Eaton, J.W.; Vercellotti, G.M. Endothelial-cell heme uptake from heme proteins: Induction of sensitization and desensitization to oxidant damage. Proc. Natl. Acad. Sci. USA 1993, 90, 9285–9289. [Google Scholar] [CrossRef]
- Balla, J.; Vercellotti, G.M.; Jeney, V.; Yachie, A.; Varga, Z.; Eaton, J.W.; Balla, G. Heme, heme oxygenase and ferritin in vascular endothelial cell injury. Mol. Nutr. Food Res. 2005, 49, 1030–1043. [Google Scholar] [CrossRef]
- Gáll, T.; Balla, G. Heme, Heme Oxygenase, and Endoplasmic Reticulum Stress—A New Insight into the Pathophysiology of Vascular Diseases. Int. J. Mol. Sci. 2019, 20, 3675. [Google Scholar] [CrossRef]
- Bunn, H.F.; Jandl, J.H. Exchange of heme among hemoglobin molecules. Proc. Natl. Acad. Sci. USA 1966, 56, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.L.; Paul, J.; Ohlsson, P.I.; Hjortsberg, K.; Paul, K.G. Heme-protein fission under nondenaturing conditions. Proc. Natl. Acad. Sci. USA 1991, 88, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Everse, J.; Hsia, N. The Toxicities of Native and Modified Hemoglobins. Free. Radic. Biol. Med. 1997, 22, 1075–1099. [Google Scholar] [CrossRef]
- Pamplona, A.; Ferreira, A.; Balla, J.; Jeney, V.; Balla, G.; Epiphanio, S.; Chora, A.; Rodrigues, C.D.; Gregoire, I.P.; Cunha-Rodrigues, M.; et al. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat. Med. 2007, 13, 703–710. [Google Scholar] [CrossRef]
- Larsen, R.; Gozzelino, R.; Jeney, V.; Tokaji, L.; Bozza, F.A.; Japiassú, A.M.; Bonaparte, D.; Cavalcante, M.M.; Chora, Â.; Ferreira, A.; et al. A Central Role for Free Heme in the Pathogenesis of Severe Sepsis. Sci. Transl. Med. 2010, 2, 51ra71. [Google Scholar] [CrossRef]
- Ishikawa, M.; Numazawa, S.; Yoshida, T. Redox regulation of the transcriptional repressor Bach1. Free. Radic. Biol. Med. 2005, 38, 1344–1352. [Google Scholar] [CrossRef]
- Nagababu, E.; Fabry, M.E.; Nagel, R.L.; Rifkind, J.M. Heme degradation and oxidative stress in murine models for hemoglobinopathies: Thalassemia, sickle cell disease and hemoglobin C disease. Blood Cells Mol. Dis. 2008, 41, 60–66. [Google Scholar] [CrossRef]
- Guéye, P.M.; Glasser, N.; Férard, G.; Lessinger, J.-M. Influence of human haptoglobin polymorphism on oxidative stress induced by free hemoglobin on red blood cells. Clin. Chem. Lab. Med. 2006, 44, 542–547. [Google Scholar] [CrossRef]
- Minneci, P.C.; Deans, K.J.; Zhi, H.; Yuen, P.S.; Star, R.A.; Banks, S.M.; Schechter, A.N.; Natanson, C.; Gladwin, M.T.; Solomon, S.B. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J. Clin. Investig. 2005, 115, 3409–3417. [Google Scholar] [CrossRef]
- Kato, G.J. Evolution of Novel Small-Molecule Therapeutics Targeting Sickle Cell Vasculopathy. JAMA 2008, 300, 2638–2646. [Google Scholar] [CrossRef]
- Krajewski, M.L.; Hsu, L.; Gladwin, M.T. The proverbial chicken or the egg? Dissection of the role of cell-free hemoglobin versus reactive oxygen species in sickle cell pathophysiology. Am. J. Physiol. Circ. Physiol. 2008, 295, H4–H7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Juhasz, B.; Varga, B.; Czompa, A.; Bak, I.; Lekli, I.; Gesztelyi, R.; Zsuga, J.; Kemeny-Beke, A.; Antal, M.; Szendrei, L.; et al. Postischemic cardiac recovery in heme oxygenase-1 transgenic ischemic/reperfused mouse myocardium. J. Cell. Mol. Med. 2011, 15, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Bak, I.; Czompa, A.; Juhasz, B.; Lekli, I.; Tosaki, A. Reduction of reperfusion-induced ventricular fibrillation and infarct size via heme oxygenase-1 overexpression in isolated mouse hearts. J. Cell. Mol. Med. 2010, 14, 2268–2272. [Google Scholar] [CrossRef] [PubMed]
- Varadi, J.; Lekli, I.; Juhász, B.; Bácskay, I.; Szabo, G.; Gesztelyi, R.; Szendrei, L.; Varga, E.; Bak, I.; Foresti, R.; et al. Beneficial effects of carbon monoxide-releasing molecules on post-ischemic myocardial recovery. Life Sci. 2007, 80, 1619–1626. [Google Scholar] [CrossRef]
- Wu, M.-L.; Ho, Y.-C.; Yet, S.-F. A Central Role of Heme Oxygenase-1 in Cardiovascular Protection. Antioxid. Redox Signal. 2011, 15, 1835–1846. [Google Scholar] [CrossRef]
- Morse, D.; Choi, A.M. Heme oxygenase-1: The “emerging molecule” has arrived. Am. J. Respir Cell Mol. Biol. 2002, 27, 8–16. [Google Scholar] [CrossRef]
- A Bertolatus, J.; Klinzman, D.; A Bronsema, D.; Ridnour, L.; Oberley, L.W. Evaluation of the role of reactive oxygen species in doxorubicin hydrochloride nephrosis. J. Lab. Clin. Med. 1991, 118, 435–445. [Google Scholar]
- Peers, C.; Steele, D.S. Carbon monoxide: A vital signalling molecule and potent toxin in the myocardium. J. Mol. Cell. Cardiol. 2012, 52, 359–365. [Google Scholar] [CrossRef]
- Ewing, J.F.; Raju, V.S.; Maines, M.D. Induction of heart heme oxygenase-1 (HSP32) by hyperthermia: Possible role in stress-mediated elevation of cyclic 3′:5′-guanosine monophosphate. J. Pharmacol. Exp. Ther. 1994, 271, 408–414. [Google Scholar]
- Haines, D.D.; Tosaki, A. Role of Heme Oxygenases in Cardiovascular Syndromes and Co-morbidities. Curr. Pharm. Des. 2018, 24, 2322–2325. [Google Scholar] [CrossRef]
- Lakkisto, P.; Palojoki, E.; Backlund, T.; Saraste, A.; Tikkanen, I.; Voipio-Pulkki, L.M.; Pulkki, K. Expression of heme oxygenase-1 in response to myocardial infarction in rats. J. Mol. Cell Cardiol. 2002, 34, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Bak, I.; Varadi, J.; Nagy, N.; Vecsernyes, M.; Tosaki, A. The role of exogenous carbon monoxide in the recovery of post-ischemic cardiac function in buffer perfused isolated rat hearts. Cell. Mol. Biol. 2005, 51, 453–459. [Google Scholar] [PubMed]
- Tosaki, A. ArrhythmoGenoPharmacoTherapy. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- Yang, Y.; Ohta, K.; Shimizu, M.; Morimoto, K.; Goto, C.; Nakai, A.; Toma, T.; Kasahara, Y.; Yachie, A.; Seki, H.; et al. Selective protection of renal tubular epithelial cells by heme oxygenase (HO)-1 during stress-induced injury. Kidney Int. 2003, 64, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Foundation, N.K. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39, S1–S246. [Google Scholar]
- Ndisang, J.F. Role of Heme Oxygenase in Inflammation, Insulin-Signalling, Diabetes and Obesity. Mediat. Inflamm. 2010, 2010, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, W.; Feng, Y.; Xu, J.; Cao, H. Edaravone attenuates experimental asthma in mice through induction of HO-1 and the Keap1/Nrf2 pathway. Exp. Ther. Med. 2019, 19, 1407–1416. [Google Scholar] [CrossRef]
- Cheng, C.F.; Lian, W.S.; Chen, S.H.; Lai, P.F.; Li, H.F.; Lan, Y.F.; Cheng, W.T.; Lin, H. Protective effects of adiponectin against renal ischemia-reperfusion injury via prostacyclin-PPARalpha-heme oxygenase-1 signaling pathway. J. Cell Physiol. 2012, 227, 239–249. [Google Scholar] [CrossRef]
- Liu, X.; Zang, P.; Han, F.; Hou, N.; Sun, X. Renal protective effects of induction of haem oxygenase-1 combined with increased adiponectin on the glomerular vascular endothelial growth factor-nitric oxide axis in obese rats. Exp. Physiol. 2015, 100, 865–876. [Google Scholar] [CrossRef]
- Mercado, N.; Thimmulappa, R.; Thomas, C.M.; Fenwick, P.S.; Chana, K.K.; Donnelly, L.E.; Biswal, S.; Ito, K.; Barnes, P.J. Decreased histone deacetylase 2 impairs Nrf2 activation by oxidative stress. Biochem. Biophys. Res. Commun. 2011, 406, 292–298. [Google Scholar] [CrossRef]
- Balogun, E.; Hoque, M.; Gong, P.; Killeen, E.; Green, C.J.; Foresti, R.; Alam, J.; Motterlini, R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003, 371, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.; Lin, L.; Choi, A.M.K.; Ryter, S.W. Heme oxygenase-1, a critical arbitrator of cell death pathways in lung injury and disease. Free. Radic. Biol. Med. 2009, 47, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Salter, B.; Pray, C.; Radford, K.; Martin, J.G.; Nair, P. Regulation of human airway smooth muscle cell migration and relevance to asthma. Respir. Res. 2017, 18, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.D.; Varga, B.; Bak, I.; Juhasz, B.; Mahmoud, F.F.; Kalantari, H.; Gesztelyi, R.; Lekli, I.; Czompa, A.; Tosaki, A. Summative interaction between astaxanthin, Ginkgo biloba extract (EGb761) and vitamin C in Suppression of respiratory inflammation: A comparison with ibuprofen. Phytother. Res. 2010, 25, 128–136. [Google Scholar] [CrossRef]
- Lv, J.; Su, W.; Yu, Q.; Zhang, M.; Di, C.; Lin, X.; Wu, M.; Xia, Z. Heme oxygenase-1 protects airway epithelium against apoptosis by targeting the proinflammatory NLRP3–RXR axis in asthma. J. Biol. Chem. 2018, 293, 18454–18465. [Google Scholar] [CrossRef]
- International Classification of Diseases (ICD). Available online: http://www.who.int/classifications/icd/en/ (accessed on 11 May 2012).
- Brito, M.A.; Brites, D.; Butterfield, D.A. A link between hyperbilirubinemia, oxidative stress and injury to neocortical synaptosomes. Brain Res. 2004, 1026, 33–43. [Google Scholar] [CrossRef]
- Hyun, H.; Won, Y.-W.; Kim, K.-M.; Lee, J.; Lee, M.; Kim, Y.-H. Therapeutic effects of a reducible poly (oligo-d-arginine) carrier with the heme oxygenase-1 gene in the treatment of hypoxic-ischemic brain injury. Biomater. 2010, 31, 9128–9134. [Google Scholar] [CrossRef]
- Song, Y.-J.; Dai, C.-X.; Li, M.; Cui, M.-M.; Ding, X.; Zhao, X.-F.; Wang, C.-L.; Li, Z.-L.; Guo, M.-Y.; Fu, Y.-Y.; et al. The potential role of HO-1 in regulating the MLK3-MKK7-JNK3 module scaffolded by JIP1 during cerebral ischemia/reperfusion in rats. Behav. Brain Res. 2019, 359, 528–535. [Google Scholar] [CrossRef]
- Saleem, S.; Zhuang, H.; Biswal, S.; Christen, Y.; Doré, S. Ginkgo Biloba Extract Neuroprotective Action Is Dependent on Heme Oxygenase 1 in Ischemic Reperfusion Brain Injury. Stroke 2008, 39, 3389–3396. [Google Scholar] [CrossRef]
- Liu, Q.; Jin, Z.; Xu, Z.; Yang, H.; Li, L.; Li, G.; Li, F.; Gu, S.; Zong, S.; Zhou, J.; et al. Antioxidant effects of ginkgolides and bilobalide against cerebral ischemia injury by activating the Akt/Nrf2 pathway in vitro and in vivo. Cell Stress Chaperon 2019, 24, 441–452. [Google Scholar] [CrossRef]
- Haines, D.D.; Bak, I.; Ferdinandy, P.; Mahmoud, F.F.; Al-Harbi, S.A.; Blasig, I.E.; Tosaki, A. Cardioprotective Effects of the Calcineurin Inhibitor FK506 and the PAF Receptor Antagonist and Free Radical Scavenger, EGb 761, in Isolated Ischemic/Reperfused Rat Hearts. J. Cardiovasc. Pharmacol. 2000, 35, 37–44. [Google Scholar] [CrossRef]
- Szabo, M.E.; Gallyas, É.; Bak, I.; Rakotovao, A.; Boucher, F.; De Leiris, J.; Nagy, N.; Varga, E.; Tosaki, A. Heme Oxygenase-1–Related Carbon Monoxide and Flavonoids in Ischemic/Reperfused Rat Retina. Investig. Opthalmology Vis. Sci. 2004, 45, 3727–3732. [Google Scholar] [CrossRef] [PubMed]
- Bak, I.; Czompa, A.; Csepanyi, E.; Juhász, B.; Kalantari, H.; Najm, K.; Aghel, N.; Varga, B.; Haines, D.D.; Tosaki, A. Evaluation of Systemic and Dermal Toxicity and Dermal Photoprotection by Sour Cherry Kernels. Phytother. Res. 2011, 25, 1714–1720. [Google Scholar] [CrossRef] [PubMed]
- Juhász, B.; Kertész, A.; Balla, J.; Balla, G.; Szabó, Z.; Bombicz, M.; Priksz, D.; Gesztelyi, R.; Varga, B.; Haines, D.D.; et al. Cardioprotective effects of sour cherry seed extract (SCSE) on the hypercholesterolemic rabbit heart. Curr. Pharm. Des. 2013, 19, 6896–6905. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, F.; Al-Awadhi, R.; Haines, D.D.; Dashti, A.; Dashti, H.; Al-Ozairi, E.; Bak, I.; Tosaki, A. Sour Cherry Seed Kernel Extract Increases Heme Oxygenase-1 Expression and Decreases Representation of CD3+ TNF-α + and CD3 + IL-8+ Subpopulations in Peripheral Blood Leukocyte Cultures from Type 2 Diabetes Patients. Phytother. Res. 2012, 27, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, F.; Haines, D.D.; Al-Awadhi, R.; Dashti, A.A.; Al-Awadhi, A.; Ibrahim, B.; Al-Zayer, B.; Juhász, B.; Tosaki, A. Sour cherry (Prunus cerasus) seed extract increases heme oxygenase-1 expression and decreases proinflammatory signaling in peripheral blood human leukocytes from rheumatoid arthritis patients. Int. Immunopharmacol. 2014, 20, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, F.; Al-Awadhi, A.M.; Haines, D.D. Amelioration of human osteoarthritis symptoms with topical ‘biotherapeutics’: A phase I human trial. Cell Stress Chaperon 2014, 20, 267–276. [Google Scholar] [CrossRef]
- Czompa, A.; Gyongyosi, A.; Czegledi, A.; Csepanyi, E.; Bak, I.; Haines, D.D.; Tosaki, A.; Lekli, I. Cardioprotection afforded by sour cherry seed kernel: The role of heme oxygenase-1. J. Cardiovasc Pharm. 2014, 64, 412–419. [Google Scholar] [CrossRef]
- Csepanyi, E.; Czompa, A.; Szabados-Furjesi, P.; Lekli, I.; Balla, J.; Balla, G.; Tosaki, A.; Bak, I. The Effects of Long-Term, Low- and High-Dose Beta-Carotene Treatment in Zucker Diabetic Fatty Rats: The Role of HO-1. Int. J. Mol. Sci. 2018, 19, 1132. [Google Scholar] [CrossRef]
- Li, C.; Lönn, M.E.; Xu, X.; Maghzal, G.J.; Frazer, D.M.; Thomas, S.R.; Halliwell, B.; Richardson, D.R.; Anderson, G.J.; Stocker, R. Sustained expression of heme oxygenase-1 alters iron homeostasis in nonerythroid cells. Free. Radic. Biol. Med. 2012, 53, 366–374. [Google Scholar] [CrossRef]
- Antognoni, F.; Potente, G.; Mandrioli, R.; Angeloni, C.; Freschi, M.; Malaguti, M.; Hrelia, S.; Lugli, S.; Gennari, F.; Muzzi, E.; et al. Fruit Quality Characterization of New Sweet Cherry Cultivars as a Good Source of Bioactive Phenolic Compounds with Antioxidant and Neuroprotective Potential. Antioxidants 2020, 9, 677. [Google Scholar] [CrossRef] [PubMed]
- Raafat, K.; El-Darra, N.; Saleh, F.A. Gastroprotective and anti-inflammatory effects of Prunus cerasus phytochemicals and their possible mechanisms of action. J. Tradit. Complement. Med. 2020, 10, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Spinnewyn, B.; Blavet, N.; Clostre, F.; Bazan, N.; Braquet, P. Involvement of platelet-activating factor (PAF) in cerebral post-ischemic phase in Mongolian gerbils. Prostaglandins 1987, 34, 337–349. [Google Scholar] [CrossRef]
- SanGiovanni, E.; Brivio, P.; Dell’Agli, M.; Calabrese, F. Botanicals as Modulators of Neuroplasticity: Focus on BDNF. Neural Plast. 2017, 2017, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gargouri, B.; Carstensen, J.; Bhatia, H.S.; Huell, M.; Dietz, G.P.; Fiebich, B.L. Anti-neuroinflammatory effects of Ginkgo biloba extract EGb761 in LPS-activated primary microglial cells. Phytomedicine 2018, 44, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.A.; Demoret, R.M.; Ohtawa, M.; Shenvi, R.A. Concise asymmetric synthesis of (−)-bilobalide. Nat. Cell Biol. 2019, 575, 643–646. [Google Scholar] [CrossRef]
- Martínez-Solís, I.; Acero, N.; Bosch-Morell, F.; Castillo, E.; González-Rosende, M.E.; Muñoz-Mingarro, D.; Ortega, T.; Sanahuja, M.A.; Villagrasa, V. Neuroprotective Potential of Ginkgo biloba in Retinal Diseases. Planta Medica 2019, 85, 1292–1303. [Google Scholar] [CrossRef]
- Wang, Y.; Pei, N.-S.; Ji, H.-X.; Wang, Y. Protective effect of a standardized Ginkgo extract (ginaton) on renal ischemia/reperfusion injury via suppressing the activation of JNK signal pathway. Phytomedicine 2008, 15, 923–931. [Google Scholar] [CrossRef]
- Sherif, I.O.; Al-Shaalan, N.H.; Sabry, D. Ginkgo Biloba Extract Alleviates Methotrexate-Induced Renal Injury: New Impact on PI3K/Akt/mTOR Signaling and MALAT1 Expression. Biomolecules 2019, 9, 691. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, M.; Zou, X. Efficacy and safety of Ginkgo biloba for patients with early diabetic nephropathy: A protocol for systematic review and meta-analysis. Medicine 2020, 99, e21959. [Google Scholar] [CrossRef]
- Benveniste, J.; Boullet, C.; Brink, C.; Labat, R. The actions of Paf-acether (platelet-activating factor) on guinea-pig isolated heart preparations. Br. J. Pharmacol. 1983, 80, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Tosaki, A.; Droy-Lefaix, M.-T.; Páli, T.; Das, D.K. Effects of SOD, catalase, and a novel antiarrhythmic drug, EGB 761, on reperfusion-induced arrhythmias in isolated rat hearts. Free. Radic. Biol. Med. 1993, 14, 361–370. [Google Scholar] [CrossRef]
- Tosaki, A.; Engelman, D.T.; Pali, T.; Engelman, R.M.; Droy-Lefaix, M.-T. Ginkgo biloba extract (EGb 761) improves postischemic function in isolated preconditioned working rat hearts. Coron. Artery Dis. 1994, 5, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Tosaki, A.; Pali, T.; Droy-Lefaix, M.-T. Effects of Ginkgo biloba extract and preconditioning on the diabetic rat myocardium. Diabetol. 1996, 39, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Varga, E.; Bodi, A.; Ferdinandy, P.; Droy-Lefaix, M.-T.; Blasig, I.E.; Tosaki, A. The Protective Effect of EGb 761 in Isolated Ischemic/Reperfused Rat Hearts: A Link Between Cardiac Function and Nitric Oxide Production. J. Cardiovasc. Pharmacol. 1999, 34, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-H.; Zhang, M.; Wu, J.-X.; Li, Y.-B.; Sun, J.-R.; Tang, S.; Bao, E. Gingko biloba extract EGB761 alleviates heat-stress damage in chicken heart tissue by stimulating Hsp70 expression in vivo in vascular endothelial cells. Br. Poult. Sci. 2020, 61, 180–187. [Google Scholar] [CrossRef]
- Ge, Y.; Xu, W.; Zhang, L.; Liu, M. Ginkgolide B attenuates myocardial infarction-induced depression-like behaviors via repressing IL-1beta in central nervous system. Int. Immunopharmacol. 2020, 85, 106652. [Google Scholar] [CrossRef]
- Tosaki, A.; Koltai, M.; Braquet, P.; Szekeres, L. Possible involvement of platelet activating factor in anaphylaxis of passively sensitised, isolated guinea pig hearts. Cardiovasc. Res. 1989, 23, 715–722. [Google Scholar] [CrossRef]
- Braquet, P.; Hosford, D. Ethnopharmacology and the development of natural PAF antagonists as therapeutic agents. J. Ethnopharmacol. 1991, 32, 135–139. [Google Scholar] [CrossRef]
- Li, C.; Liu, K.; Liu, S.; Aerqin, Q.; Wu, X. Role of Ginkgolides in the Inflammatory Immune Response of Neurological Diseases: A Review of Current Literatures. Front. Syst. Neurosci. 2020, 14, 45. [Google Scholar] [CrossRef]
- Kusmic, C.; Basta, G.; Lazzerini, G.; Vesentini, N.; Barsacchi, R. The effect of Ginkgo biloba in isolated ischemic/reperfused rat heart: A link between vitamin E preservation and prostaglandin biosynthesis. J. Cardiovasc. Pharm. 2004, 44, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Yang, X.; Zhang, L.; Ansari, I.A.; Khan, M.S.; Han, S.; Feng, Y. Ginkgolide B ameliorates oxidized low-density lipoprotein-induced endothelial dysfunction via modulating Lectin-like ox-LDL-receptor-1 and NADPH oxidase 4 expression and inflammatory cascades. Phytother. Res. 2018, 32, 2417–2427. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiong, Y.; Zhang, H.; Li, J.; Wang, N.; Chen, W.; Yuan, X.; Su, Q.; Li, W.; Huang, H.; et al. Ginkgo biloba extract EGb761 attenuates brain death-induced renal injury by inhibiting pro-inflammatory cytokines and the SAPK and JAK-STAT signalings. Sci. Rep. 2017, 7, 45192. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Liu, Y.; Liu, Y.; Chen, K.; Lyu, S. Ginkgo biloba Leaf Extract Protects against Myocardial Injury via Attenuation of Endoplasmic Reticulum Stress in Streptozotocin-Induced Diabetic ApoE(-/-) Mice. Oxid. Med. Cell Longev. 2018, 2018, 2370617. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-J.; Ren, S.-M.; Dong, J.-Z.; Qiu, C.-G.; Chen, Y.-W.; Tao, H.-L. Ginkgo biloba extract-761 protects myocardium by regulating Akt/Nrf2 signal pathway. Drug Des. Dev. Ther. 2019, 13, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Popal, M.S.; Liu, Y.; Gao, R.; Lyu, S.; Chen, K.; Liu, Y. Ginkgo Biloba Leaf Extract Attenuates Atherosclerosis in Streptozotocin-Induced Diabetic ApoE-/- Mice by Inhibiting Endoplasmic Reticulum Stress via Restoration of Autophagy through the mTOR Signaling Pathway. Oxid. Med. Cell Longev. 2019, 2019, 8134678. [Google Scholar]
- Zhang, W.; Song, J.; Yan, R.; Li, L.; Xiao, Z.-Y.; Zhou, W.-X.; Wang, Z.-Z.; Xiao, W.; Du, G. Diterpene ginkgolides protect against cerebral ischemia/reperfusion damage in rats by activating Nrf2 and CREB through PI3K/Akt signaling. Acta Pharmacol. Sin. 2018, 39, 1259–1272. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haines, D.D.; Tosaki, A. Heme Degradation in Pathophysiology of and Countermeasures to Inflammation-Associated Disease. Int. J. Mol. Sci. 2020, 21, 9698. https://doi.org/10.3390/ijms21249698
Haines DD, Tosaki A. Heme Degradation in Pathophysiology of and Countermeasures to Inflammation-Associated Disease. International Journal of Molecular Sciences. 2020; 21(24):9698. https://doi.org/10.3390/ijms21249698
Chicago/Turabian StyleHaines, Donald David, and Arpad Tosaki. 2020. "Heme Degradation in Pathophysiology of and Countermeasures to Inflammation-Associated Disease" International Journal of Molecular Sciences 21, no. 24: 9698. https://doi.org/10.3390/ijms21249698
APA StyleHaines, D. D., & Tosaki, A. (2020). Heme Degradation in Pathophysiology of and Countermeasures to Inflammation-Associated Disease. International Journal of Molecular Sciences, 21(24), 9698. https://doi.org/10.3390/ijms21249698



