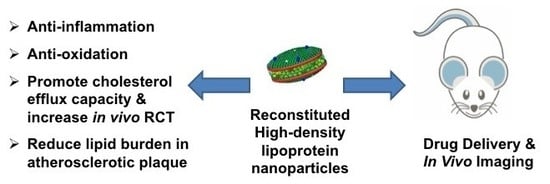
Roles of Reconstituted High-Density Lipoprotein Nanoparticles in Cardiovascular Disease: A New Paradigm for Drug Discovery
Abstract
1. Introduction
2. Atheroprotective Functions of HDL
3. Therapeutic Approaches Targeting Increasing HDL Particles
4. ApoAI Mimetic Peptide
5. Reconstituted High-Density Lipoprotein (rHDL) Nanoparticles
6. Reconstituted ApoAI Milano/Palmitoyl-Oleoyl Phosphatidyl Choline (POPC)
7. MDCO-216
8. CER-001
9. CSL-111
10. CSL-112
11. rHDL Nanoparticles as a Drug Delivery Vehicle
12. Delivery of Oligonucleotides Using rHDL Nanoparticles
13. Molecular Imaging of rHDL-Based Nanoparticles in Atherosclerosis
14. Concluding Remarks
Funding
Conflicts of Interest
Abbreviations
| AA | Arachidonic acid |
| ACS | Acute coronary syndrome |
| COX-2 | Cycloxygenase-2 |
| HDL | High-density lipoprotein |
| HDL-C | High-density lipoprotein cholesterol |
| rHDL | Reconstituted HDL |
| ApoAI | Apolipoprotein AI |
| ApoA-IM | ApoAI Milano |
| ApoB | Apolipoprotein B |
| EPA | Eicosapentaenoic acid |
| DHA | Docosahexaenoic acid |
| DPPG | Dipalmitoylphosphatidyl glycerol |
| HCP | Host cell proteins |
| HFD | High-fat diet |
| RCT | Reverse cholesterol transport |
| LCAT | Lecithin cholesterol acyltransferase |
| REVEAL | Randomized Evaluation of the Effects of Anacetrapib through Lipid modification |
| CETP | Cholesteryl ester transfer protein |
| CE | Cholesteryl ester |
| LDL | Low-density lipoprotein |
| ABCA1 | ATP-binding cassette transporter A1 |
| ABCG1 | ATP-binding cassette transporter G1 |
| POPC | Palmitoyl-oleoyl phosphatidyl choline |
| SR-BI | Scavenger receptor class B type I |
| SPM | Sphingomyelin |
| SAA | Serum amyloid A |
| CVD | Cardiovascular Disease |
| ASCVD | Atherosclerotic Cardiovascular Disease |
| FH | Familial hypercholesterolemia |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| MACE | Major adverse cardiovascular events |
| MPO | Myeloperoxidase |
| MCP-1 | Monocyte chemotactic factor 1 |
| PON1 | Paraoxonase1 |
| LXR | Liver X receptors |
| LT | Lovastatin |
| BET | Bromodomain and extraterminal domain |
| ROS | Reactive oxygen species |
| I/R | Ischemic reperfusion |
| IVUS | Intravascular ultrasonography |
| QCA | Quantitative coronary angiography |
| PET/CT | Positron emission tomography–computed tomography |
| 89Zr | Zirconium-89 |
References
- Linton, M.R.F.; Yancey, P.G.; Davies, S.S.; Jerome, W.G.; Linton, E.F.; Song, W.L.; Doran, A.C.; Vickers, K.C. The Role of Lipids and Lipoproteins in Atherosclerosis. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Grossman, A., Hershman, J.M., Kaltsas, G., Koch, C., Kopp, P., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- McNamara, J.J.; Molot, M.A.; Stremple, J.F.; Cutting, R.T. Coronary artery disease in combat casualties in Vietnam. JAMA 1971, 216, 1185–1187. [Google Scholar] [CrossRef] [PubMed]
- Stary, H.C. Evolution and progression of atherosclerotic lesions in coronary arteries of children and young adults. Arteriosclerosis 1989, 9, I19–I32. [Google Scholar] [PubMed]
- Berenson, G.S.; Srinivasan, S.R.; Bao, W.; Newman, W.P.; Tracy, R.E.; Wattigney, W.A. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. New Engl. J. Med. 1998, 338, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Tuzcu, E.M.; Kapadia, S.R.; Tutar, E.; Ziada, K.M.; Hobbs, R.E.; McCarthy, P.M.; Young, J.B.; Nissen, S.E. High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: Evidence from intravascular ultrasound. Circulation 2001, 103, 2705–2710. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Puri, R.; Anderson, T.; Ballantyne, C.M.; Cho, L.; Kastelein, J.J.; Koenig, W.; Somaratne, R.; Kassahun, H.; Yang, J.; et al. Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients: The GLAGOV Randomized Clinical Trial. JAMA 2016, 316, 2373–2384. [Google Scholar] [CrossRef]
- Puri, R.; Nissen, S.E.; Somaratne, R.; Cho, L.; Kastelein, J.J.; Ballantyne, C.M.; Koenig, W.; Anderson, T.J.; Yang, J.; Kassahun, H.; et al. Impact of PCSK9 inhibition on coronary atheroma progression: Rationale and design of Global Assessment of Plaque Regression with a PCSK9 Antibody as Measured by Intravascular Ultrasound (GLAGOV). Am Heart J. 2016, 176, 83–92. [Google Scholar] [CrossRef]
- Galvani, S.; Hla, T. Quality Versus Quantity: Making HDL Great Again. Arter. Thromb. Vasc. Biol. 2017, 37, 1018–1019. [Google Scholar] [CrossRef]
- Brownell, N.; Rohatgi, A. Modulating cholesterol efflux capacity to improve cardiovascular disease. Curr. Opin. Lipidol. 2016, 27, 398–407. [Google Scholar] [CrossRef]
- Davidson, W.S. HDL-C vs HDL-P: How changing one letter could make a difference in understanding the role of high-density lipoprotein in disease. Clin. Chem. 2014, 60, e1–e3. [Google Scholar] [CrossRef]
- Rader, D.J. New Therapeutic Approaches to the Treatment of Dyslipidemia. Cell Metab. 2016, 23, 405–412. [Google Scholar] [CrossRef]
- Feig, J.E.; Hewing, B.; Smith, J.D.; Hazen, S.L.; Fisher, E.A. High-density lipoprotein and atherosclerosis regression: Evidence from preclinical and clinical studies. Circ. Res. 2014, 114, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, I.; Goldbourt, U.; Boyko, V.; Behar, S.; Reicher-Reiss, H.; Group, B.I.P.S. Relation between on-treatment increments in serum high-density lipoprotein cholesterol levels and cardiac mortality in patients with coronary heart disease (from the Bezafibrate Infarction Prevention trial). Am. J. Cardiol. 2006, 97, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Jahagirdar, R.; Zhang, H.; Azhar, S.; Tobin, J.; Attwell, S.; Yu, R.; Wu, J.; McLure, K.G.; Hansen, H.C.; Wagner, G.S.; et al. A novel BET bromodomain inhibitor, RVX-208, shows reduction of atherosclerosis in hyperlipidemic ApoE deficient mice. Atherosclerosis 2014, 236, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Valenta, D.T.; Bulgrien, J.J.; Banka, C.L.; Curtiss, L.K. Overexpression of human ApoAI transgene provides long-term atheroprotection in LDL receptor-deficient mice. Atherosclerosis 2006, 189, 255–263. [Google Scholar] [CrossRef] [PubMed]
- McLure, K.G.; Gesner, E.M.; Tsujikawa, L.; Kharenko, O.A.; Attwell, S.; Campeau, E.; Wasiak, S.; Stein, A.; White, A.; Fontano, E.; et al. RVX-208, an inducer of ApoA-I in humans, is a BET bromodomain antagonist. PLoS ONE 2013, 8, e83190. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Gordon, A.; Johansson, J.; Wolski, K.; Ballantyne, C.M.; Kastelein, J.J.; Taylor, A.; Borgman, M.; Nissen, S.E. Efficacy and safety of a novel oral inducer of apolipoprotein a-I synthesis in statin-treated patients with stable coronary artery disease a randomized controlled trial. J. Am. Coll. Cardiol. 2011, 57, 1111–1119. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Puri, R.; Wolski, K.; Ballantyne, C.M.; Barter, P.J.; Brewer, H.B.; Kastelein, J.J.; Hu, B.; Uno, K.; Kataoka, Y.; et al. Effect of the BET Protein Inhibitor, RVX-208, on Progression of Coronary Atherosclerosis: Results of the Phase 2b, Randomized, Double-Blind, Multicenter, ASSURE Trial. Am. J. Cardiovasc. Drugs 2016, 16, 55–65. [Google Scholar] [CrossRef]
- Tsujikawa, L.M.; Fu, L.; Das, S.; Halliday, C.; Rakai, B.D.; Stotz, S.C.; Sarsons, C.D.; Gilham, D.; Daze, E.; Wasiak, S.; et al. Apabetalone (RVX-208) reduces vascular inflammation in vitro and in CVD patients by a BET-dependent epigenetic mechanism. Clin. Epigenetics 2019, 11, 102. [Google Scholar] [CrossRef]
- Kingwell, B.A.; Chapman, M.J.; Kontush, A.; Miller, N.E. HDL-targeted therapies: Progress, failures and future. Nat. Rev. Drug Discov. 2014, 13, 445–464. [Google Scholar] [CrossRef]
- Hung, A.M.; Tsuchida, Y.; Nowak, K.L.; Sarkar, S.; Chonchol, M.; Whitfield, V.; Salas, N.; Dikalova, A.; Yancey, P.G.; Huang, J.; et al. IL-1 Inhibition and Function of the HDL-Containing Fraction of Plasma in Patients with Stages 3 to 5 CKD. Clin. J. Am. Soc. Nephrol. 2019, 14, 702–711. [Google Scholar] [CrossRef]
- Navab, M.; Imes, S.S.; Hama, S.Y.; Hough, G.P.; Ross, L.A.; Bork, R.W.; Valente, A.J.; Berliner, J.A.; Drinkwater, D.C.; Laks, H.; et al. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J. Clin. Invest. 1991, 88, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Marathe, G.K.; Zimmerman, G.A.; McIntyre, T.M. Platelet-activating factor acetylhydrolase, and not paraoxonase-1, is the oxidized phospholipid hydrolase of high density lipoprotein particles. J. Biol. Chem. 2003, 278, 3937–3947. [Google Scholar] [CrossRef] [PubMed]
- Kontush, A.; Chapman, M.J. Antiatherogenic function of HDL particle subpopulations: Focus on antioxidative activities. Curr. Opin. Lipidol. 2010, 21, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, A.; de Lemos, J.A.; Shaul, P.W. HDL cholesterol efflux capacity and cardiovascular events. New Engl. J. Med. 2015, 372, 1871–1872. [Google Scholar] [CrossRef]
- Rohatgi, A.; Khera, A.; Berry, J.D.; Givens, E.G.; Ayers, C.R.; Wedin, K.E.; Neeland, I.J.; Yuhanna, I.S.; Rader, D.R.; de Lemos, J.A.; et al. HDL cholesterol efflux capacity and incident cardiovascular events. New Engl. J. Med. 2014, 371, 2383–2393. [Google Scholar] [CrossRef]
- Ebtehaj, S.; Gruppen, E.G.; Bakker, S.J.L.; Dullaart, R.P.F.; Tietge, U.J.F. HDL (High-Density Lipoprotein) Cholesterol Efflux Capacity Is Associated with Incident Cardiovascular Disease in the General Population. Arter. Thromb. Vasc. Biol. 2019, 39, 1874–1883. [Google Scholar] [CrossRef]
- Bellanger, N.; Orsoni, A.; Julia, Z.; Fournier, N.; Frisdal, E.; Duchene, E.; Bruckert, E.; Carrie, A.; Bonnefont-Rousselot, D.; Pirault, J.; et al. Atheroprotective reverse cholesterol transport pathway is defective in familial hypercholesterolemia. Arter. Thromb. Vasc. Biol. 2011, 31, 1675–1681. [Google Scholar] [CrossRef]
- Ogura, M.; Hori, M.; Harada-Shiba, M. Association Between Cholesterol Efflux Capacity and Atherosclerotic Cardiovascular Disease in Patients with Familial Hypercholesterolemia. Arter. Throm. Vas. 2016, 36, 181–188. [Google Scholar] [CrossRef]
- Huang, L.H.; Zinselmeyer, B.H.; Chang, C.H.; Saunders, B.T.; Elvington, A.; Baba, O.; Broekelmann, T.J.; Qi, L.; Rueve, J.S.; Swartz, M.A.; et al. Interleukin-17 Drives Interstitial Entrapment of Tissue Lipoproteins in Experimental Psoriasis. Cell Metab. 2019, 29, 475–487.e477. [Google Scholar] [CrossRef]
- Huang, Y.; DiDonato, J.A.; Levison, B.S.; Schmitt, D.; Li, L.; Wu, Y.; Buffa, J.; Kim, T.; Gerstenecker, G.S.; Gu, X.; et al. An abundant dysfunctional apolipoprotein A1 in human atheroma. Nat. Med. 2014, 20, 193–203. [Google Scholar] [CrossRef]
- May-Zhang, L.S.; Yermalitsky, V.; Huang, J.; Pleasent, T.; Borja, M.S.; Oda, M.N.; Jerome, W.G.; Yancey, P.G.; Linton, M.F.; Davies, S.S. Modification by isolevuglandins, highly reactive gamma-ketoaldehydes, deleteriously alters high-density lipoprotein structure and function. J. Biol. Chem. 2018, 293, 9176–9187. [Google Scholar] [CrossRef] [PubMed]
- Mani, P.; Rohatgi, A. Niacin Therapy, HDL Cholesterol, and Cardiovascular Disease: Is the HDL Hypothesis Defunct? Curr. Atheroscler. Rep. 2015, 17, 43. [Google Scholar] [CrossRef] [PubMed]
- Tall, A.R.; Rader, D.J. Trials and Tribulations of CETP Inhibitors. Circ. Res. 2018, 122, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Group, H.T.R.C.; Bowman, L.; Hopewell, J.C.; Chen, F.; Wallendszus, K.; Stevens, W.; Collins, R.; Wiviott, S.D.; Cannon, C.P.; Braunwald, E.; et al. Effects of Anacetrapib in Patients with Atherosclerotic Vascular Disease. New Engl. J. Med. 2017, 377, 1217–1227. [Google Scholar] [CrossRef]
- Marsche, G. It’s Time to Reassess the High-Density Lipoprotein (HDL) Hypothesis: CSL112, a Novel Promising Reconstituted HDL Formulation. J. Am. Heart Assoc. 2015, 4, e002371. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, A. High-Density Lipoprotein Function Measurement in Human Studies: Focus on Cholesterol Efflux Capacity. Prog. Cardiovasc. Dis. 2015, 58, 32–40. [Google Scholar] [CrossRef]
- Sacks, F.M.; Jensen, M.K. From High-Density Lipoprotein Cholesterol to Measurements of Function: Prospects for the Development of Tests for High-Density Lipoprotein Functionality in Cardiovascular Disease. Arter. Thromb. Vasc. Biol. 2018, 38, 487–499. [Google Scholar] [CrossRef]
- Zanoni, P.; Khetarpal, S.A.; Larach, D.B.; Hancock-Cerutti, W.F.; Millar, J.S.; Cuchel, M.; DerOhannessian, S.; Kontush, A.; Surendran, P.; Saleheen, D.; et al. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science 2016, 351, 1166–1171. [Google Scholar] [CrossRef]
- Timon-Zapata, J.; Laserna-Mendieta, E.J.; Pineda-Tenor, D.; Agudo-Macazaga, M.; Narros-Cecilia, C.; Rocha-Bogas, M.J.; Ruiz-Martin, G.; Gomez-Serranillos, M. Extreme concentrations of high density lipoprotein cholesterol affect the calculation of low density lipoprotein cholesterol in the Friedewald formula and other proposed formulas. Clin. Biochem. 2011, 44, 1451–1456. [Google Scholar] [CrossRef]
- Madsen, C.M.; Varbo, A.; Tybjaerg-Hansen, A.; Frikke-Schmidt, R.; Nordestgaard, B.G. U-shaped relationship of HDL and risk of infectious disease: Two prospective population-based cohort studies. Eur. Heart J. 2018, 39, 1181–1190. [Google Scholar] [CrossRef]
- Zhu, L.; Luu, T.; Emfinger, C.H.; Parks, B.A.; Shi, J.; Trefts, E.; Zeng, F.; Kuklenyik, Z.; Harris, R.C.; Wasserman, D.H.; et al. CETP Inhibition Improves HDL Function but Leads to Fatty Liver and Insulin Resistance in CETP-Expressing Transgenic Mice on a High-Fat Diet. Diabetes 2018, 67, 2494–2506. [Google Scholar] [CrossRef]
- Linton, M.F.; Babaev, V.R.; Huang, J.; Linton, E.F.; Tao, H.; Yancey, P.G. Macrophage Apoptosis and Efferocytosis in the Pathogenesis of Atherosclerosis. Circ. J. 2016, 80, 2259–2268. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Huang, J.; Gui, T.; Yang, Y.; Feng, T.; Tzvetkov, N.T.; Xu, T.; Gai, Z.; Zhou, Y.; Zhang, J.; et al. SR-BI as a target of natural products and its significance in cancer. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ditiatkovski, M.; Palsson, J.; Chin-Dusting, J.; Remaley, A.T.; Sviridov, D. Apolipoprotein A-I Mimetic Peptides: Discordance Between In Vitro and In Vivo Properties—Brief Report. Arter. Thromb. Vasc. Biol. 2017, 37, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Van Lenten, B.J.; Wagner, A.C.; Jung, C.L.; Ruchala, P.; Waring, A.J.; Lehrer, R.I.; Watson, A.D.; Hama, S.; Navab, M.; Anantharamaiah, G.M.; et al. Anti-inflammatory apoA-I-mimetic peptides bind oxidized lipids with much higher affinity than human apoA-I. J. Lipid Res. 2008, 49, 2302–2311. [Google Scholar] [CrossRef] [PubMed]
- Navab, M.; Anantharamaiah, G.M.; Reddy, S.T.; Hama, S.; Hough, G.; Grijalva, V.R.; Wagner, A.C.; Frank, J.S.; Datta, G.; Garber, D.; et al. Oral D-4F causes formation of pre-beta high-density lipoprotein and improves high-density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation 2004, 109, 3215–3220. [Google Scholar] [CrossRef]
- Qin, S.; Kamanna, V.S.; Lai, J.H.; Liu, T.; Ganji, S.H.; Zhang, L.; Bachovchin, W.W.; Kashyap, M.L. Reverse D4F, an apolipoprotein-AI mimetic peptide, inhibits atherosclerosis in ApoE-null mice. J. Cardiovasc Pharm. Ther. 2012, 17, 334–343. [Google Scholar] [CrossRef]
- Dunbar, R.L.; Movva, R.; Bloedon, L.T.; Duffy, D.; Norris, R.B.; Navab, M.; Fogelman, A.M.; Rader, D.J. Oral Apolipoprotein A-I Mimetic D-4F Lowers HDL-Inflammatory Index in High-Risk Patients: A First-in-Human Multiple-Dose, Randomized Controlled Trial. Clin. Transl. Sci. 2017, 10, 455–469. [Google Scholar] [CrossRef]
- Marinko, J.T.; Huang, H.; Penn, W.D.; Capra, J.A.; Schlebach, J.P.; Sanders, C.R. Folding and Misfolding of Human Membrane Proteins in Health and Disease: From Single Molecules to Cellular Proteostasis. Chem. Rev. 2019, 119, 5537–5606. [Google Scholar] [CrossRef]
- Shah, S.; Chib, R.; Raut, S.; Bermudez, J.; Sabnis, N.; Duggal, D.; Kimball, J.D.; Lacko, A.G.; Gryczynski, Z.; Gryczynski, I. Photophysical characterization of anticancer drug valrubicin in rHDL nanoparticles and its use as an imaging agent. J. Photochem. Photobiol. B 2016, 155, 60–65. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, D.L.; Liu, X.B.; Bi, S.J.; Zhao, T.; Sui, S.J.; Ji, X.P.; Lu, Q.H. Darapladib, a Lipoprotein-Associated Phospholipase A2 Inhibitor, Reduces Rho Kinase Activity in Atherosclerosis. Yonsei Med. J. 2016, 57, 321–327. [Google Scholar] [CrossRef]
- Stewart, R.A.; White, H.D. The role of lipoprotein-associated phospholipase a(2) as a marker and potential therapeutic target in atherosclerosis. Curr. Atheroscler. Rep. 2011, 13, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, G.; Sirtori, C.R.; Capurso, A., 2nd; Weisgraber, K.H.; Mahley, R.W. A-IMilano apoprotein. Decreased high density lipoprotein cholesterol levels with significant lipoprotein modifications and without clinical atherosclerosis in an Italian family. J. Clin. Invest. 1980, 66, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Uno, K.; Kataoka, Y.; Nissen, S.E. ETC-216 for coronary artery disease. Expert Opin. Biol. Ther. 2011, 11, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Tsunoda, T.; Tuzcu, E.M.; Schoenhagen, P.; Cooper, C.J.; Yasin, M.; Eaton, G.M.; Lauer, M.A.; Sheldon, W.S.; Grines, C.L.; et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: A randomized controlled trial. JAMA 2003, 290, 2292–2300. [Google Scholar] [CrossRef]
- Ibanez, B.; Giannarelli, C.; Cimmino, G.; Santos-Gallego, C.G.; Alique, M.; Pinero, A.; Vilahur, G.; Fuster, V.; Badimon, L.; Badimon, J.J. Recombinant HDL(Milano) exerts greater anti-inflammatory and plaque stabilizing properties than HDL(wild-type). Atherosclerosis 2012, 220, 72–77. [Google Scholar] [CrossRef]
- Reijers, J.A.A.; Kallend, D.G.; Malone, K.E.; Jukema, J.W.; Wijngaard, P.L.J.; Burggraaf, J.; Moerland, M. MDCO-216 Does Not Induce Adverse Immunostimulation, in Contrast to Its Predecessor ETC-216. Cardiovasc. Drugs Ther. 2017, 31, 381–389. [Google Scholar] [CrossRef]
- Kempen, H.J.; Schranz, D.B.; Asztalos, B.F.; Otvos, J.; Jeyarajah, E.; Drazul-Schrader, D.; Collins, H.L.; Adelman, S.J.; Wijngaard, P.L. Incubation of MDCO-216 (ApoA-IMilano/POPC) with Human Serum Potentiates ABCA1-Mediated Cholesterol Efflux Capacity, Generates New Prebeta-1 HDL, and Causes an Increase in HDL Size. J. Lipids 2014, 2014, 923903. [Google Scholar] [CrossRef]
- Kempen, H.J.; Asztalos, B.F.; Moerland, M.; Jeyarajah, E.; Otvos, J.; Kallend, D.G.; Bellibas, S.E.; Wijngaard, P.L. High-Density Lipoprotein Subfractions and Cholesterol Efflux Capacities After Infusion of MDCO-216 (Apolipoprotein A-IMilano/Palmitoyl-Oleoyl-Phosphatidylcholine) in Healthy Volunteers and Stable Coronary Artery Disease Patients. Arter. Thromb. Vasc. Biol. 2016, 36, 736–742. [Google Scholar] [CrossRef]
- Kallend, D.G.; Reijers, J.A.; Bellibas, S.E.; Bobillier, A.; Kempen, H.; Burggraaf, J.; Moerland, M.; Wijngaard, P.L. A single infusion of MDCO-216 (ApoA-1 Milano/POPC) increases ABCA1-mediated cholesterol efflux and pre-beta 1 HDL in healthy volunteers and patients with stable coronary artery disease. Eur. Heart J. Cardiovasc. Pharmacother. 2016, 2, 23–29. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Puri, R.; Ballantyne, C.M.; Jukema, J.W.; Kastelein, J.J.P.; Koenig, W.; Wright, R.S.; Kallend, D.; Wijngaard, P.; Borgman, M.; et al. Effect of Infusion of High-Density Lipoprotein Mimetic Containing Recombinant Apolipoprotein A-I Milano on Coronary Disease in Patients With an Acute Coronary Syndrome in the MILANO-PILOT Trial: A Randomized Clinical Trial. Jama Cardiol. 2018, 3, 806–814. [Google Scholar] [CrossRef]
- Tardy, C.; Goffinet, M.; Boubekeur, N.; Ackermann, R.; Sy, G.; Bluteau, A.; Cholez, G.; Keyserling, C.; Lalwani, N.; Paolini, J.F.; et al. CER-001, a HDL-mimetic, stimulates the reverse lipid transport and atherosclerosis regression in high cholesterol diet-fed LDL-receptor deficient mice. Atherosclerosis 2014, 232, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.C.; Ballantyne, C.M.; Barter, P.; Dasseux, J.L.; Fayad, Z.A.; Guertin, M.C.; Kastelein, J.J.; Keyserling, C.; Klepp, H.; Koenig, W.; et al. Effects of the high-density lipoprotein mimetic agent CER-001 on coronary atherosclerosis in patients with acute coronary syndromes: A randomized trial. Eur. Heart J. 2014, 35, 3277–3286. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Andrews, J.; Kastelein, J.J.P.; Merkely, B.; Nissen, S.E.; Ray, K.K.; Schwartz, G.G.; Worthley, S.G.; Keyserling, C.; Dasseux, J.L.; et al. Effect of Serial Infusions of CER-001, a Pre-beta High-Density Lipoprotein Mimetic, on Coronary Atherosclerosis in Patients Following Acute Coronary Syndromes in the CER-001 Atherosclerosis Regression Acute Coronary Syndrome Trial: A Randomized Clinical Trial. JAMA Cardiol. 2018, 3, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Richart, A.; Reddy, M.; Natoli, A.; Heywood, S.; Khalaji, M.; Lancaster, G.; Diditchenko, S.; Navdaev, A.; Kingwell, B. ApoA-I nanoparticles (CSL-111) Directly Modulates Inflammatory Cells After Myocardial Infarction in Mice. Arterioscler. Thromb. Vasc. Biol. 2019, 39, A329. [Google Scholar]
- Bhushan, S.; Kondo, K.; Predmore, B.L.; Zlatopolsky, M.; King, A.L.; Pearce, C.; Huang, H.; Tao, Y.X.; Condit, M.E.; Lefer, D.J. Selective beta2-adrenoreceptor stimulation attenuates myocardial cell death and preserves cardiac function after ischemia-reperfusion injury. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1865–1874. [Google Scholar] [CrossRef][Green Version]
- Bhushan, S.; Kondo, K.; Polhemus, D.J.; Otsuka, H.; Nicholson, C.K.; Tao, Y.X.; Huang, H.; Georgiopoulou, V.V.; Murohara, T.; Calvert, J.W.; et al. Nitrite therapy improves left ventricular function during heart failure via restoration of nitric oxide-mediated cytoprotective signaling. Circ. Res. 2014, 114, 1281–1291. [Google Scholar] [CrossRef]
- Tardif, J.C.; Gregoire, J.; L’Allier, P.L.; Ibrahim, R.; Lesperance, J.; Heinonen, T.M.; Kouz, S.; Berry, C.; Basser, R.; Lavoie, M.A.; et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: A randomized controlled trial. JAMA 2007, 297, 1675–1682. [Google Scholar] [CrossRef]
- Diditchenko, S.; Gille, A.; Pragst, I.; Stadler, D.; Waelchli, M.; Hamilton, R.; Leis, A.; Wright, S.D. Novel formulation of a reconstituted high-density lipoprotein (CSL112) dramatically enhances ABCA1-dependent cholesterol efflux. Arter. Thromb. Vasc. Biol. 2013, 33, 2202–2211. [Google Scholar] [CrossRef]
- Waksman, R.; Torguson, R.; Kent, K.M.; Pichard, A.D.; Suddath, W.O.; Satler, L.F.; Martin, B.D.; Perlman, T.J.; Maltais, J.A.; Weissman, N.J.; et al. A first-in-man, randomized, placebo-controlled study to evaluate the safety and feasibility of autologous delipidated high-density lipoprotein plasma infusions in patients with acute coronary syndrome. J. Am. Coll. Cardiol. 2010, 55, 2727–2735. [Google Scholar] [CrossRef]
- Marsche, G.; Saemann, M.D.; Heinemann, A.; Holzer, M. Inflammation alters HDL composition and function: Implications for HDL-raising therapies. Pharm. Ther. 2013, 137, 341–351. [Google Scholar] [CrossRef]
- Han, C.Y.; Tang, C.; Guevara, M.E.; Wei, H.; Wietecha, T.; Shao, B.; Subramanian, S.; Omer, M.; Wang, S.; O’Brien, K.D.; et al. Serum amyloid A impairs the antiinflammatory properties of HDL. J. Clin. Invest. 2016, 126, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Birner-Gruenberger, R.; Schittmayer, M.; Holzer, M.; Marsche, G. Understanding high-density lipoprotein function in disease: Recent advances in proteomics unravel the complexity of its composition and biology. Prog. Lipid Res. 2014, 56, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Milton, A.; Arnold, R.D.; Huang, H.; Smith, F.; Panizzi, J.R.; Panizzi, P. Methods for measuring myeloperoxidase activity toward assessing inhibitor efficacy in living systems. J. Leukoc. Biol. 2016, 99, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Lacko, A.G.; Sabnis, N.A.; Nagarajan, B.; McConathy, W.J. HDL as a drug and nucleic acid delivery vehicle. Front. Pharm. 2015, 6, 247. [Google Scholar] [CrossRef] [PubMed]
- Raut, S.; Mooberry, L.; Sabnis, N.; Garud, A.; Dossou, A.S.; Lacko, A. Reconstituted HDL: Drug Delivery Platform for Overcoming Biological Barriers to Cancer Therapy. Front. Pharm. 2018, 9, 1154. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, J.; Bower, J.; Corbin, I.R. Lipoprotein Drug Delivery Vehicles for Cancer: Rationale and Reason. Int. J. Mol. Sci. 2019, 20, 6327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; He, J.; Jiang, C.; Zhang, W.; Yang, Y.; Wang, Z.; Liu, J. Plaque-hyaluronidase-responsive high-density-lipoprotein-mimetic nanoparticles for multistage intimal-macrophage-targeted drug delivery and enhanced anti-atherosclerotic therapy. Int. J. Nanomed. 2017, 12, 533–558. [Google Scholar] [CrossRef]
- Yu, M.; Amengual, J.; Menon, A.; Kamaly, N.; Zhou, F.; Xu, X.; Saw, P.E.; Lee, S.J.; Si, K.; Ortega, C.A.; et al. Targeted Nanotherapeutics Encapsulating Liver X Receptor Agonist GW3965 Enhance Antiatherogenic Effects without Adverse Effects on Hepatic Lipid Metabolism in Ldlr(−/−) Mice. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef]
- Tang, J.; Baxter, S.; Menon, A.; Alaarg, A.; Sanchez-Gaytan, B.L.; Fay, F.; Zhao, Y.; Ouimet, M.; Braza, M.S.; Longo, V.A.; et al. Immune cell screening of a nanoparticle library improves atherosclerosis therapy. Proc. Natl. Acad. Sci. USA 2016, 113, E6731–E6740. [Google Scholar] [CrossRef]
- Duivenvoorden, R.; Tang, J.; Cormode, D.P.; Mieszawska, A.J.; Izquierdo-Garcia, D.; Ozcan, C.; Otten, M.J.; Zaidi, N.; Lobatto, M.E.; van Rijs, S.M.; et al. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat. Commun. 2014, 5, 3065. [Google Scholar] [CrossRef]
- Tang, J.; Lobatto, M.E.; Hassing, L.; van der Staay, S.; van Rijs, S.M.; Calcagno, C.; Braza, M.S.; Baxter, S.; Fay, F.; Sanchez-Gaytan, B.L.; et al. Inhibiting macrophage proliferation suppresses atherosclerotic plaque inflammation. Sci. Adv. 2015, 1. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yuan, W.; Yu, B.; Kuai, R.; Hu, W.; Morin, E.E.; Garcia-Barrio, M.T.; Zhang, J.; Moon, J.J.; Schwendeman, A.; et al. Synthetic High-Density Lipoprotein-Mediated Targeted Delivery of Liver X Receptors Agonist Promotes Atherosclerosis Regression. EBioMedicine 2018, 28, 225–233. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Liu, L.; Bai, H.; Wang, J.; Zhang, Y.; Zhang, W.; Zhang, M.; Wu, Z.; Liu, J. Arachidonic acid-modified lovastatin discoidal reconstituted high density lipoprotein markedly decreases the drug leakage during the remodeling behaviors induced by lecithin cholesterol acyltransferase. Pharm. Res. 2014, 31, 1689–1709. [Google Scholar] [CrossRef]
- Michell, D.L.; Vickers, K.C. HDL and microRNA therapeutics in cardiovascular disease. Pharm. Ther. 2016, 168, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.J.; Lee, R.G.; Brandt, T.A.; Tai, L.J.; Fu, W.; Peralta, R.; Yu, R.; Hurh, E.; Paz, E.; McEvoy, B.W.; et al. Cardiovascular and Metabolic Effects of ANGPTL3 Antisense Oligonucleotides. New Engl. J. Med. 2017, 377, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.B. Dyslipidaemia: ANGPTL3: A therapeutic target for atherosclerosis. Nat. Rev. Cardiol. 2017, 14, 381. [Google Scholar] [CrossRef]
- Wang, D.; Atanasov, A.G. The microRNAs Regulating Vascular Smooth Muscle Cell Proliferation: A Minireview. Int. J. Mol. Sci. 2019, 20, 324. [Google Scholar] [CrossRef]
- Davalos, A.; Chroni, A. Antisense oligonucleotides, microRNAs, and antibodies. Handb. Exp. Pharm. 2015, 224, 649–689. [Google Scholar] [CrossRef]
- Tabet, F.; Vickers, K.C.; Cuesta Torres, L.F.; Wiese, C.B.; Shoucri, B.M.; Lambert, G.; Catherinet, C.; Prado-Lourenco, L.; Levin, M.G.; Thacker, S.; et al. HDL-transferred microRNA-223 regulates ICAM-1 expression in endothelial cells. Nat. Commun. 2014, 5, 3292. [Google Scholar] [CrossRef]
- Raut, S.; Dasseux, J.L.; Sabnis, N.A.; Mooberry, L.; Lacko, A. Lipoproteins for therapeutic delivery: Recent advances and future opportunities. Ther. Deliv. 2018, 9, 257–268. [Google Scholar] [CrossRef]
- Kanter, J.E. Monocyte Recruitment Versus Macrophage Proliferation in Atherosclerosis. Circ. Res. 2017, 121, 1109–1110. [Google Scholar] [CrossRef] [PubMed]
- Babaev, V.R.; Huang, J.; Ding, L.; Zhang, Y.; May, J.M.; Linton, M.F. Loss of Rictor in Monocyte/Macrophages Suppresses Their Proliferation and Viability Reducing Atherosclerosis in LDLR Null Mice. Front. Immunol. 2018, 9, 215. [Google Scholar] [CrossRef] [PubMed]
- DelBove, C.E.; Strothman, C.E.; Lazarenko, R.M.; Huang, H.; Sanders, C.R.; Zhang, Q. Reciprocal modulation between amyloid precursor protein and synaptic membrane cholesterol revealed by live cell imaging. Neurobiol. Dis. 2019, 127, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Wildgruber, M.; Swirski, F.K.; Zernecke, A. Molecular imaging of inflammation in atherosclerosis. Theranostics 2013, 3, 865–884. [Google Scholar] [CrossRef]
- Huang, J.; Smith, F.; Panizzi, J.R.; Goodwin, D.C.; Panizzi, P. Inactivation of myeloperoxidase by benzoic acid hydrazide. Arch. Biochem. Biophys. 2015, 570, 14–22. [Google Scholar] [CrossRef][Green Version]
- Huang, J.; Smith, F.; Panizzi, P. Ordered cleavage of myeloperoxidase ester bonds releases active site heme leading to inactivation of myeloperoxidase by benzoic acid hydrazide analogs. Arch. Biochem. Biophys. 2014, 548, 74–85. [Google Scholar] [CrossRef]
- Cheng, D.; Talib, J.; Stanley, C.P.; Rashid, I.; Michaelsson, E.; Lindstedt, E.L.; Croft, K.D.; Kettle, A.J.; Maghzal, G.J.; Stocker, R. Inhibition of MPO (Myeloperoxidase) Attenuates Endothelial Dysfunction in Mouse Models of Vascular Inflammation and Atherosclerosis. Arter. Thromb. Vasc. Biol. 2019, 39, 1448–1457. [Google Scholar] [CrossRef]
- Ronald, J.A.; Chen, J.W.; Chen, Y.; Hamilton, A.M.; Rodriguez, E.; Reynolds, F.; Hegele, R.A.; Rogers, K.A.; Querol, M.; Bogdanov, A.; et al. Enzyme-sensitive magnetic resonance imaging targeting myeloperoxidase identifies active inflammation in experimental rabbit atherosclerotic plaques. Circulation 2009, 120, 592–599. [Google Scholar] [CrossRef]
- Breckwoldt, M.O.; Chen, J.W.; Stangenberg, L.; Aikawa, E.; Rodriguez, E.; Qiu, S.; Moskowitz, M.A.; Weissleder, R. Tracking the inflammatory response in stroke in vivo by sensing the enzyme myeloperoxidase. Proc. Natl. Acad. Sci. USA 2008, 105, 18584–18589. [Google Scholar] [CrossRef]
- Chen, J.W.; Breckwoldt, M.O.; Aikawa, E.; Chiang, G.; Weissleder, R. Myeloperoxidase-targeted imaging of active inflammatory lesions in murine experimental autoimmune encephalomyelitis. Brain 2008, 131, 1123–1133. [Google Scholar] [CrossRef]
- Sanchez-Gaytan, B.L.; Fay, F.; Lobatto, M.E.; Tang, J.; Ouimet, M.; Kim, Y.; van der Staay, S.E.; van Rijs, S.M.; Priem, B.; Zhang, L.; et al. HDL-mimetic PLGA nanoparticle to target atherosclerosis plaque macrophages. Bioconjug. Chem. 2015, 26, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, S.; Sabnis, N.A.; Raut, S.; Lacko, A.G. Superparamagnetic reconstituted high-density lipoprotein nanocarriers for magnetically guided drug delivery. Int. J. Nanomed. 2017, 12, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.H.; van der Valk, F.M.; Smits, L.P.; Sandberg, M.; Dasseux, J.L.; Baron, R.; Barbaras, R.; Keyserling, C.; Coolen, B.F.; Nederveen, A.J.; et al. HDL mimetic CER-001 targets atherosclerotic plaques in patients. Atherosclerosis 2016, 251, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Perez-Medina, C.; Tang, J.; Abdel-Atti, D.; Hogstad, B.; Merad, M.; Fisher, E.A.; Fayad, Z.A.; Lewis, J.S.; Mulder, W.J.; Reiner, T. PET Imaging of Tumor-Associated Macrophages with 89Zr-Labeled High-Density Lipoprotein Nanoparticles. J. Nucl. Med. 2015, 56, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.M.; Ye, J.; Jarr, K.U.; Hosseini-Nassab, N.; Smith, B.R.; Leeper, N.J. Nanoparticle Therapy for Vascular Diseases. Arter. Thromb. Vasc. Biol. 2019, 39, 635–646. [Google Scholar] [CrossRef] [PubMed]


| Mimetic | Protein to Phospholipid Ratio | Dose Duration | Population Size | Clinical Outcomes | Reference |
|---|---|---|---|---|---|
| ETC-216 | reconstituted apoAI Milano/POPC complex = 1:1.1 | 5 weekly infusions of ETC-216 at 45 mg/kg | n = 47 | Modest regression of coronary plaque in the individual | [56] |
| MDCO-216 | reconstituted apoAI Milano/POPC complex = 1:1.1 | 5 weekly, 20 mg/kg | (n = 59) placebo (n = 67) in statin-treated patients | Failed to produce an incremental plaque regression in statin therapy | [62] |
| CER-001 | reconstituted human apoAI to SPM and DPPG (32:1) = 1:2.7 | 10 weekly, 3 mg/kg, in addition to statins | CER-001 (n = 135) or placebo (n = 137) in patients with ACS | Failed to promote regression of coronary atherosclerosis | [65] |
| CER-001 | recombinant human apoAI to SPM and DPPG (32:1) = 1:2.7 | 6 weekly, 12 mg/kg | placebo n = 113, CER-001 n = 100 | Failed to reduce coronary atherosclerosis on IVUS | [64] |
| CSL-111 | human apoAI with soybean phosphatidylcholine (CSL-111) | 4 weekly, 40 mg/kg, 80 mg/kg | n = 111 | Significant improvement in the plaque characterization index | [69] |
| CSL-112 | plasma-derived apoAI to mixed PCs isolated from soybean = 1:1.4 | weekly infusions of CSL-112 | Results to be concluded in 2022 | CSL-112 are feasible, well tolerated | [71] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Wang, D.; Huang, L.-H.; Huang, H. Roles of Reconstituted High-Density Lipoprotein Nanoparticles in Cardiovascular Disease: A New Paradigm for Drug Discovery. Int. J. Mol. Sci. 2020, 21, 739. https://doi.org/10.3390/ijms21030739
Huang J, Wang D, Huang L-H, Huang H. Roles of Reconstituted High-Density Lipoprotein Nanoparticles in Cardiovascular Disease: A New Paradigm for Drug Discovery. International Journal of Molecular Sciences. 2020; 21(3):739. https://doi.org/10.3390/ijms21030739
Chicago/Turabian StyleHuang, Jiansheng, Dongdong Wang, Li-Hao Huang, and Hui Huang. 2020. "Roles of Reconstituted High-Density Lipoprotein Nanoparticles in Cardiovascular Disease: A New Paradigm for Drug Discovery" International Journal of Molecular Sciences 21, no. 3: 739. https://doi.org/10.3390/ijms21030739
APA StyleHuang, J., Wang, D., Huang, L.-H., & Huang, H. (2020). Roles of Reconstituted High-Density Lipoprotein Nanoparticles in Cardiovascular Disease: A New Paradigm for Drug Discovery. International Journal of Molecular Sciences, 21(3), 739. https://doi.org/10.3390/ijms21030739