BMAL1 Modulates Epidermal Healing in a Process Involving the Antioxidative Defense Mechanism
Abstract
:1. Introduction
2. Results
2.1. Depletion of Bmal1 Delays Epidermal Healing
2.2. Bmal1−/− Mice Present High Levels of ROS and SOD upon Injury
2.3. Topical Delivery of NAC Halts ROS and SOD Accumulation Driven by BMAL1 Depletion
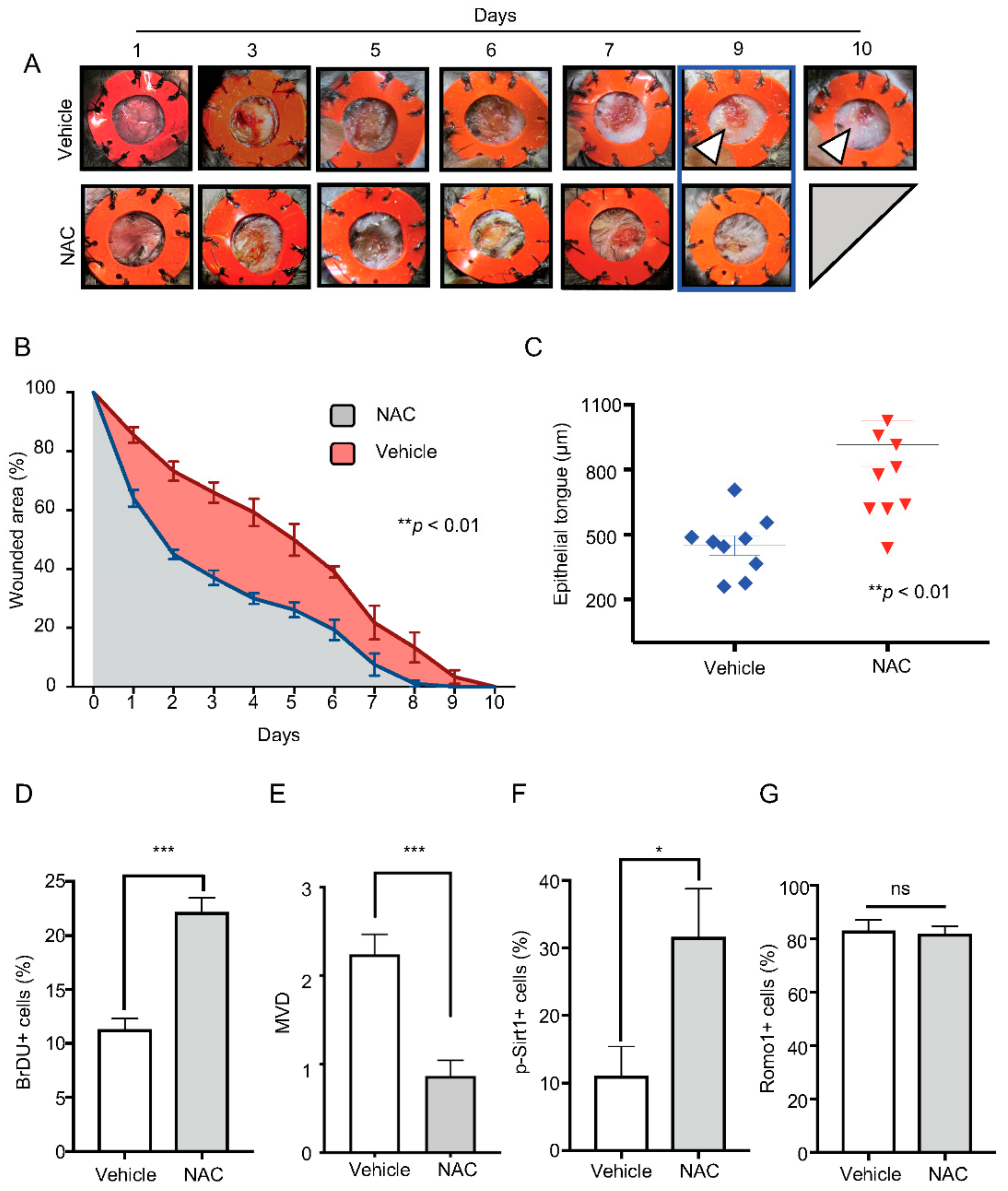
2.4. Administration of NAC Rescues Bmal1−/− Healing Phenotype
3. Discussion
4. Materials and Methods
4.1. Wound Healing Assay and Experimental Mice
4.2. Histology and Immunofluorescence
4.3. Microvessel Density
4.4. Bromodeoxyuridine (BrdU) Incorporation
4.5. Single-cell isolation from skin
4.6. ROS/SOD Assay and FACS Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bass, J.; Takahashi, J.S. Circadian integration of metabolism and energetics. Science 2010, 330, 1349–1354. [Google Scholar] [CrossRef] [Green Version]
- Baba, K.; Piano, I.; Lyuboslavsky, P.; Chrenek, M.A.; Sellers, J.T.; Zhang, S.; Gargini, C.; He, L.; Tosini, G.; Iuvone, P.M. Removal of clock gene Bmal1 from the retina affects retinal development and accelerates cone photoreceptor degeneration during aging. Proc. Natl. Acad. Sci. USA 2018, 115, 13099–13104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Early, J.O.; Menon, D.; Wyse, C.A.; Cervantes-Silva, M.P.; Zaslona, Z.; Carroll, R.G.; Palsson-McDermott, E.M.; Angiari, S.; Ryan, D.G.; Corcoran, S.E.; et al. Circadian clock protein BMAL1 regulates IL-1beta in macrophages via NRF2. Proc. Natl. Acad. Sci. USA 2018, 115, E8460–E8468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalska, E.; Ripperger, J.A.; Hoegger, D.C.; Bruegger, P.; Buch, T.; Birchler, T.; Mueller, A.; Albrecht, U.; Contaldo, C.; Brown, S.A. NONO couples the circadian clock to the cell cycle. Proc. Natl. Acad. Sci. USA 2013, 110, 1592–1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, S.; Yin, H.; Nam, D.; Li, Y.; Ma, K. Brain and muscle Arnt-like 1 promotes skeletal muscle regeneration through satellite cell expansion. Exp. Cell Res. 2015, 331, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Haus, E.; Smolensky, M. Biological clocks and shift work: Circadian dysregulation and potential long-term effects. Cancer Causes Control 2006, 17, 489–500. [Google Scholar] [CrossRef]
- Kunimoto, T.; Okubo, N.; Minami, Y.; Fujiwara, H.; Hosokawa, T.; Asada, M.; Oda, R.; Kubo, T.; Yagita, K. A PTH-responsive circadian clock operates in ex vivo mouse femur fracture healing site. Sci. Rep. 2016, 6, 22409–22415. [Google Scholar] [CrossRef] [Green Version]
- Antunes, L.C.; Levandovski, R.; Dantas, G.; Caumo, W.; Hidalgo, M.P. Obesity and shift work: Chronobiological aspects. Nutr. Res. Rev. 2010, 23, 155–168. [Google Scholar] [CrossRef] [Green Version]
- Pan, A.; Schernhammer, E.S.; Sun, Q.; Hu, F.B. Rotating night shift work and risk of type 2 diabetes: Two prospective cohort studies in women. PLoS Med. 2011, 8, e1001141. [Google Scholar] [CrossRef] [Green Version]
- Hoyle, N.P.; Seinkmane, E.; Putker, M.; Feeney, K.A.; Krogager, T.P.; Chesham, J.E.; Bray, L.K.; Thomas, J.M.; Dunn, K.; Blaikley, J.; et al. Circadian actin dynamics drive rhythmic fibroblast mobilization during wound healing. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Gu, F.; Han, J.; Laden, F.; Pan, A.; Caporaso, N.E.; Stampfer, M.J.; Kawachi, I.; Rexrode, K.M.; Willett, W.C.; Hankinson, S.E.; et al. Total and cause-specific mortality of U.S. nurses working rotating night shifts. Am. J. Prev. Med. 2015, 48, 241–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schernhammer, E.S.; Laden, F.; Speizer, F.E.; Willett, W.C.; Hunter, D.J.; Kawachi, I.; Colditz, G.A. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J. Natl. Cancer Inst. 2001, 93, 1563–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knutsson, A.; Alfredsson, L.; Karlsson, B.; Akerstedt, T.; Fransson, E.I.; Westerholm, P.; Westerlund, H. Breast cancer among shift workers: Results of the WOLF longitudinal cohort study. Scand. J. Work. Health 2013, 39, 170–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masri, S.; Sassone-Corsi, P. The emerging link between cancer, metabolism, and circadian rhythms. Nat. Med. 2018, 24, 1795–1803. [Google Scholar] [CrossRef]
- Verlande, A.; Masri, S. Circadian Clocks and Cancer: Timekeeping Governs Cellular Metabolism. Trends Endocrinol. Metab. 2019, 30, 445–458. [Google Scholar] [CrossRef]
- Bjarnason, G.A.; Mackenzie, R.G.; Nabid, A.; Hodson, I.D.; El-Sayed, S.; Grimard, L.; Brundage, M.; Wright, J.; Hay, J.; Ganguly, P.; et al. Comparison of toxicity associated with early morning versus late afternoon radiotherapy in patients with head-and-neck cancer: A prospective randomized trial of the National Cancer Institute of Canada Clinical Trials Group (HN3). Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 166–172. [Google Scholar] [CrossRef]
- Yang, Y.; Adebali, O.; Wu, G.; Selby, C.P.; Chiou, Y.Y.; Rashid, N.; Hu, J.; Hogenesch, J.B.; Sancar, A. Cisplatin-DNA adduct repair of transcribed genes is controlled by two circadian programs in mouse tissues. Proc. Natl. Acad. Sci. USA 2018, 115, E4777–E4785. [Google Scholar] [CrossRef] [Green Version]
- Menet, J.S.; Pescatore, S.; Rosbash, M. CLOCK:BMAL1 is a pioneer-like transcription factor. Genes Dev. 2014, 28, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Young, M.E.; Brewer, R.A.; Peliciari-Garcia, R.A.; Collins, H.E.; He, L.; Birky, T.L.; Peden, B.W.; Thompson, E.G.; Ammons, B.J.; Bray, M.S.; et al. Cardiomyocyte-specific BMAL1 plays critical roles in metabolism, signaling, and maintenance of contractile function of the heart. J. Biol. Rhythm. 2014, 29, 257–276. [Google Scholar] [CrossRef]
- Liu, Y.; Johnson, B.P.; Shen, A.L.; Wallisser, J.A.; Krentz, K.J.; Moran, S.M.; Sullivan, R.; Glover, E.; Parlow, A.F.; Drinkwater, N.R.; et al. Loss of BMAL1 in ovarian steroidogenic cells results in implantation failure in female mice. Proc. Natl. Acad. Sci. USA 2014, 111, 14295–14300. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, C.S.; Almeida, L.O.; Guimaraes, D.M.; Martins, M.D.; Papagerakis, P.; Papagerakis, S.; Leopoldino, A.M.; Castilho, R.M.; Squarize, C.H. PI3K-PTEN dysregulation leads to mTOR-driven upregulation of the core clock gene BMAL1 in normal and malignant epithelial cells. Oncotarget 2016, 7, 42393–42407. [Google Scholar] [CrossRef] [PubMed]
- Zagni, C.; Almeida, L.O.; Balan, T.; Martins, M.T.; Rosselli-Murai, L.K.; Papagerakis, P.; Castilho, R.M.; Squarize, C.H. PTEN Mediates Activation of Core Clock Protein BMAL1 and Accumulation of Epidermal Stem Cells. Stem Cell Rep. 2017, 9, 304–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cano Sanchez, M.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting Oxidative Stress and Mitochondrial Dysfunction in the Treatment of Impaired Wound Healing: A Systematic Review. Antioxidants (Basel) 2018, 7, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Liu, P.; Wang, H.; Xiao, C.; Lin, C.; Liu, J.; Dong, D.; Fu, T.; Yang, Y.; Wang, Z.; et al. Modulation of Circadian Rhythms Affects Corneal Epithelium Renewal and Repair in Mice. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1865–1874. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Lu, X.T.; Li, Y.G.; Liu, Y.; Yan, W.; Li, N.; Sun, Y.Y. Effect of Period 2 on the proliferation, apoptosis and migration of osteosarcoma cells, and the corresponding mechanisms. Oncol. Lett. 2018, 16, 2668–2674. [Google Scholar] [CrossRef] [Green Version]
- Chiou, Y.Y.; Yang, Y.; Rashid, N.; Ye, R.; Selby, C.P.; Sancar, A. Mammalian Period represses and de-represses transcription by displacing CLOCK-BMAL1 from promoters in a Cryptochrome-dependent manner. Proc. Natl. Acad. Sci. USA 2016, 113, E6072–E6079. [Google Scholar] [CrossRef] [Green Version]
- Chung, Y.M.; Lee, S.B.; Kim, H.J.; Park, S.H.; Kim, J.J.; Chung, J.S.; Yoo, Y.D. Replicative senescence induced by Romo1-derived reactive oxygen species. J. Biol. Chem. 2008, 283, 33763–33771. [Google Scholar] [CrossRef] [Green Version]
- Na, A.R.; Chung, Y.M.; Lee, S.B.; Park, S.H.; Lee, M.S.; Yoo, Y.D. A critical role for Romo1-derived ROS in cell proliferation. Biochem. Biophys. Res. Commun. 2008, 369, 672–678. [Google Scholar] [CrossRef]
- Kim, H.J.; Jo, M.J.; Kim, B.R.; Kim, J.L.; Jeong, Y.A.; Na, Y.J.; Park, S.H.; Lee, S.Y.; Lee, D.H.; Kim, B.H.; et al. Overexpression of Romo1 is an unfavorable prognostic biomarker and a predictor of lymphatic metastasis in non-small cell lung cancer patients. Oncotargets Ther. 2018, 11, 4233–4246. [Google Scholar] [CrossRef] [Green Version]
- Tamaru, T.; Hattori, M.; Ninomiya, Y.; Kawamura, G.; Vares, G.; Honda, K.; Mishra, D.P.; Wang, B.; Benjamin, I.; Sassone-Corsi, P.; et al. ROS stress resets circadian clocks to coordinate pro-survival signals. PLoS ONE 2013, 8, e82006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asher, G.; Gatfield, D.; Stratmann, M.; Reinke, H.; Dibner, C.; Kreppel, F.; Mostoslavsky, R.; Alt, F.W.; Schibler, U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 2008, 134, 317–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, S. “Clocks” in the NAD World: NAD as a metabolic oscillator for the regulation of metabolism and aging. Biochim. Biophys. Acta 2010, 1804, 1584–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galiano, R.D.; Michaels, J.; Dobryansky, M.; Levine, J.P.; Gurtner, G.C. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004, 12, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Webber, L.P.; Yip, B.; Nascimento Filho, C.; Park, H.B.; Castilho, R.M.; Squarize, C.H. Topical delivery of mTOR inhibitor halts scarring. J. Dermatol. Sci. 2019, 95, 76–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Squarize, C.H.; Castilho, R.M.; Abrahao, A.C.; Molinolo, A.; Lingen, M.W.; Gutkind, J.S. PTEN deficiency contributes to the development and progression of head and neck cancer. Neoplasia 2013, 15, 461–471. [Google Scholar] [CrossRef] [Green Version]
- Castilho, R.M.; Squarize, C.H.; Chodosh, L.A.; Williams, B.O.; Gutkind, J.S. mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell. 2009, 5, 279–289. [Google Scholar] [CrossRef] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silveira, E.J.D.; Nascimento Filho, C.H.V.; Yujra, V.Q.; Webber, L.P.; Castilho, R.M.; Squarize, C.H. BMAL1 Modulates Epidermal Healing in a Process Involving the Antioxidative Defense Mechanism. Int. J. Mol. Sci. 2020, 21, 901. https://doi.org/10.3390/ijms21030901
Silveira EJD, Nascimento Filho CHV, Yujra VQ, Webber LP, Castilho RM, Squarize CH. BMAL1 Modulates Epidermal Healing in a Process Involving the Antioxidative Defense Mechanism. International Journal of Molecular Sciences. 2020; 21(3):901. https://doi.org/10.3390/ijms21030901
Chicago/Turabian StyleSilveira, Ericka J. D., Carlos H. V. Nascimento Filho, Veronica Q. Yujra, Liana P. Webber, Rogerio M. Castilho, and Cristiane H. Squarize. 2020. "BMAL1 Modulates Epidermal Healing in a Process Involving the Antioxidative Defense Mechanism" International Journal of Molecular Sciences 21, no. 3: 901. https://doi.org/10.3390/ijms21030901




