Controversial Roles of Gut Microbiota-Derived Short-Chain Fatty Acids (SCFAs) on Pancreatic β-Cell Growth and Insulin Secretion
Abstract
1. Literature Review
2. Cross-Talks between Microbiota and Their Host through the Production of Mucus, Antimicrobial Signals, and SCFAs
3. SCFAs Serve as Energy Substrates, Especially for Colonocytes
SCFAs Improve Host Metabolism by Regulating Appetite and Energy Expenditure
4. SCFAs Interact with G-Protein-Coupled, Nutrient-Sensing Receptors and Histone Deacetylases (HDACs)
5. Conflicting Results of SCFA Receptor Knockouts on Glucose Tolerance, Insulin Secretion, and β-Cell Mass
6. Acetate and Propionate Protect β-Cells from Cytokine-Induced Damage
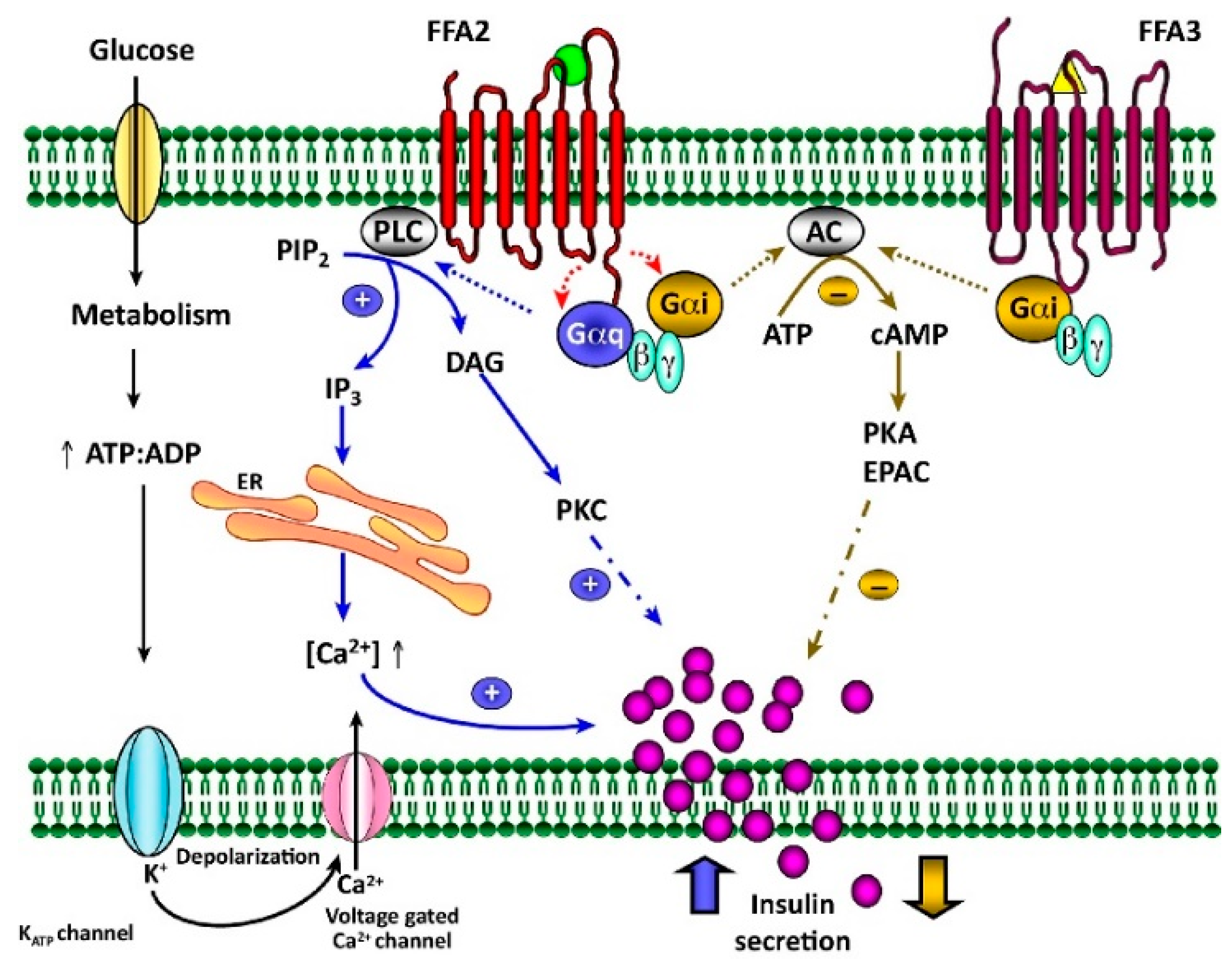
7. Conflicting Evidence That SCFAs Regulate Insulin Secretion from Pancreatic β-Cells
7.1. FFA2 Agonist (PA) Potentiates Insulin Secretion through Gαq/PLC-Mediated IP3 and Ca2+ Activations
7.2. Propionate and Acetate Potentiate GSIS in Human and Mouse Islets
7.3. Contradictory Receptor- and G-Protein-Dependent Effects of Acetate and Propionate on GSIS
7.4. Our Interpretation
8. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.A.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut Microbiota: The Neglected Endocrine Organ. Mol. Endocrinol. 2014, 28, 1221–1238. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Ryan, P.M.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Gut microbiota, obesity and diabetes. Postgrad. Med. J. 2016, 92, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Rios-Covian, D.; Salazar, N.; Gueimonde, M.; de los Reyes-Gavilan, C.G. Shaping the Metabolism of Intestinal Bacteroides Population through Diet to Improve Human Health. Front. Microbiol. 2017, 8, 376. [Google Scholar] [CrossRef]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Udayappan, S.D.; Hartstra, A.V.; Dallinga-Thie, G.M.; Nieuwdorp, M. Intestinal microbiota and faecal transplantation as treatment modality for insulin resistance and type 2 diabetes mellitus. Clin. Exp. Immunol. 2014, 177, 24–29. [Google Scholar] [CrossRef]
- Remely, M.; Aumueller, E.; Merold, C.; Dworzak, S.; Hippe, B.; Zanner, J.; Pointner, A.; Brath, H.; Haslberger, A.G. Effects of short chain fatty acid producing bacteria on epigenetic regulation of FFAR3 in type 2 diabetes and obesity. Gene 2014, 537, 85–92. [Google Scholar] [CrossRef]
- Chakraborti, C.K. New-found link between microbiota and obesity. World J. Gastrointest. Pathophysiol. 2015, 6, 110–119. [Google Scholar] [CrossRef]
- Kasubuchi, M.; Hasegawa, S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 2015, 7, 2839–2849. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Knip, M.; Siljander, H. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2016, 12, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Microbiota and diabetes: An evolving relationship. Gut 2014, 63, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Fujisawa, K.; Ito, E.; Idei, S.; Kawaguchi, N.; Kimoto, M.; Hiemori, M.; Tsuji, H. Improvement of obesity and glucose tolerance by acetate in Type 2 diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biosci. Biotechnol. Biochem. 2007, 71, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M.; Brummer, R.J.; Derrien, M.; MacDonald, T.T.; Troost, F.; Cani, P.D.; Theodorou, V.; Dekker, J.; Meheust, A.; de Vos, W.M.; et al. Homeostasis of the gut barrier and potential biomarkers. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G171–G193. [Google Scholar] [CrossRef]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Wicksteed, B.; Schiltz, G.E.; Gilchrist, A.; Layden, B.T. SCFA Receptors in Pancreatic β Cells: Novel Diabetes Targets? Trends Endocrinol. Metabol. 2016, 27, 653–664. [Google Scholar] [CrossRef]
- Boets, E.; Gomand, S.V.; Deroover, L.; Preston, T.; Vermeulen, K.; De Preter, V.; Hamer, H.M.; Van den Mooter, G.; De Vuyst, L.; Courtin, C.M.; et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: A stable isotope study. J. Physiol. 2017, 595, 541–555. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Backhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Knauf, C. How gut microbes talk to organs: The role of endocrine and nervous routes. Mol. Metabol. 2016, 5, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Offermanns, S. FFA2 and FFA3 in Metabolic Regulation. In Free Fatty Acid Receptors; Milligan, G., Kimura, I., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 205–220. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut microb. 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Vogensen, F.K.; Van Den Berg, F.W.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Sugiyama, T.; Yamaguchi, Y.; Tamura, Y.; Izawa, S.; Hijikata, Y.; Ebi, M.; Funaki, Y.; Ogasawara, N.; Goto, C.; et al. Gut microbiota disorders cause type 2 diabetes mellitus and homeostatic disturbances in gut-related metabolism in Japanese subjects. J. Clin. Biochem. Nutr. 2019, 64, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, T.F.S.; Grześkowiak, Ł.; Franceschini, S.C.C.; Bressan, J.; Ferreira, C.L.L.F.; Peluzio, M.C.G. Higher level of faecal SCFA in women correlates with metabolic syndrome risk factors. Br. J. Nutr. 2013, 109, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Hinnebusch, B.F.; Meng, S.; Wu, J.T.; Archer, S.Y.; Hodin, R.A. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J. Nutr. 2002, 132, 1012–1017. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The Microbiome and Butyrate Regulate Energy Metabolism and Autophagy in the Mammalian Colon. Cell metabol. 2011, 13, 517–526. [Google Scholar] [CrossRef]
- Soliman, M.L.; Rosenberger, T.A. Acetate supplementation increases brain histone acetylation and inhibits histone deacetylase activity and expression. Mol. Cell. Biochem. 2011, 352, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Li, Z.; Yi, C.-X.; Katiraei, S.; Kooijman, S.; Zhou, E.; Chung, C.K.; Gao, Y.; van den Heuvel, J.K.; Meijer, O.C.; Berbée, J.F. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut 2017, gutjnl-2017-314050. [Google Scholar] [CrossRef] [PubMed]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Trajkovski, M.; Wollheim, C.B. Physiology: Microbial signals to the brain control weight. Nature 2016, 534, 185–187. [Google Scholar] [CrossRef]
- McNelis, J.C.; Lee, Y.S.; Mayoral, R.; van der Kant, R.; Johnson, A.M.; Wollam, J.; Olefsky, J.M. GPR43 Potentiates beta-Cell Function in Obesity. Diabetes 2015, 64, 3203–3217. [Google Scholar] [CrossRef]
- Moran, B.M.; Flatt, P.R.; McKillop, A.M. G protein-coupled receptors: Signalling and regulation by lipid agonists for improved glucose homoeostasis. Acta Diabetol. 2016, 53, 177–188. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Villa, S.R.; Fuller, M.; Wicksteed, B.; Mackay, C.R.; Alquier, T.; Poitout, V.; Mancebo, H.; Mirmira, R.G.; Gilchrist, A.; et al. An Acetate-Specific GPCR, FFAR2, Regulates Insulin Secretion. Mol. Endocrinol. 2015, 29, 1055–1066. [Google Scholar] [CrossRef]
- Ulven, T. Short-chain free fatty acid receptors FFA2/GPR43 and FFA3/GPR41 as new potential therapeutic targets. Front. Endocrinol. 2012, 3. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Navarro, G.; Layden, B.T. Gut Microbiota: FFAR Reaching Effects on Islets. Endocrinology 2018, 159, 2495–2505. [Google Scholar] [CrossRef]
- Ang, Z.; Xiong, D.; Wu, M.; Ding, J.L. FFAR2-FFAR3 receptor heteromerization modulates short-chain fatty acid sensing. FASEB J. 2018, 32, 289–303. [Google Scholar] [CrossRef]
- Miyamoto, J.; Hasegawa, S.; Kasubuchi, M.; Ichimura, A.; Nakajima, A.; Kimura, I. Nutritional Signaling via Free Fatty Acid Receptors. Int. J. Mol. Sci. 2016, 17, 450. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, A.; Hasegawa, S.; Kasubuchi, M.; Kimura, I. Free fatty acid receptors as therapeutic targets for the treatment of diabetes. Front. Pharmacol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Milligan, G.; Bolognini, D.; Sergeev, E. Ligands at the Free Fatty Acid Receptors 2/3 (GPR43/GPR41). Handbook Exp. Pharmacol. 2017, 236, 17–32. [Google Scholar] [CrossRef]
- Orgaard, A.; Jepsen, S.L.; Holst, J.J. Short-chain fatty acids and regulation of pancreatic endocrine secretion in mice. Islets 2019, 11, 103–111. [Google Scholar] [CrossRef]
- Villa, S.R.; Mishra, R.K.; Zapater, J.L.; Priyadarshini, M.; Gilchrist, A.; Mancebo, H.; Schiltz, G.E.; Layden, B.T. Homology modeling of FFA2 identifies novel agonists that potentiate insulin secretion. J. Invest. Med. 2017, 65, 1116–1124. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Layden, B.T. FFAR3 modulates insulin secretion and global gene expression in mouse islets. Islets 2015, 7, e1045182. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. Chapter Three—The Role of Short-Chain Fatty Acids in Health and Disease. In Advances in Immunology; Alt, F.W., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 121, pp. 91–119. [Google Scholar]
- Pingitore, A.; Chambers, E.S.; Hill, T.; Maldonado, I.R.; Liu, B.; Bewick, G.; Morrison, D.J.; Preston, T.; Wallis, G.A.; Tedford, C.; et al. The diet-derived short chain fatty acid propionate improves beta-cell function in humans and stimulates insulin secretion from human islets in vitro. Diabetes Obes. Metab. 2017, 19, 257–265. [Google Scholar] [CrossRef]
- Villa, S.R.; Priyadarshini, M.; Fuller, M.H.; Bhardwaj, T.; Brodsky, M.R.; Angueira, A.R.; Mosser, R.E.; Carboneau, B.A.; Tersey, S.A.; Mancebo, H.; et al. Loss of Free Fatty Acid Receptor 2 leads to impaired islet mass and beta cell survival. Sci. Rep. 2016, 6, 28159. [Google Scholar] [CrossRef]
- Fuller, M.; Priyadarshini, M.; Gibbons, S.M.; Angueira, A.R.; Brodsky, M.; Hayes, M.G.; Kovatcheva-Datchary, P.; Bäckhed, F.; Gilbert, J.A.; Lowe, W.L., Jr.; et al. The short-chain fatty acid receptor, FFA2, contributes to gestational glucose homeostasis. Am. J. Physiol.-Endocrinol. Metab. 2015, 309, E840–E851. [Google Scholar] [CrossRef]
- Bjursell, M.; Admyre, T.; Göransson, M.; Marley, A.E.; Smith, D.M.; Oscarsson, J.; Bohlooly-Y, M. Improved glucose control and reduced body fat mass in free fatty acid receptor 2-deficient mice fed a high-fat diet. Am. J. Physiol.-Endocrinol. Metab. 2011, 300, E211–E220. [Google Scholar] [CrossRef]
- Tang, C.; Ahmed, K.; Gille, A.; Lu, S.; Gröne, H.-J.; Tunaru, S.; Offermanns, S. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat. Med. 2015, 21, 173. [Google Scholar] [CrossRef]
- Veprik, A.; Laufer, D.; Weiss, S.; Rubins, N.; Walker, M.D. GPR41 modulates insulin secretion and gene expression in pancreatic β-cells and modifies metabolic homeostasis in fed and fasting states. FASEB J. 2016, 30, 3860–3869. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.M.; Perez Chumbiauca, C.N.; Mather, K.J.; Mirmira, R.G.; Tersey, S.A. Detection of Islet β-Cell Death in Vivo by Multiplex PCR Analysis of Differentially Methylated DNA. Endocrinology 2013, 154, 3476–3481. [Google Scholar] [CrossRef]
- Pingitore, A.; Gonzalez-Abuin, N.; Ruz-Maldonado, I.; Huang, G.C.; Frost, G.; Persaud, S.J. Short chain fatty acids stimulate insulin secretion and reduce apoptosis in mouse and human islets in vitro: Role of free fatty acid receptor 2. Diabetes Obes. Metab. 2019, 21, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Philippe, J.; Drucker, D.J.; Chick, W.L.; Habener, J.F. Transcriptional regulation of genes encoding insulin, glucagon, and angiotensinogen by sodium butyrate in a rat islet cell line. Mol. Cell. Biol. 1987, 7, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Jordan, H.N.; Phillips, R.W. Effect of fatty acids on isolated ovine pancreatic islets. Am. J. Physiol. 1978, 234, E162–E167. [Google Scholar] [CrossRef] [PubMed]
- Tiengo, A.; Valerio, A.; Molinari, M.; Meneghel, A.; Lapolla, A. Effect of ethanol, acetaldehyde, and acetate on insulin and glucagon secretion in the perfused rat pancreas. Diabetes 1981, 30, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.G.; Singh, S.P. Effect of ethanol and its metabolites on glucose mediated insulin release from isolated islets of rats. Metabolism 1979, 28, 85–89. [Google Scholar] [CrossRef]
- Hu, J.; Lin, S.; Zheng, B.; Cheung, P.C.K. Short-chain fatty acids in control of energy metabolism. Crit. Rev. Food Sci. Nutr. 2018, 58, 1243–1249. [Google Scholar] [CrossRef]
- Sun, J.; Xu, M.; Ortsater, H.; Lundeberg, E.; Juntti-Berggren, L.; Chen, Y.Q.; Haeggstrom, J.Z.; Gudmundsson, G.H.; Diana, J.; Agerberth, B. Cathelicidins positively regulate pancreatic beta-cell functions. FASEB J. 2016, 30, 884–894. [Google Scholar] [CrossRef]
- Pound, L.D.; Patrick, C.; Eberhard, C.E.; Mottawea, W.; Wang, G.-S.; Abujamel, T.; Vandenbeek, R.; Stintzi, A.; Scott, F.W. Cathelicidin Antimicrobial Peptide: A Novel Regulator of Islet Function, Islet Regeneration, and Selected Gut Bacteria. Diabetes 2015, 64, 4135–4147. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Furio, L.; Mecheri, R.; van der Does, A.M.; Lundeberg, E.; Saveanu, L.; Chen, Y.; van Endert, P.; Agerberth, B.; Diana, J. Pancreatic β-Cells Limit Autoimmune Diabetes via an Immunoregulatory Antimicrobial Peptide Expressed under the Influence of the Gut Microbiota. Immunity 2015, 43, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ponton, A.; Okamoto, H.; Takasawa, S.; Herrera, P.L.; Liu, J.L. Activation of the Reg family genes by pancreatic-specific IGF-I gene deficiency and after streptozotocin-induced diabetes in mouse pancreas. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E50–E58. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Wang, X.; Li, B.; Chowdhury, S.; Lu, Y.; Srikant, C.B.; Ning, G.; Liu, J.L. Pancreatic islet-specific overexpression of Reg3β protein induced the expression of pro-islet genes and protected mice against streptozotocin-induced diabetes. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E669–E680. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, Y.; Srikant, C.B.; Gao, Z.H.; Liu, J.L. Intestinal adaptation and Reg gene expression induced by antidiabetic duodenal-jejunal bypass surgery in Zucker fatty rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G635–G645. [Google Scholar] [CrossRef]
- Li, Q.; Wang, H.; Zogopoulos, G.; Shao, Q.; Dong, K.; Lv, F.; Nwilati, K.; Gui, X.Y.; Cuggia, A.; Liu, J.L.; et al. Reg proteins promote acinar-to-ductal metaplasia and act as novel diagnostic and prognostic markers in pancreatic ductal adenocarcinoma. Oncotarget 2016, 7, 77838–77853. [Google Scholar] [CrossRef]
- Luo, C.; Yu, L.T.; Yang, M.Q.; Li, X.; Zhang, Z.Y.; Alfred, M.O.; Liu, J.L.; Wang, M. Recombinant Reg3beta protein protects against streptozotocin-induced beta-cell damage and diabetes. Sci. Rep. 2016, 6, 35640. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, B.; Miao, X.; Ramgattie, C.; Gao, Z.H.; Liu, J.L. Reg2 Expression Is Required for Pancreatic Islet Compensation in Response to Aging and High-Fat Diet-Induced Obesity. Endocrinology 2017, 158, 1634–1644. [Google Scholar] [CrossRef]
- Ogawa, H.; Fukushima, K.; Naito, H.; Funayama, Y.; Unno, M.; Takahashi, K.; Kitayama, T.; Matsuno, S.; Ohtani, H.; Takasawa, S.; et al. Increased expression of HIP/PAP and regenerating gene III in human inflammatory bowel disease and a murine bacterial reconstitution model. Inflamm. Bowel. Dis. 2003, 9, 162–170. [Google Scholar] [CrossRef]
- Lee, K.S.; Kalantzis, A.; Jackson, C.B.; O’Connor, L.; Murata-Kamiya, N.; Hatakeyama, M.; Judd, L.M.; Giraud, A.S.; Menheniott, T.R. Helicobacter pylori CagA triggers expression of the bactericidal lectin REG3gamma via gastric STAT3 activation. PLoS ONE 2012, 7, e30786. [Google Scholar] [CrossRef]
- Lepage, D.; Bruneau, J.; Brouillard, G.; Jones, C.; Lussier, C.R.; Remillard, A.; Lemieux, E.; Asselin, C.; Boudreau, F. Identification of GATA-4 as a novel transcriptional regulatory component of regenerating islet-derived family members. Biochim. Biophys. Acta 2015, 1849, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Sanna, S.; van Zuydam, N.R.; Mahajan, A.; Kurilshikov, A.; Vich Vila, A.; Võsa, U.; Mujagic, Z.; Masclee, A.A.M.; Jonkers, D.M.A.E.; Oosting, M.; et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Gene. 2019, 51, 600–605. [Google Scholar] [CrossRef] [PubMed]
| GPR41/FFA3 | GPR43/FFA2 | |
|---|---|---|
| Ligand affinity (EC50, µM) | ||
| Acetate | >1000 | 35 to 431 |
| Propionate | 6 to 127 | 14 to 290 |
| Butyrate | 42 to 158 | 28 to 371 |
| Ligand preference | Propionate > Butyrate > Acetate | Acetate = Propionate > Butyrate |
| Coupled G-proteins | Gαi/o | Gαq/11, Gαi/o, β arrestin |
| Agonists | MCPC or MCP, C1 to C6, Compound 4 [45] | CMTB, phenylacetamide (PA; Comp 58), CFMB [45], SCA14/15, C1 to C5, A [46] |
| Orthosteric agonist | AR19 [44] | Compound (1) and (2) [44] Compound (3) [44] |
| Orthosteric antagonist | GLPG0974 = (4) [44] CATPB = (5) [44] | |
| Allosteric agonist | (12), AR420626 = (13) [44] | 4-CMTB = (10) [44] |
| Antagonists | 1 [36,39], β-hydroxybutyrate (BHB)? [44,47] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.-L.; Segovia, I.; Yuan, X.-L.; Gao, Z.-h. Controversial Roles of Gut Microbiota-Derived Short-Chain Fatty Acids (SCFAs) on Pancreatic β-Cell Growth and Insulin Secretion. Int. J. Mol. Sci. 2020, 21, 910. https://doi.org/10.3390/ijms21030910
Liu J-L, Segovia I, Yuan X-L, Gao Z-h. Controversial Roles of Gut Microbiota-Derived Short-Chain Fatty Acids (SCFAs) on Pancreatic β-Cell Growth and Insulin Secretion. International Journal of Molecular Sciences. 2020; 21(3):910. https://doi.org/10.3390/ijms21030910
Chicago/Turabian StyleLiu, Jun-Li, Irina Segovia, Xiao-Lin Yuan, and Zu-hua Gao. 2020. "Controversial Roles of Gut Microbiota-Derived Short-Chain Fatty Acids (SCFAs) on Pancreatic β-Cell Growth and Insulin Secretion" International Journal of Molecular Sciences 21, no. 3: 910. https://doi.org/10.3390/ijms21030910
APA StyleLiu, J.-L., Segovia, I., Yuan, X.-L., & Gao, Z.-h. (2020). Controversial Roles of Gut Microbiota-Derived Short-Chain Fatty Acids (SCFAs) on Pancreatic β-Cell Growth and Insulin Secretion. International Journal of Molecular Sciences, 21(3), 910. https://doi.org/10.3390/ijms21030910





