Riboflavin: The Health Benefits of a Forgotten Natural Vitamin
Abstract
:1. Introduction
2. Beneficial Health Effects of RF
2.1. Antioxidant Properties
2.2. Reperfusion Oxidative Injury
| Model | Dose | Antioxidant Enzymes | Key Findings | References |
|---|---|---|---|---|
| Anti-aging in Drosophila melanogaster (fruit fly) | RF at 120 µg/mL | SOD1 ↑; CAT ↑; lipofuscin (LF) ↓ | RF prolonged the life span and increased reproductive capacity through anti-oxidative stress pathway involving enhancing the activity of SOD1 and CAT and inhibiting lipofuscin accumulation | [11] |
| Keratoconus corneal stroma cells | Keratoconus cells were treated with low dose of RF at 0.167 µg/mL | Increasing gene expression of antioxidant enzymes: aldehyde dehydrogenase 3A1, CAT, enolase 1, GPx 1, haem oxygenase 1, SOD 1 and transketolase | RF improved the synthesis of a normal extracellular matrix and downregulated ROS level in keratoconus. It was quatified by the total collagen protein in the keratoconic stroma. | [12] |
| Diabetes-induced cardiac dysfunction | RF at 20 mg/kg was treated after streptozotocin-induced diabetes type I. | SOD↑, MDA↓, HO-1 protein level↑ | RFK can reduced the risk of cardiac dysfunction by increasing antioxidant, HO-1 and decreasing CTGF levels as well as improving lipid profile | [16] |
| Diabetes mellitus type-2 | RF at 10 and 20 mg/kg was treated after alloxan-induced DM | SOD↑, catalase↑, GSH↑, MDA↓ | Decreased pancreatic activity, restored ant-oxidant enzyme activity, decreased FBG level while calcium level and GLUT-4 expression was increased | [17] |
| Cardiac abnormalities in experimental atherosclerosis in rat | RF at 40 mg/kg together with CoRNS after hypolipidemic induction | SOD↑, CAT↑, GPx↑ | CoRNS significant reduced lipid profile: LDL and cardiac enzymes (LDH, ALT, AST, ALP) with enhanced levels of HDL and antioxidants. | [18] |
| GTN-induced brain oxidative toxicity | RF at 100 mg/kg was treated before GTN-induced migraine | Lipid peroxidation↓, GSH↑, GPx↑ | RF with selenium administration protected against GTN-induced brain oxidative toxicity by protecting brain MMCA activity, inhibiting free radicals and supporting the antioxidant redox system. | [19] |
| Migraine model | RF 100 mg/kg was treated before GTN-induced migraine | Lipid peroxidation↓, GSH↑ | RF and vitamin E had a protective effect on the GTN-induced brain injury by inhibiting free radical production, regulation of calcium-dependent processes, and supporting the antioxidant redox system. | [20] |
| Model | RF Dose | Key findings | Conclusions | References |
|---|---|---|---|---|
| Stroke-induced brain damage (neuroprotection against excitotoxicity) | RF at 0.05–0.5 mM before glutamate or NMDA treatment | RF at the concentrations of 0.2, 0.3, and 0.4 mM were significantly neuroprotective against glutamate and NMDA. | RF ameliorate glutamate or NMDA-mediated excitotoxicity to CGCs | [21] |
| Cortical contusion injury (CCI) | RF treatment with 7.5 mg/kg, i.p; n = 7, 15 min after injury. A second dose was applied after 24h after injury. | Reducing brain edema formation, and inhibit GFAP+ expression, improve behavioral function. | Administration of RF following CCI of the frontal cortex improves recovery of function following injury | [22] |
| Cortical contusion injury (CCI). | RF was treated after CCI:a combination of 1 mmol/kg MgCl2 and 7.5 mg/kg RF | The combination of MgCl2 and RF improved the functional recovery while the half-dose combination did not. | RF and magnesium infusions improved functional recovery to a greater extent than either alone following a frontal cortical contusion injury in rats | [23] |
2.3. Malaria Infection
2.4. Immune System
2.5. Photosensitizing Properties of RF
2.6. Cancer
2.7. Migraine
2.8. Cataract
2.9. Premenstrual Syndrome (PMS)
2.10. Bone
2.11. Neuropathy
2.12. Anemia
2.13. Diabetes Mellitus
2.14. Cardiac Abnormalities
2.15. Hypertension
3. Side Effects of Lack or Excess of RF
4. Mechanism of Antioxidant Protection
5. Perspective Use of RF in Complementary Medicine: Administration via Functional Food and Nanocapsules
5.1. RF in Food
5.2. RF Encapsulation
| Encapsulation Techniques | Wall Material | Illustration of Characteristics | Purpose | Size | References |
|---|---|---|---|---|---|
| Cold-set gelation | Whey protein isolated | 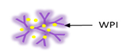 | Proofing suitability of encapsulation system for intestinal delivery using in vitro and in vivo models | 1.8 mm | [11] |
| Cross-linking of HIU-treated SPI with TGase | Soy protein isolated (SPI) | 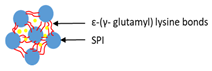 | Demonstrating of HIU-treated SPI–TGase cold gel for longer retention in the gastrointestinal system | 3 mm | [111] |
| Ionotropic gelation | Alginate/chitosan nanoparticles | 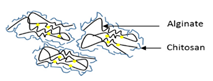 | Establishing of alginate/chitosan nanoparticle for controlled release in different temperature and pH conditions | 119.5 ± 49.9 nm | [112] |
| Ultrasonication | Soy protein/dextran nanogel | 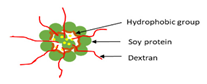 | Providing basic design of soy protein/dextran nanogel for effective and suitable carriers for bioactive compounds | 143.3 nm | [113] |
| Bioconjugation | Phenylalanine ethyl ester–alginate conjugated (PEA) | 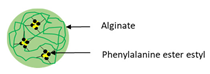 | Illustrating a sonication method of self-assembled nanoparticles formed by PEA conjugate without cytotoxicity against cell lines | 200 nm | [114] |
| Supercritical fluid technology | Fully hydrogenated canola oil | 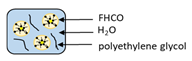 | Evaluating surfactant and molecular weight of stabilizer from supercritical fluid technology for development of solid lipid nanoparticles | 104 ± 5.7 nm | [115] |
| Coprecipitation-Crosslinking-Dissolution technique (CCD-technique) | Human serum albumin | 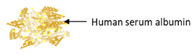 | Demonstrating a simple coprecipitation method of albumin submicron particles with good biocompatibility | 900 ± 1000 nm | [116] |
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| RF | Riboflavin |
| FMN | Flavin mononucleotide |
| FAD | Flavin adenine dinucleotide |
| SOD | Superoxide dismutase |
| ROS | Reactive oxygen species |
| GR | Glutathione reductase |
| GPx | Glutathione peroxidase |
| GSSG | Oxidized glutathione |
| GSH | Reduced glutathione |
| OGD | Oxygen glucose deprivation |
| LDH | Lactate dehydrogenase |
| Hb | Haemoglobin |
| TNF-α | Tumor necrosis factor alpha |
| NO | Nitric oxide |
| NF-κB | Nuclear factor factor kappa B |
| IκB | Inhibitory kappa B |
| LPS | Lipopolysaccharide |
| IL-6 | Interleukin-6 |
| MCP-1 | Monocyte chemo attractant protein 1 |
| MIP-2 | Macrophage inflammatory protein ()), |
| HMGB1 | High-mobility group protein B1 |
| MDA | Malondialdehyde level |
| MPO | Myeloperoxidase |
| CAT | Catalase |
| HSP25 | Heat shock protein 25 |
| RFK | Riboflavin Kinase |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| Nox2 | NADPH oxidase 2 |
| TNFR1 | necrosis factor receptor 1 |
| CRC | Colorectal cancer |
| MTHFR | Methylenetetrahydrofolate reductase |
| CCL4 | Carbon tetrachloride |
| PMS | Premenstrual syndrome |
| RFVT2 | RF transporter protein 2 |
| MMCA | Microsomal membrane Ca2+-ATPase |
| GTN | Glyceryl trinitrate |
| SE | Selenium |
| CCI | Cortical contusion injury linear dichroism |
| GFAP+ | Glial fibrillary acidic protein |
| T2DM | Type-2-diabetes mellitus |
| PTL | Peritoneal leukocytes |
| SPI | Soy protein isolate |
| HIU | High intensity ultrasound |
| TGase | Transglutaminase |
| SIF | Simulated intestinal fluid |
| SGF | Simulated gastric fluid |
| EE% | Entrapment efficiency |
| LC | Loading capacity |
| PEA | Phenylalanine ethyl ester–alginate conjugate |
| CCD-technique | Coprecipitation-Crosslinking-Dissolution technique |
References
- Cardoso, D.R.; Libardi, S.H.; Skibsted, L.H. Riboflavin as a photosensitizer. Effects on human health and food quality. Food Funct. 2012, 3, 487–502. [Google Scholar] [CrossRef]
- Dym, O.; Eisenberg, D. Sequence-structure analysis of FAD-containing proteins. Protein Sci. 2001, 10, 1712–1728. [Google Scholar] [CrossRef]
- Buehler, B.A. Vitamin B2: Riboflavin. J. Evid. Based. Complementary Altern. Med. 2011, 16, 88–90. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, W.E.; Yan, J.Q.; Liu, M.; Zhou, Y.; Shen, X.; Ma, Y.L.; Feng, X.S.; Yang, J.; Li, G.H. A review of the extraction and determination methods of thirteen essential vitamins to the human body: An update from 2010. Molecules 2018, 23, 1484. [Google Scholar] [CrossRef] [Green Version]
- Gul, W.N.; Anwar, Z.; Qadeer, K. Methods of analysis of riboflavin ( vitamin B2 ): A review. J. Pharm. Pharm. Sci. 2014, 2, 10–21. [Google Scholar]
- Antal, I.P.; Bazel, Y.R.; Kormosh, Z.A. Electrochemical methods for determining group B vitamins. J. Anal. Chem. 2013, 68, 565–576. [Google Scholar] [CrossRef]
- Powers, H.J. Riboflavin (vitamin B-2) and health. Am. J. Clin. Nutr. 2003, 77, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Toyosawa, T.; Suzuki, M.; Kodama, K.; Araki, S. Effects of intravenous infusion of highly purified vitamin B2 on lipopolysaccharide-induced shock and bacterial infection in mice. Eur. J. Pharmacol. 2004, 492, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Toyosawa, T.; Suzuki, M.; Kodama, K.; Araki, S. Potentiation by amino acid of the therapeutic effect of highly purified vitamin B2 in mice with lipopolysaccharide-induced shock. Eur. J. Pharmacol. 2004, 493, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Toyasaki, T. Antioxidant effect of riboflavin in enzymic lipid peroxidation. J. Agric. Food Chem. 1992, 40, 1727–1730. [Google Scholar] [CrossRef]
- Zou, Y.; Ruan, M.; Luan, J.; Feng, X.; Chen, S.; Chu, Z. Anti-aging effect of riboflavin via endogenous antioxidant in fruit fly Drosophila melanogaster. J. Nutr. Health Aging 2015, 21, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Cheung, I.M.Y.; Mcghee, C.N.J.; Sherwin, T. Beneficial effect of the antioxidant riboflavin on gene expression of extracellular matirix elements, antioxidants and oxidases in keratoconic stromal cells. Clin. Exp. Optom. 2014, 97, 349–355. [Google Scholar] [PubMed]
- Sanches, S.C.; Naira, L.; Ramalho, Z.; Mendes-Braz, M.; Terra, V.A.; Cecchini, R.; Augusto, M.J.; Ramalho, F.S. Riboflavin (vitamin B-2) reduces hepatocellular injury following liver ischaemia and reperfusion in mice. Food Chem. Toxicol. 2014, 67, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Pompella, A.; Visvikis, A.; Paolicchi, A.; De Tata, V.; Casini, A.F. The changing faces of glutathione, a cellular protagonist. Biochem. Pharmacol. 2003, 66, 1499–1503. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Dwivedi, A.; Pal, M.K.; Rastogi, N.; Gupta, P.; Ali, S.; Prabhu Bh, M.; Kushwaha, H.N.; Singh Ray, R.; Singh, S.K.; et al. Attenuated neuroprotective effect of riboflavin under UV-B irradiation via miR-203/c-Jun signaling pathway in vivo and in vitro. J. Biomed. Sci. 2014, 21, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Li, W.; Lu, X.; Zhao, X. Riboflavin alleviates cardiac failure in Type I diabetic cardiomyopathy. Heart Int. 2011, 6, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.M.; Iqbal, S.; Naseem, I. Ameliorative effect of riboflavin on hyperglycemia, oxidative stress and DNA damage in type-2 diabetic mice: Mechanistic and therapeutic strategies. Arch. Biochem. Biophys. 2015, 584, 10–19. [Google Scholar] [CrossRef]
- Indumathi, U.; Kanchana, K.; Sachdanandam, P. Protective role of coenzyme Q10, riboflavin, niacin, selenium (CoRNS) and Emblica officinalis on cardiac abnormalities in experimental atherosclerosis. Biomed. Prev. Nutr. 2013, 3, 313–318. [Google Scholar] [CrossRef]
- Nazıroğlu, M.; Çelik, Ö.; Uğuz, A.C.; Bütün, A. Protective effects of riboflavin and selenium on brain microsomal Ca2+-ATPase and oxidative damage caused by glyceryl trinitrate in a rat headache model. Biol. Trace Elem. Res. 2014, 164, 72–79. [Google Scholar] [CrossRef]
- Bütün, A.; Nazıroğlu, M.; Demirci, S.; Çelik, Ö.; Uğuz, A.C. Riboflavin and vitamin E increase brain calcium and antioxidants, and microsomal calcium-ATP-ase values in rat headache models induced by glyceryl trinitrate. J. Membr. Biol. 2015, 248, 205–213. [Google Scholar]
- Lin, Y.; Desbois, A.; Jiang, S.; Hou, S.T. Group B vitamins protect murine cerebellar granule cells from glutamate/NMDA toxicity. Neuroreport 2004, 15, 2241–2244. [Google Scholar] [CrossRef] [PubMed]
- Hoane, M.R.; Wolyniak, J.G.; Akstulewicz, S.L. Administration of riboflavin improves behavioral outcome and reduces edema formation and glial fibrillary acidic protein expression after traumatic brain injury. J. Neurotrauma 2005, 22, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Barbre, A.B.; Hoane, M.R. Magnesium and riboflavin combination therapy following cortical contusion injury in the rat. Brain Res. Bull. 2006, 69, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Seekamp, A.; Hultquist, D.E.; Till, G.O. Protection by vitamin B2 against oxidant-mediated acute lung injury. Inflammation 1999, 23, 449–460. [Google Scholar] [CrossRef]
- Betz, A.L.; Ren, X.D.; Ennis, S.R.; Hultquist, D.E. Riboflavin reduces edema in focal cerebral ischemia. Acta Neurochir. Suppl. (Wien). 1994, 60, 314–317. [Google Scholar]
- Mack, C.P.; Hultquist, D.E.; Shlafer, M. Myocardial flavin reductase and riboflavin: A potential role in decreasing reoxygenation injury. Biochem. Biophys. Res. Commun. 1995, 212, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.G.; Suryakar, A.N.; Sardeshmukh, A.S.; Rathi, D.B. Studies on biochemical changes with special reference to and antioxidants in malaria patients. Indian J. Clin. Biochem. 2003, 18, 136–149. [Google Scholar] [CrossRef] [Green Version]
- Akompong, T.; Ghori, N.; Haldar, K. In vitro activity of riboflavin against the human malaria parasite Plasmodium falciparum. Antimicrob. Agents Chemother. 2000, 44, 88–96. [Google Scholar] [CrossRef] [Green Version]
- George, B.O.; Ojegbemi, O. Oxidative stress and the effect of riboflavin supplementation in individuals with uncomplicated malaria infection. African J. Biotechnol. 2009, 8, 849–853. [Google Scholar]
- Araki, S.; Suzuki, M.; Fujimoto, M.; Kimura, M. Enhancement of resistance to bacterial infection in mice by vitamin b2. J. Vet. Med. Sci. 1995, 57, 599–602. [Google Scholar] [CrossRef] [Green Version]
- Mazur-Bialy, A.I.; Buchala, B.; Plytycz, B. Riboflavin deprivation inhibits macrophage viability and activity - a study on the RAW 264.7 cell line. Br. J. Nutr. 2013, 110, 509–514. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, A.A.; Reis, J.C.; Qureshi, N.; Papasian, C.J.; Morrison, D.C.; Schaefer, D.M. δ-Tocotrienol and quercetin reduce serum levels of nitric oxide and lipid parameters in female chickens. Lipids Health Dis. 2011, 10, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdrengh, M.; Tarkowski, A. Riboflavin in innate and acquired immune responses. Inflamm. Res. 2005, 9, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, K.; Hasegawa, T.; Hultquist, D.E.; Harada, H.; Yoshikawa, Y.; Yanamadala, S.; Liao, H.; Visovatti, S.H.; Pinsky, D.J. Riboflavin-mediated reduction of oxidant injury, rejection, and vasculopathy after cardiac allotransplantation. Transplantation 2007, 83, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.A.; Tan, X.; Reis, J.C.; Badr, M.Z.; Papasian, C.J.; Morrison, D.C.; Qureshi, N. Suppression of nitric oxide induction and pro-inflammatory cytokines by novel proteasome inhibitors in various experimental models. Lipids Health Dis. 2011, 10, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertollo, C.M.; Oliveira, A.C.P.; Rocha, L.T.S.; Costa, K.A.; Nascimento, E.B.; Coelho, M.M. Characterization of the antinociceptive and anti-inflammatory activities of riboflavin in different experimental models. Eur. J. Pharmacol. 2006, 547, 184–191. [Google Scholar] [CrossRef] [PubMed]
- França, D.S.; Souza, A.L.S.; Almeida, K.R.; Dolabella, S.S.; Martinelli, C.; Coelho, M.M. B vitamins induce an antinociceptive effect in the acetic acid and formaldehyde models of nociception in mice. Eur. J. Pharmacol. 2001, 421, 157–164. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I.; Majka, A.; Wojtas, L.; Kolaczkowska, E.; Plytycz, B. Strain-specific effects of riboflavin supplementation on zymosan-induced peritonitis in C57BL/6J, BALB/c and CBA mice. Life Sci. 2011, 88, 265–271. [Google Scholar] [CrossRef]
- Mazur, A.I.; Natorska, J.; Wypasek, E.; Kołaczkowska, E.; Płytycz, B. Anti-inflammatory effects of riboflavin and morphine on zymosan-induced peritonitis in Swiss mice. Cent. J. Immunol. 2008, 33, 98–101. [Google Scholar]
- Mazur-Bialy, A.I.; Kolaczkowska, E.; Plytycz, B. Modulation of zymosan-induced peritonitis by riboflavin co-injection, pre-injection or post-injection in male Swiss mice. Life Sci. 2012, 91, 1351–1357. [Google Scholar] [CrossRef]
- Granados-Soto, V.; Terán-Rosales, F.; Rocha-González, H.I.; Reyes-García, G.; Medina-Santillán, R.; Rodríguez-Silverio, J.; Flores-Murrieta, F.J. Riboflavin reduces hyperalgesia and inflammation but not tactile allodynia in the rat. Eur. J. Pharmacol. 2004, 492, 35–40. [Google Scholar] [CrossRef]
- Toyosawa, T.; Suzuki, M.; Kodama, K.; Araki, S. Highly Purified Vitamin B2 Presents a Promising Therapeutic Strategy for Sepsis and Septic Shock. Infect. Immun. 2004, 72, 1820–1823. [Google Scholar] [CrossRef] [PubMed]
- Kodama, K.; Suzuki, M.; Toyosawa, T.; Araki, S. Inhibitory mechanisms of highly purified vitamin B2 on the productions of proinflammatory cytokine and NO in endotoxin-induced shock in mice. Life Sci. 2005, 78, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.K.; Chen, C.M.; Chen, C.Y.O.; Liu, J.F.; Lin, H.W.; Chou, H.T.; Li, S.C. Riboflavin protects mice against liposaccharide-induced shock through expression of heat shock protein 25. Food Chem. Toxicol. 2010, 48, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, N.O.; Imam, F.; Nadeem, A.; Al-Harbi, M.M.; Korashy, H.M.; Sayed-Ahmed, M.M.; Hafez, M.M.; Al-Shabanah, O.A.; Nagi, M.N.; Bahashwan, S. Riboflavin attenuates lipopolysaccharide-induced lung injury in rats. Toxicol. Mech. Methods 2015, 25, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Mazur-bialy, A.I.; Pochec, E. HMGB1 inhibition during zymosan-induced inflammation: The potential therapeutic action of riboflavin. Arch. Immunol. Ther. Exp. 2015, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.-H.; Yin, Y.-J.; Zhang, J.-X. Sepsis and immune response. World J. Emerg. Med. 2011, 2, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Mal, P.; Ghosh, D.; Bandyopadhyay, D.; Dutta, K.; Bishayi, B. Ampicillin alone and in combination with riboflavin modulates Staphylococcus aureus infection induced septic arthritis in mice. Indian J. Exp. Biol. 2012, 50, 677–689. [Google Scholar]
- Dey, S.; Bishayi, B. Riboflavin along with antibiotics balances reactive oxygen species and inflammatory cytokines and controls Staphylococcus aureus infection by boosting murine macrophage function and regulates inflammation. J. Inflamm. 2016, 13, 36. [Google Scholar] [CrossRef] [Green Version]
- Mal, P.; Dutta, K.; Bandyopadhyay, D.; Basu, A.; Khan, R.; Bishayi, B. Azithromycin in combination with riboflavin decreases the severity of Staphylococcus aureus infection induced septic arthritis by modulating the production of free radicals and endogenous cytokines. Inflamm. Res. 2013, 62, 259–273. [Google Scholar] [CrossRef]
- Schramm, M.; Wiegmann, K.; Schramm, S.; Gluschko, A.; Herb, M.; Utermöhlen, O.; Krönke, M. Riboflavin (vitamin B2) deficiency impairs NADPH oxidase 2 (Nox2) priming and defense against Listeria monocytogenes. Eur. J. Immunol. 2014, 44, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Wooley, J.G.; Sebrell, W. Nutritional deficiency and infection: I. influence of riboflavin or thiamin deficiency on fatal experimental pneumococcal infection in white mice. Public Health Rep. 1942, 57, 149–161. [Google Scholar] [CrossRef]
- Thaimuta, Z.L. Riboflavin protective role against mitochondrial toxicity and lipodystrophy due to stavudine and lamivudine. Ph.D. Thesis, University of Nairobi, Eldoret, Kenya, 2014. [Google Scholar]
- Corbin, F. Pathogen inactivation of blood components: Current status and introduction of an approach using riboflavin as a photosensitizer. Int. J. Hematol. 2002, 76 (Suppl. S2), 253–257. [Google Scholar] [CrossRef] [PubMed]
- Ruane, P.H.; Edrich, R.; Gampp, D.; Keil, S.D.; Leonard, R.L.; Goodrich, R.P. Photochemical inactivation of selected viruses and bacteria in platelet concentrates using riboflavin and light. Transfusion 2004, 44, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Cardo, L.J.; Rentas, F.J.; Ketchum, L.; Salata, J.; Harman, R.; Melvin, W.; Weina, P.J.; Mendez, J.; Reddy, H.; Goodrich, R. Pathogen inactivation of Leishmania donovani infantum in plasma and platelet concentrates using riboflavin and ultraviolet light. Vox Sang. 2006, 90, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.P.R.; Transue, S.; Snyder, E.L. Pathogen inactivation techniques. Best Pract. Res. Clin. Haematol. 2006, 19, 205–242. [Google Scholar] [CrossRef]
- Shrubsole, M.J.; Shu, X.O.; Li, H.L.; Cai, H.; Yang, G.; Gao, Y.T.; Gao, J.; Zheng, W. Dietary B vitamin and methionine intakes and breast cancer risk among Chinese women. Am. J. Epidemiol. 2011, 173, 1171–1182. [Google Scholar] [CrossRef] [Green Version]
- Chaves Neto, A.H.; Pelizzaro-Rocha, K.J.; Fernandes, M.N.; Ferreira-Halder, C.V. Antitumor activity of irradiated riboflavin on human renal carcinoma cell line 786-O. Tumor Biol. 2015, 36, 595–604. [Google Scholar] [CrossRef]
- Machado, D.; Shishido, S.M.; Queiroz, K.C.S.; Oliveira, D.N.; Faria, A.L.C.; Catharino, R.R.; Spek, C.A.; Ferreira, C.V. Irradiated riboflavin diminishes the aggressiveness of melanoma in vitro and in vivo. PLoS ONE 2013, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- De Vogel, S.; Dindore, V.; van Engeland, M.; Goldbohm, R.A.; van den Brandt, P.A.; Weijenberg, M.P. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectalc cancer. J. Nutr. 2008, 138, 2372–2378. [Google Scholar] [CrossRef] [Green Version]
- Zschäbitz, S.; Cheng, T.D.; Neuhouser, M.L.; Zheng, Y.; Ray, R.M.; Miller, J.W.; Song, X.; Maneval, D.R.; Beresford, S.A.A.; Lane, D.; et al. B vitamin intakes and incidence of colorectal cancer: Results from the women’s health initiative observational study cohort. Am. J. Clin. Nutr. 2013, 97, 332–343. [Google Scholar]
- Kabat, G.C.; Miller, A.B.; Jain, M.; Rohan, T.E. Dietary intake of selected B vitamins in relation to risk of major cancers in women. Br. J. Cancer 2008, 99, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Takata, Y.; Cai, Q.; Beeghly-Fadiel, A.; Li, H.; Shrubsole, M.J.; Ji, B.T.; Yang, G.; Chow, W.H.; Gao, Y.T.; Zheng, W.; et al. Dietary B vitamin and methionine intakes and lung cancer risk among female never smokers in China. Cancer Causes Control 2012, 23, 1965–1975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, B.Y.; McDuffie, K.; Wilkens, L.R.; Kamemoto, L.; Goodman, M.T. Diet and premalignant lesions of the cervix: Evidence of a protective role for folate, riboflavin, thiamin, and vitamin B12. Cancer Causes Control 2003, 14, 859–870. [Google Scholar] [CrossRef] [Green Version]
- Powers, H.J. Interaction among folate, riboflavin, genotype, and cancer, with reference to colorectal and cervical cancer. J. Nutr. 2005, 135, 2960S–2966S. [Google Scholar] [CrossRef] [Green Version]
- Rivlin, R.S. Riboflavin and cancer: A review. Cancer Res. 1973, 33, 1977–1986. [Google Scholar]
- Foy, H.; Kondi, A.; Verjee, Z.H.M. Relation of riboflavin deficiency to corticosteroid metabolism and red cell hypoplasia in baboons. J. Nutr. 1972, 102, 571–582. [Google Scholar] [CrossRef]
- Bareford, L.M.; Phelps, M.A.; Foraker, A.B.; Swaan, P.W. Intracellular processing of riboflavin in human breast cancer cells. Mol. Pharm. 2008, 5, 839–848. [Google Scholar] [CrossRef]
- Webster, R.P.; Gawde, M.D.; Bhattacharya, R.K. Modulation of carcinogen-induced DNA damage and repair enzyme activity by dietary riboflavin. Cancer Lett. 1996, 98, 129–135. [Google Scholar] [CrossRef]
- Hassan, I.; Chibber, S.; Naseem, I. Vitamin B2: A promising adjuvant in cisplatin based chemoradiotherapy by cellular redox management. Food Chem. Toxicol. 2013, 59, 715–723. [Google Scholar] [CrossRef]
- Naseem, I.; Hassan, I.; Alhazza, I.M.; Chibber, S. Protective effect of riboflavin on cisplatin induced toxicities: A gender-dependent study. J. Trace Elem. Med. Biol. 2015, 29, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Z.; An, Y.; Yang, D.; Wang, S.; Xu, Q.; Yuan, S. Exploration of the protection of riboflavin laurate on oral mucositis induced by chemotherapy or radiotherapy at the cellular level: What is the leading contributor? Int. J. Mol. Sci. 2013, 14, 4722–4733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Harbi, N.O.; Imam, F.; Nadeem, A.; Al-Harbi, M.M.; Iqbal, M.; Ahmad, S.F. Carbon tetrachloride-induced hepatotoxicity in rat is reversed by treatment with riboflavin. Int. Immunopharmacol. 2014, 21, 383–388. [Google Scholar] [CrossRef]
- Thakur, K.; Tomar, S.K.; Singh, A.K.; Mandal, S.; Arora, S.; Division, D.M.; Division, D.T.; Division, D.C.; Microbiology, D. Riboflavin and health: A review of recent human research. Crit. Rev. Food Sci. Nutr. 2017, 57, 3650–3660. [Google Scholar] [CrossRef] [PubMed]
- Sparaco, M.; Feleppa, M.; Lipton, R.B.; Rapoport, A.M.; Bigal, M.E. Mitochondrial dysfunction and migraine: Evidence and hypotheses. Cephalalgia 2006, 26, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, M.; Goldman, R.D. Effectiveness of riboflavin in pediatric migraine prevention. Can. Fam. Physician 2014, 60, 244–246. [Google Scholar] [PubMed]
- Condó, M.; Posar, A.; Arbizzani, A.; Parmeggiani, A. Riboflavin prophylaxis in pediatric and adolescent migraine. J. Headache Pain 2009, 10, 361–365. [Google Scholar] [CrossRef] [Green Version]
- Maizels, M.; Blumenfeld, A.; Burchette, R. A combination of riboflavin, magnesium, and feverfew for migraine prophylaxis: A randomized trial. Headache 2004, 44, 885–890. [Google Scholar] [CrossRef]
- Gaul, C.; Diener, H.-C.; Danesch, U. Improvement of migraine symptoms with a proprietary supplement containing riboflavin, magnesium and Q10: A randomized, placebo-controlled, double-blind, multicenter trial. J. Headache Pain 2015, 16, 516. [Google Scholar] [CrossRef] [Green Version]
- Jacques, P.F.; Chylack, L.T.; Hankinson, S.E.; Khu, P.M.; Rogers, G.; Friend, J.; Tung, W.; Wolfe, J.K.; Padhye, N.; Willett, W.C.; et al. Long-term nutrient intake and early age-related nuclear lens opacities. Arch. Ophthalmol. 2001, 119, 1009–1019. [Google Scholar] [CrossRef] [Green Version]
- Skalka, H.W.; Prchal, J.T. Cataracts and riboflavin deficiency. Am. J. Clin. Nutr. 1981, 34, 861–863. [Google Scholar] [CrossRef] [PubMed]
- SEETHARAM BHAT, K. Nutritional status of thiamin riboflavin and pyridoxine in cataract patients. Nutr. Rep. Int. 1987, 36, 685–692. [Google Scholar]
- Chocano-bedoya, P.O.; Manson, J.E.; Hankinson, S.E.; Willett, W.C.; Johnson, S.R.; Chasan-taber, L.; Ronnenberg, A.G.; Bigelow, C.; Bertone-johnson, E.R. Dietary B vitamin intake and incident premenstrual syndrome. Am. J. Clin. Nutr. 2011, 93, 1080–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaves Neto, A.H.; Yano, C.L.; Paredes-Gamero, E.J.; Machado, D.; Justo, G.Z.; Peppelenbosch, M.P.; Ferreira, C.V. Riboflavin and photoproducts in MC3T3-E1 differentiation. Toxicol. Vitr. 2010, 24, 1911–1919. [Google Scholar] [CrossRef] [PubMed]
- Ogunleye, A.J.; Odutuga, A.A. The effect of riboflavin deficiency on cerebrum and cerebellum of developing rat brain. J. Nutr. Sci. Vitaminol. (Tokyo). 1989, 35, 193–197. [Google Scholar] [CrossRef]
- Foley, A.R.; Menezes, M.P.; Pandraud, A.; Gonzalez, M.A.; Al-Odaib, A.; Abrams, A.J.; Sugano, K.; Yonezawa, A.; Manzur, A.Y.; Burns, J.; et al. Treatable childhood neuronopathy caused by mutations in riboflavin transporter RFVT2. Brain A J. Neurol. 2014, 137, 44–56. [Google Scholar] [CrossRef] [Green Version]
- Boisvert, W.A.; Castañeda, C.; Mendoza, I.; Langeloh, G.; Solomons, N.W.; Gershoff, S.N.; Russell, R.M. Prevalence of riboflavin deficiency among Guatemalan elderly people and its relationship to milk intake. Am. J. Clin. Nutr. 1993, 58, 85–90. [Google Scholar] [CrossRef]
- Powers, H.J.; Weaver, L.T.; Austin, S.; Beresford, J.K. A proposed intestinal mechanism for the effect of riboflavin deficiency on iron loss in the rat. Br. J. Nutr. 1993, 69, 553–561. [Google Scholar] [CrossRef] [Green Version]
- Powers, H.J.; Bates, C.J.; Prentice, A.M.; Lamb, W.H.; Jepson, M.; Bowman, H. The relative effectiveness of iron and iron with riboflavin in correcting a microcytic anaemia in men and children in rural Gambia. Hum. Nutr. Clin. Nutr. 1983, 37, 413–425. [Google Scholar]
- Powers, H.J.; Hill, M.H.; Mushtaq, S.; Dainty, J.R.; Majsak-Newman, G.; Williams, E.A. Correcting a marginal riboflavin deficiency improves hematologic status in young women in the United Kingdom (RIBOFEM). Am. J. Clin. Nutr. 2011, 93, 1274–1284. [Google Scholar] [CrossRef] [Green Version]
- Lopez, A.D.; Mathers, C.D.; Ezzati, M.; Jamison, D.T.; Murray, C.J. Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data. Lancet 2006, 367, 1747–1757. [Google Scholar] [CrossRef]
- Lawes, C.M.M.; Vander Hoorn, S.; Rodgers, A. Global burden of blood-pressure-related disease, 2001. Lancet 2008, 371, 1513–1518. [Google Scholar] [CrossRef]
- França, C.F.; Vianna, L.M. The response of young and adult rats to the riboflavin supplementation. Braz. Arch. Biol. Technol. 2010, 53, 855–860. [Google Scholar] [CrossRef] [Green Version]
- Leblanc, J.G.; Laiño, J.E.; del Valle, M.J.; Vannini, V.; van Sinderen, D.; Taranto, M.P.; de Valdez, G.F.; de Giori, G.S.; Sesma, F. B-Group vitamin production by lactic acid bacteria—Current knowledge and potential applications. J. Appl. Microbiol. 2011, 111, 1297–1309. [Google Scholar] [CrossRef]
- Mensink, G.B.M.; Fletcher, R.; Gurinovic, M.; Huybrechts, I.; Lafay, L.; Serra-Majem, L.; Szponar, L.; Tetens, I.; Verkaik-Kloosterman, J.; Baka, A.; et al. Mapping low intake of micronutrients across Europe. Br. J. Nutr. 2013, 110, 755–773. [Google Scholar] [CrossRef] [Green Version]
- Flynn, A.; Moreiras, O.; Stehle, P.; Fletcher, R.J.; Müller, D.J.G.; Rolland, V. Vitamins and minerals: A model for safe addition to foods. Eur. J. Nutr. 2003, 42, 118–130. [Google Scholar] [CrossRef]
- Ashoori, M.; Saedisomeolia, A. Riboflavin (vitamin B2) and oxidative stress: A review. Br. J. Nutr. 2014, 111, 1985–1991. [Google Scholar] [CrossRef] [Green Version]
- LeBlanc, J.G.; Burgess, C.; Sesma, F.; de Giori, G.S.; van Sinderen, D. Ingestion of milk fermented by genetically modified Lactococcus lactis improves the riboflavin status of deficient rats. J. Dairy Sci. 2005, 88, 3435–3442. [Google Scholar] [CrossRef] [Green Version]
- Capozzi, V.; Menga, V.; Digesù, A.M.; De Vita, P.; Van Sinderen, D.; Cattivelli, L.; Fares, C.; Spano, G. Biotechnological production of vitamin B2-enriched bread and pasta. J. Agric. Food Chem. 2011, 59, 8013–8020. [Google Scholar] [CrossRef]
- Juarez del Valle, M.; Laiño, J.E.; Savoy de Giori, G.; LeBlanc, J.G. Riboflavin producing lactic acid bacteria as a biotechnological strategy to obtain bio-enriched soymilk. Food Res. Int. 2014, 62, 1015–1019. [Google Scholar] [CrossRef]
- Russo, P.; de Chiara, M.L.V.; Capozzi, V.; Arena, M.P.; Amodio, M.L.; Rascón, A.; Dueñas, M.T.; López, P.; Spano, G. Lactobacillus plantarum strains for multifunctional oat-based foods. LWT Food Sci. Technol. 2016, 68, 288–294. [Google Scholar] [CrossRef] [Green Version]
- Russo, P.; Capozzi, V.; Arena, M.P.; Spadaccino, G.; Dueñas, M.T.; López, P.; Fiocco, D.; Spano, G. Riboflavin-overproducing strains of Lactobacillus fermentum for riboflavin-enriched bread. Appl. Microbiol. Biotechnol. 2014, 98, 3691–3700. [Google Scholar] [CrossRef] [PubMed]
- Burgess, C.M.; Smid, E.J.; Rutten, G.; van Sinderen, D. A general method for selection of riboflavin-overproducing food grade micro-organisms. Microb. Cell Fact. 2006, 5, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeBlanc, J.G.; Rutten, G.; Bruinenberg, P.; Sesma, F.; de Giori, G.S.; Smid, E.J. A novel dairy product fermented with Propionibacterium freudenreichii improves the riboflavin status of deficient rats. Nutrition 2006, 22, 645–651. [Google Scholar] [CrossRef]
- Berry Ottaway, P. Stability of vitamins during food processing and storage. In Chemical Deterioration and Physical Instability of Food and Beverages; Elsevier: Amsterdam, The Netherlands, 2010; pp. 539–560. [Google Scholar]
- Bou, R.; Cofrades, S.; Jiménez-Colmenero, F. Physicochemical properties and riboflavin encapsulation in double emulsions with different lipid sources. LWT Food Sci. Technol. 2014, 59, 621–628. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Subirade, M. Alginate-whey protein granular microspheres as oral delivery vehicles for bioactive compounds. Biomaterials 2006, 27, 4646–4654. [Google Scholar] [CrossRef]
- Chen, L.; Subirade, M. Effect of preparation conditions on the nutrient release properties of alginate-whey protein granular microspheres. Eur. J. Pharm. Biopharm. 2007, 65, 354–362. [Google Scholar] [CrossRef]
- O’Neill, G.J.; Jacquier, J.C.; Mukhopadhya, A.; Egan, T.; O’Sullivan, M.; Sweeney, T.; O’Riordan, E.D. In vitro and in vivo evaluation of whey protein hydrogels for oral delivery of riboflavin. J. Funct. Foods 2015, 19, 512–521. [Google Scholar] [CrossRef]
- Hu, H.; Zhu, X.; Hu, T.; Cheung, I.W.Y.; Pan, S.; Li-Chan, E.C.Y. Effect of ultrasound pre-treatment on formation of transglutaminase-catalysed soy protein hydrogel as a riboflavin vehicle for functional foods. J. Funct. Foods 2015, 19, 182–193. [Google Scholar] [CrossRef]
- Azevedo, M.A.; Bourbon, A.I.; Vicente, A.A.; Cerqueira, M.A. Alginate/chitosan nanoparticles for encapsulation and controlled release of vitamin B2. Int. J. Biol. Macromol. 2014, 71, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Jin, B.; Zhou, X.; Li, X.; Lin, W.; Chen, G.; Qiu, R. Self-assembled modified soy protein/dextran nanogel induced by ultrasonication as a delivery vehicle for riboflavin. Molecules 2016, 21, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Zhao, S.R.; Li, J.X.; Hong, L.; Raja, M.A.; Yu, L.J.; Liu, C.G. Nanoparticles based on phenylalanine ethyl ester-alginate conjugate asvVitamin B2 delivery system. J. Biomater. Appl. 2016, 31, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Couto, R.; Alvarez, V.; Temelli, F. Encapsulation of vitamin B2 in solid lipid nanoparticles using supercritical CO2. J. Supercrit. Fluids 2017, 120, 432–442. [Google Scholar] [CrossRef]
- Suwannasom, N.; Smuda, K.; Kloypan, C.; Kaewprayoon, W.; Baisaeng, N.; Prapan, A.; Chaiwaree, S.; Georgieva, R.; Bäumler, H.; Suwannasom, N.; et al. Albumin Submicron Particles with Entrapped Riboflavin—Fabrication and Characterization. Nanomaterials 2019, 9, 482. [Google Scholar] [CrossRef] [Green Version]









| Riboflavin | FAD | FMN | |
|---|---|---|---|
| Skin | 7.6 × 10−6 | — | — |
| Cerebral cortex | 7.2 × 10−6 | — | — |
| Myocardium | 3.2 × 10−5 | — | — |
| Pectoral muscle | 7.2 × 10−6 | — | — |
| Aortic tissue | 4.8 × 10−7 | 9.7 × 10−7 | 2.2 × 10−7 |
| Erythrocyte | 1.4 × 10−7 | 4.3 × 10−7 | 2.8 × 10−8 |
| Plasma | 1.0 × 10−8 | 6.3 × 10−8 | 7.5 × 10−9 |
| Eye-fluid | 4.5 × 10−6 | — | — |
| Animal Model | RF Doses/Models | Major Outcome | References |
|---|---|---|---|
| Inflammation-Related Pain | |||
| Acetic acid-induced abdominal constructions, formaldehyde-induced nociceptive response and hot-plate models in mice | RF at 3–100 mg/kg i.p. injection 1 h before acetic acid-induced model, RF at 6 or12 mg/kg i.p. injection 1 h before formaldehyde-induced nociceptive response, and RF at 50 mg/kg i.p. injection 1 h before formaldehyde-induced hindpaw edema | A dose-dependent RF inhibited the nociceptive response produced by acetic acid. Pre-treatment RF remarkably reduced the acute nociceptive response induced by formaldehyde in the second phase, but not in the hot-plate model. RF moderately inhibited formaldehyde-induced hindpaw edema. | [37] |
| Formalin-induced and carrageenan-induced paw edema, and spinal nerve ligation models in rat | RF at 1–50 mg/kg oral administration 30 min before formalin test and 6.25–150 mg/kg immediately after carrageenan injection | Second phase treatment with RF produced a significant dose-dependent inhibition in flinching behavior produced by formalin and RF at 25 mg/kg dose had peak antinociceptive effect in formalin-induced model. RF reduced hyperalgesic effect, highest effect at 75 mg/kg dose. In addition, a dose- and time-dependent RF treatment reduced by carrageenan-induced edema, but not tactile allodynia in the spinal nerve ligation models. Moreover, antinociceptive effect of RF can be reversed by glibenclamide and NG-L-nitro-aeginie methyl ester. | [41] |
| Formalin-induced nociceptive response, carrageenan-induced paw edema, LPS-induced febrile response, and cotton pellet-induced formation of fibrovascular tissue models in rat | RF at 25, 50, 100 mg/kg i.p. injection 30 min before formalin-induced nociceptive response, carrageenan-induced paw edema, RF at 50 or 100 mg/kg immediately or 2 hr after LPS-induced the febrile response, and RF at 50 or 100 mg/kg i.p. 7 days after s.c. implantation of a cotton pellet-induced fibrovascular tissue | RF inhibited the nociceptive response in the mouse formalin test, markedly in second phase. RF was dose-dependently reduced the mechanical allodynia and the paw edema induced by carrageenan and inhibited the fever induced by LPS. Moreover, the formation of fibrovascular tissue induced by s.c. implant of a cotton pellet was inhibited. Therefore, RF prevents prolonged inflammatory response. | [36] |
| Zymosan-induced peritonitis in Swiss mice | RF at 20, 50, 100 mg/kg i.p. injection 30 min before zymosan administration; RF at 50 mg/kg in combination with 5 mg/kg morphine | RF at 50 and 100 mg/kg induced antinociceptive-related body writhes and RF at 100 mg/kg dose suppressed intraperitoneal PMN influx. On the other hand, RF co-injected with morphine at low dose had antinociceptive effect and also reduced levels of proinflammatory cytikines such as TNF-α, IL-12p07, and IFN-γ according to RF dose and the time of injection. | [39] |
| Anti-Inflammatory Effect | |||
| Toxin-induced shock (LPS-induced shock and S. aureus enterotoxin B (SEB)-induced shock) and bacterial infection in mice | RF at 2.5, 5, 10, and 20 mg/kg bolus injection 6 h after LPS injection or SEB–D-galactosamine injection. RF at 2.5, 5, 10, 20 mg/kg 1 day before E. coli inoculation or 1 and 2 days after S. aureus inoculation. | RF decreased the mortality of endotoxin- and exotoxin-induced shock, gram-negative and gram-positive bacterial infection including long-term treatment. In addition, RF reduced levels of plasma inflammatory cytokines, including TNF-, IL-1β, IL-6, IFN-γ, MCP-1, MIP-2, and NO level. Moreover, co-administration RF with APC ameliorated survival rate of toxin-induced shock. | [42] |
| LPS-induced shock model and bacterial infection model in mice | RF at 2.5, 5, 10, 20, 40, and 80 mg/kg/6h i.v. infusion after 6 h LPS injection. RF at 80 mg/kg/6 h after 1 h E.coli infection or RF at 20, 40, 80 mg/kg/6 h after 1 h S.aureus infection. | RF protected mice against the mortality in both toxin shock and infection models, but RF reduced only the level of IL-6 and NO in plasma. In addition, RF decreased the elevation of TNF-α, IL-1β, MPC-1, IL-6, and NO level in plasma. | [8] |
| LPS-induced shock model in mice | RF at 2.5 or 10 mg/kg for 6 h continuous i.v bolus administration with or without aminolevane® or single dose injection with or without amino acids or valine after 6 h LPS injection. | RF at 10 mg/kg administered continuously for 6 h reduced morbidities on LPS- induced shock model, and was better with aminolevane® combination treatment. RF treatment in combination with tryptophan, isoleucine, proline, threonine, alanine or valine had improved the survival rate, but only valine was significantly effective. Moreover, RF reduced IL-6, lactic acid level, but increased glucose level. | [9] |
| Endotoxin-induced shock in mice | RF at 20 mg/kg i.v. administered after 6 h LPS injection | RF decreased the number of IL-6 and MIP-2 and NO levels in plasma. RF also reduced IL-6 and MIP-2 levels in lung, but inhibited only MIP-2 level in liver. However, RF reduced IL-6 mRNA expression in lung, but MIP-2 mRNA expression was inhibited in liver and kidney. Additionally, iNO expression was inhibited by RF. | [43] |
| Olive oil-triggered paw swelling and collagen-induced arthritis models in mice | RF at 20 mg/kg i.p. administration before oil injection or after collagen-induced arthritis | RF inhibited the paw swelling induced by olive oil, affecting a reduction in neutrophil-dependent reaction. However, RF could not inhibit delayed type hypersensitivity reactivity and collagen II arthritis. | [33] |
| LPS-induced shock model in mice | RF at 1 and 10 mg/kg i.p. injection at 2 and 0 h before LPS administration | RF significantly suppressed the LPS-induced lethality in mice septic shock model and RF have protective effect through up-regulated the expression of HSP25 in the lung and heart. | [44] |
| Zymosan-induced peritonitis in different C57BL/6J, BALB/c and CBA mice strains | RF at 50 mg/kg i.p. co-injection with zymosan (40 mg/kg) | RF co-treatment with zymosan reduced pain symptoms. Anti-inflammatory effects of RF are strain-specific manner. Particularly, peritoneal leukocytes (PTL) accumulation was inhibited in BALB/c and CBA strains, but was prolonged in C57BL/6J strain. The expression of iNOS was delayed (C57BL/6J) or inhibited (BALB/c and CBA) in PTL lysates as well as the prolonged (C57BL/6) or inhibited (BALB/c) intraperitoneal accumulation of MMP-9. | [38] |
| Zymosan-induced peritonitis in Swiss mice | RF at 0, 20, 50, or 100 mg/kg by co-injection, pre-injection or post-injection in zymosan-induced peritonitis | RF itself induced nociceptive-related body writhes, but effectively reduces zymosan-induced writhing response on influence of pre-injection or post- injection. RF also reduced the evaluation number of PLTs, mainly PMN and an increase in inflammation-related cytokines and MMP-9 with dose- and administration time-dependent effect. | [40] |
| LPS-induced acute lungs injury in rat | RF at 30 mg/kg, p.o. for 7 days before LPS intranasally (i.n.) | RF reduced interstitial edema, hemorrhage, infiltration of inflammatory PMNs, and destruction of lung parenchyma as well as decreased the iNOS level, but enhanced GSH, GR, GRx, and CAT expression. | [45] |
| Zymosan-induced inflammation in mice and in vitro macrophages | RF at 50 mg/kg i.p. injection 30 min either before zymosan, together with zymosan, or 2, 4, 6 h after i.p. zymosan injection. | RF causes both the inhibition of expression and release of HMGB1 in time-dependent manner. | [46] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suwannasom, N.; Kao, I.; Pruß, A.; Georgieva, R.; Bäumler, H. Riboflavin: The Health Benefits of a Forgotten Natural Vitamin. Int. J. Mol. Sci. 2020, 21, 950. https://doi.org/10.3390/ijms21030950
Suwannasom N, Kao I, Pruß A, Georgieva R, Bäumler H. Riboflavin: The Health Benefits of a Forgotten Natural Vitamin. International Journal of Molecular Sciences. 2020; 21(3):950. https://doi.org/10.3390/ijms21030950
Chicago/Turabian StyleSuwannasom, Nittiya, Ijad Kao, Axel Pruß, Radostina Georgieva, and Hans Bäumler. 2020. "Riboflavin: The Health Benefits of a Forgotten Natural Vitamin" International Journal of Molecular Sciences 21, no. 3: 950. https://doi.org/10.3390/ijms21030950
APA StyleSuwannasom, N., Kao, I., Pruß, A., Georgieva, R., & Bäumler, H. (2020). Riboflavin: The Health Benefits of a Forgotten Natural Vitamin. International Journal of Molecular Sciences, 21(3), 950. https://doi.org/10.3390/ijms21030950






