Advances in Chemistry and Bioactivity of Magnoflorine and Magnoflorine-Containing Extracts
Abstract
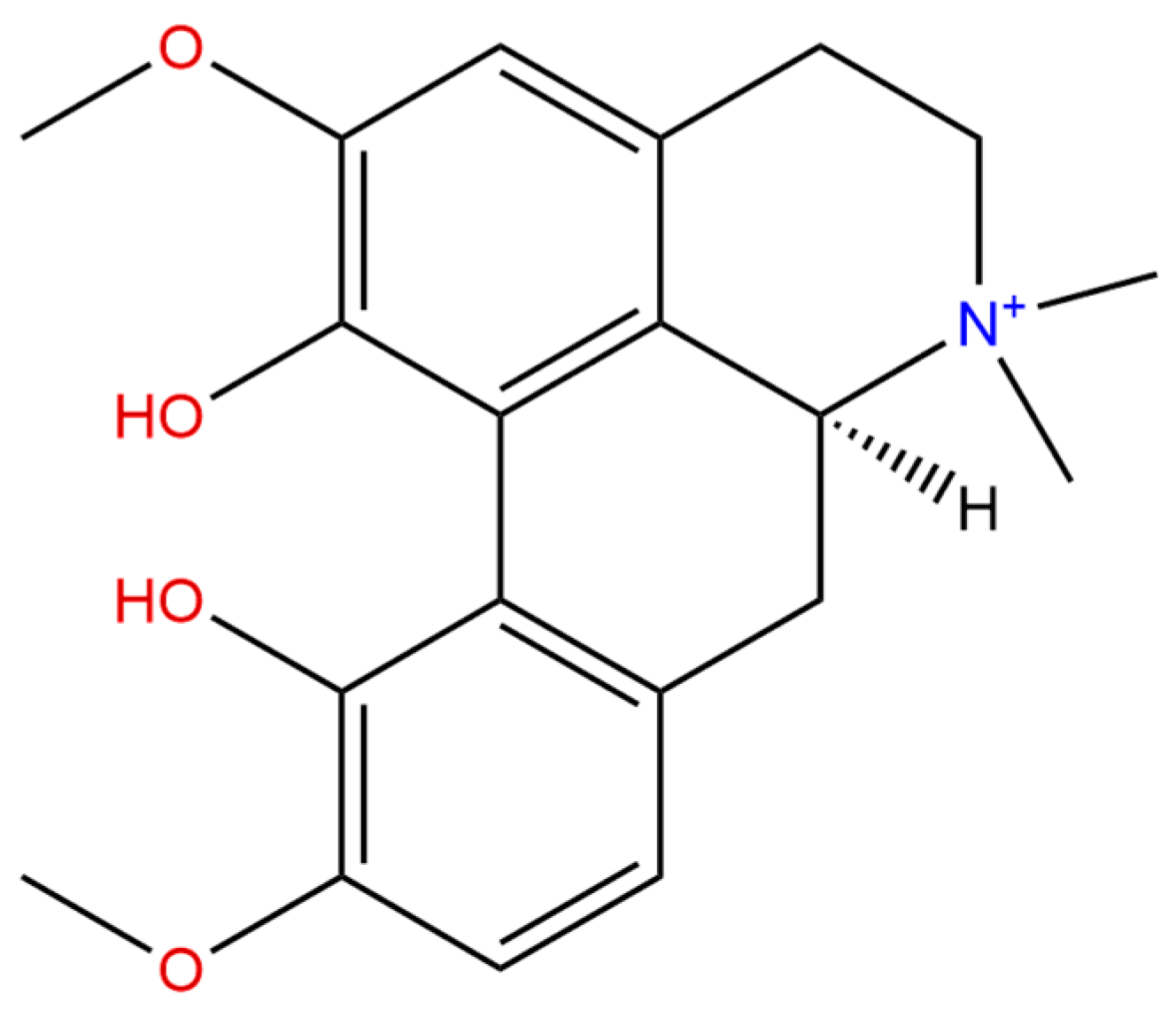
:1. Introduction
2. Chemistry and Pharmacokinetics of Magnoflorine
Pharmacokinetics of Magnoflorine
3. Biological Activities
3.1. The Effect on Carbohydrate–Lipid Metabolism
Hypercholesterolemia and Obesity
3.2. Antioxidant Properties
3.3. Anti-Alzheimier’s and Anti-Aging Effect
3.4. Anti-Inflammatory, Immunomodulatory, and Anti-Allergic Effects
3.5. Anticancer Activity
3.6. Depressant Effect on the Central Nervous System
3.7. Antidepressant Effect
3.8. Antiosteoporosis Effect
3.9. Cardiovascular Effects
3.10. Anti-Microbial Activity
3.10.1. Antibacterial Activity
3.10.2. Anti-Fungal Activity
3.10.3. Antiviral Activity
4. Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Kukula-Koch, W.; Widelski, J. Alkaloids. In Pharmacognosy: Fundamentals, Applications and Strategy; Badal, S., Delgoda, R., Eds.; Academic Press: London, UK, 2017; pp. 163–198. ISBN 9780128021040. [Google Scholar]
- Cubukcu, B. Quarternary alkaloids of Berberis crataegina and Berberis cretica. Plantes Med. Phytother. 1968, 2, 272–280. [Google Scholar]
- Kukula-Koch, W.; Mroczek, T. Application of hydrostatic CCC-TLC-HPLC-ESI-TOF-MS for the bioguided fractionation of anticholinesterase alkaloids from Argemone mexicana L. roots. Anal. Bioanal. Chem. 2015, 407, 2581–2589. [Google Scholar] [CrossRef] [Green Version]
- Kukula-Koch, W.; Kruk-Słomka, M.; Stępnik, K.; Szalak, R.; Biała, G. The evaluation of pro-cognitive and antiamnestic properties of berberine and magnoflorine isolated from barberry species by Centrifugal Partition Chromatography (CPC), in relation to QSAR modeling. Int. J. Mol. Sci 2017, 18, 2511. [Google Scholar] [CrossRef] [Green Version]
- Jossang, A.; Leboeuf, M.; Cave, A. Alkaloids of annonaceae XVII: Alkaloids of Enantia polycarpa Engl. et Diels. Planta Med. 1977, 32, 249–257. [Google Scholar] [CrossRef]
- Kukula-Koch, W. The elevation of LC-ESI-Q-TOF-MS response in the analysis of isoquinoline alkaloids from some Papaveraceae and Berberidaceae representatives. J. Anal. Meth. Chem. 2017, 2017, 8384107. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.; Eisenbrand, G. Chinese Drugs of Plant Origin. Chemistry, Pharmacology and Use in Traditional and Modern Medicine; Springer: Berlin, Germany, 1992. [Google Scholar]
- Duan, J.-X.; Li, G.-Y.; Lü, S.-W.; Su, H.; Xu, D.; Guo, Y.-Y.; Kuang, H.-X.; Wang, Q.-H. Analysis of bioactive components and pharmacokinetics of Caulophyllum robustum in rat plasma after oral administration by UPLC-ESI-MS/MS. J. Asia Nat. Prod. Res. 2018, 1–13. [Google Scholar] [CrossRef]
- Singh, A.; Bajpai, V.; Kumar, S.; Singh Rawat, A.K.; Kumar, B. Analysis of isoquinoline alkaloids from Mahonia leschenaultia and Mahonia napaulensis roots using UHPLC-Orbitrap-MSn and UHPLC-QqQLIT-MS/MS. J. Pharm. Anal. 2017, 7, 77–86. [Google Scholar] [CrossRef] [PubMed]
- He, J.-M.; Mu, Q. The medicinal uses of the genus Mahonia in traditional Chinese medicine: An ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2015, 175, 668–683. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Yin, Y.; Wei, J.; Chen, X.; Ouyang, H.; Chang, Y.; Gao, X.; He, J. Development and validation of a HPLC-MS/MS method for simultaneous determination of twelve bioactive compounds in epimedium: Application to a pharmacokinetic study in rats. Molecules 2018, 23, 1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, C.-Y.; Liu, J.-Q.; Zhang, R.; Shu, J.-C. A new alkaloid from the fruit of Nandina domestica Thunb. Nat. Prod. Res. 2014, 28, 1159–1164. [Google Scholar] [CrossRef]
- Bala, M.; Kumar, S.; Pratap, K.; Verma, P.K.; Padwad, Y.; Singh, B. Bioactive isoquinoline alkaloids from Cissampelos pareira. Nat. Prod. Res. 2018, 33, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-M.; Wang, L.-J.; Pang, H.-Q.; Guo, Y.; Xiao, P.T.; Chu, C.; Guo, L.; Liu, E.-H. Rapid profiling of alkaloid analogues in Sinomenii caulis by an integrated characterization strategy and quantitative analysis. J. Pharm. Biomed. Anal. 2019, 174, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, Y.; He, J.; Kang, Y.; Wang, Y.; Yang, P.; Guo, J.; Huang, J. Structural characterisation of alkaloids in leaves and roots of Stephania kwangsiensis by LC-QTOF-MS. Phytochem. Anal. 2018, 29, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Gorpenchenko, T.Y.; Grigorchuk, V.P.; Fedoreyev, S.A.; Tarbeeva, G.K.; Tchernoded, G.K.; Bulgakov, V.P. Stepharine production in morphogenic cell cultures of Stephania glabra (ROXB.) miers. Plant Cell Tissue Organ Cult. 2017, 128, 67–76. [Google Scholar] [CrossRef]
- Yin, X.; Bai, R.; Guo, Q.; Su, G.; Wang, J.; Yang, X.; Li, L.; Tu, P.; Chai, X. Hendersine A, a novel isoquinoline alkaloid from Corydalis hendersonii. Tetrahedron Lett. 2016, 57, 4858–4862. [Google Scholar] [CrossRef]
- Bournine, L.; Bensalem, S.; Wauters, J.; Iguer-Ouada, M.; Maiza-Benabdesselam, F.; Bedjou, F.; Castronovo, V.; Bellahcene, A.; Tits, M.; Frédérich, M. Identification and quantification of the main active anticancer alkaloids from the root of Glaucium flavum. Int. J. Mol. Sci. 2013, 14, 23533–23544. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, Z.; Wu, C.; Dong, H.; Gan, C.; Fan, L.; Wang, W.; Yang, C. Ultrahigh-Performance liquid chromatography with tandem mass spectrometry for the determination of 10 alkaloids in beagle plasma after the oral administration of the three Coptidis rhizoma extracts. J. Ethnopharmacol. 2019, 239, 111896. [Google Scholar] [CrossRef]
- Qi, L.; Ma, Y.; Zhong, F.; Shen, C. Comprehensive quality assessment for Rhizoma coptidis based on quantitative and qualitative metabolic profiles using high performance liquid chromatography, Fourier transform near-infrared and Fourier transform mid-infrared combined with multivariate statistical analysis. J. Pharm. Biomed. Anal. 2018, 161, 436–443. [Google Scholar]
- Kubo, S.; Kuroda, M.; Matsuo, Y.; Masatani, D.; Sakagami, H.; Mimaki, Y. New cardenolides from the seeds of Adonis aestivalis. Chem. Pharm. Bull. 2012, 60, 1275–1282. [Google Scholar] [CrossRef] [Green Version]
- Hao, D.-C.; Xiao, P.-G.; Ma, H.-Y.; Peng, Y.; He, C.-N. Mining chemodiversity from biodiversity: Pharmacophylogeny of medicinal plants of Ranunculaceae. Chin. J. Nat. Med. 2015, 13, 507–520. [Google Scholar] [CrossRef]
- Mushtaq, S.; Aga, M.A.; Qazi, P.H.; Alli, M.N.; Shah, A.M.; Lone, S.A.; Shah, A.; Hussain, A.; Rasool, F.; Dar, H.; et al. Isolation, characterization and HPLC quantification of compounds from Aquilegia fragrans Benth: Their In Vitro antibacterial activities against bovine mastitis pathogens. J. Ethnopharmacol. 2016, 178, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Tuzimski, T.; Petruczynik, A.; Misiurek, J. Separation of a mixture of eleven alkaloids by 2D-TLC on multi-K CS5 plates and identifcation of analytes in Thalictrum foetidum root extract by TLC and HPLC-DAD. J. Plan. Chromatogr. Mod. TLC 2017, 30, 142–147. [Google Scholar] [CrossRef]
- Jenkins, A.L.; Hedgepeth, W.A. Analysis of chiral pharmaceuticals by HPLC. Chirality 2005, 17, S24–S29. [Google Scholar] [CrossRef] [PubMed]
- Parveen, A.; Wang, Y.-H.; Fantoukh, O.; Alhusban, M.; Raman, V.; Ali, Z.; Khan, I.A. Development of a chemical fingerprint as a tool to distinguish closely related Tinospora species and quantitation of marker compounds. J. Pharm. Biomed. Anal. 2020, 178, 112894. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Chi, Y.; Liu, J.; Wang, M.; Qin, J.; Ou, L.; Chen, W.; Zhao, Z.; Zhan, R.; Xu, H. Profiling and pharmacokinetic studies of alkaloids in rats after oral administration of Zanthoxylum nitidum decoction by UPLC-Q-TOF-MS/MS and HPLC-MS/MS. Molecules 2019, 24, 585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, T.; Emori, W.; Zhang, R.-H.; Okafor, P.C.; Yang, M.; Cheng, C.-R. Detailed characterization of Phellodendron chinense Schneid and its application in the corrosion inhibition of carbon steel in acidic media. Bioelectrochem 2019, 130, 107332. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.; Zhang, Y.; Zhang, X.; Zhang, Z.; Liao, Y.; Zhang, B. A new method for simultaneous determination of phenolic acids, alkaloids and limonoids in Phellodendri amurensis cortex. Molecules 2019, 24, 709. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Wei, P.; Peng, Q.; Qin, S.; Zhou, Y.; Zhu, C.; Zhang, L. Simultaneous qualitative and quantitative evaluation of Toddalia asiatica root by using HPLC-DAD and UPLC-QTOF-MS/MS. Phytochem. Anal. 2019, 30, 164–181. [Google Scholar] [CrossRef]
- Bai, L.; Zhang, H.; Liu, Q.; Zhao, Y.; Cui, X.; Guo, S.; Zhang, L.; Ho, C.-T.; Bai, N. Chemical characterization of the main bioactive constituents from fruits of Ziziphus jujube. Food Funct. 2016, 7, 2870–2877. [Google Scholar] [CrossRef]
- Cordeiro, K.W.; Felipe, J.L.; Malange, K.F.; Do Prado, P.R.; De Oliveira Figueiredo, P.; Garcez, F.R.; De Cassia Freitas, K.; Garcez, W.S.; Toffoli-Kadri, M.C. Anti-Inflammatory and antinociceptive activities of Croton urucurana Baillon bark. J. Ethnopharmacol. 2016, 183, 128–135. [Google Scholar] [CrossRef]
- Guo, K.; Tong, C.; Fu, Q.; Xu, J.; Shi, S.; Xiao, Y. Identification of minor lignans, alkaloids, and phenylpropanoid glycosides in Magnolia officinalis by HPLC-DAD-QTOF-MS/MS. J. Pharm. Biomed. Anal. 2019, 170, 153–160. [Google Scholar] [CrossRef]
- Tian, X.; Guo, S.; He, K.; Roller, M.; Yang, M.; Liu, Q.; Zhang, L.; Ho, C.-T.; Bai, N. Qualitative and quantitative analysis of chemical constituents of Ptychopetalum olacoides Benth. Nat. Prod. Res. 2018, 32, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Lv, L.; Di, X.; Xue, J.; Gao, Z.; Zhang, G.; Zhang, H. The compatibility of six alkaloids in ermiao pill explored by a comparative pharmacokinetic and network pharmacological study. Biomed. Chromatogr. 2019, 33, e4509. [Google Scholar] [CrossRef] [PubMed]
- Manske, R.H.F. The alkaloids. In Chemistry and Physiology; Academic Press: New York, NY, USA, 1967; Volume 9. [Google Scholar]
- Karimov, A. Berberis alkaloids. Chem. Nat. Compd. 1993, 24, 3. [Google Scholar] [CrossRef]
- Iwasa, K.; Takahashi, T.; Nishiyama, Y.; Moriyasu, M.; Sugiura, M.; Takeuchi, A.; Tode, C.; Tokuda, H.; Takeda, K. Online structural elucidation of alkaloids and other constituents in crude extracts and cultured cells of Nandina domestica by combination of LC-MS/MS, LC-NMR, and LC-CD analyses. J. Nat. Prod. 2008, 71, 1376–1385. [Google Scholar] [CrossRef]
- Bentley, K.W. The Chemistry of Natural Products. Volume 1: The Alkaloids; Interscience Publishers: New York, NY, USA, 1957. [Google Scholar]
- Shamma, M. The Isoquinoline Alkaloids Chemistry and Pharmacology; Academic Press: London, UK, 1972. [Google Scholar]
- Ludwiczuk, A.; Skalicka-Woźniak, K.; Georgiev, M. Terpenoids. In Pharmacognosy: Fundamentals, Applications and Strategy; Badal, S., Delgoda, R., Eds.; Academic Press: London, UK, 2017; pp. 233–266. [Google Scholar]
- Avula, B.; Bae, J.-Y.; Majrashi, T.; Wu, T.Y.; Wang, Y.H.; Wang, M.; Ali, Z.; Wu, Y.-C.; Khan, I.A. Targeted and non-targeted analysis of annonaceous alkaloids and acetogenins from Asimina and Annona species using UHPLC-QToF-MS. J. Pharm. Biomed. Anal. 2018, 159, 548–566. [Google Scholar] [CrossRef]
- Widelski, J.; Mroczek, T.; Głowniak, K. Optimization of extraction of pyrrolizidine alkaloids from plant material. Chem. Anal. 2006, 51, 567–580. [Google Scholar]
- Xue, B.; Zhao, Y.; Su, J.; Miao, Q.; Miao, P.; Chen, N.; Wang, Z.; Zhang, Y.; Ma, S. In Vitro intestinal absorption and metabolism of Magnoflorine and its potential interaction in Coptidis Rhizoma decoction in rat. Eur. J. Drug Metab. Pharm. 2017, 42, 281–293. [Google Scholar] [CrossRef]
- Yang, T.C.; Chao, H.F.; Shi, L.S.; Chang, T.C.; Lin, H.C.; Chang, W.L. Alkaloids from Coptis chinensis root promote glucose uptake in C2C12 myotubes. Fitoterapia 2014, 93, 239–244. [Google Scholar] [CrossRef]
- Tang, X.-Y.; Dai, Z.-Q.; Wu, Q.-C.; Zeng, J.-X.; Gao, M.-X.; Xiao, H.-H.; Yao, Z.-H.; Dai, Y.; Yao, X.-S. Simultaneous determination of multiple components in rat plasma and pharmacokinetic studies at a pharmacodynamic dose of Xian-Ling-Gu-Bao capsule by UPLC-MS/MS. J. Pharm. Biomed. Anal. 2020, 177, 112836. [Google Scholar] [CrossRef]
- Jin, J.; Xue, H.; Sun, X.; Zan, B.; Li, Y.; Wang, T.; Shi, R.; Ma, Y. Simultaneous determination of multiple compounds of Da-Huang-Xiao-Shi decoction in rat plasma by LC-MS/MS and its application in a pharmacokinetic study. J. Pharm. Biomed. Anal. 2019, 174, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Guo, C.-E.; Chen, H.; Chen, J.; Bi, X.; Li, H.; Zhu, H.; Ma, P.; Zhang, Y.; Lin, H. Simultaneous determination of six coptis alkaloids in urine and feces by LC-MS/MS and its application to excretion kinetics and the compatibility mechanism of Jiao-Tai-Wan in insomniac rats. Biomed. Chromatogr. 2018, 32, e4248. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Han, L.; Cao, B.; Yang, X.; Zhu, X.; Yang, B.; Zhao, H.; Qiao, W. Use of magnoflorine-phospholipid complex to permeate blood-brain barrier and treat depression in the CUMS animal model. Drug Deliv. 2019, 26, 566–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, T.M.; Na, M.; Min, B.S.; Zhang, X.; Lee, I.; Ngoc, T.M.; Thuong, P.T.; Sok, D.E.; Bae, K. Protective effect of magnoflorine isolated from Coptidis rhizoma on Cu2+-induced oxidation of human low density lipoprotein. Planta Med. 2007, 73, 1281–1284. [Google Scholar] [CrossRef]
- Hung, T.M.; Lee, J.P.; Min, B.S.; Choi, J.S.; Na, M.; Zhang, X.; Ngoc, T.M.; Lee, I.; Bae, K. Magnoflorine from Coptidis rhizoma protects high density lipoprotein during oxidant stress. Biol. Pharm. Bull. 2007, 30, 1157–1160. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.J.; Jiang, Z.M.; Xiao, P.T.; Sun, J.B.; Bi, Z.M.; Liu, E.H. Identification of anti-inflammatory components in Sinomenii caulis based on spectrum-effect relationship and chemometric methods. J. Pharm. Biomed. Anal. 2019, 167, 38–48. [Google Scholar] [CrossRef]
- Shen, Y.; Li, C.G.; Zhou, S.F.; Pang, E.C.; Story, D.F.; Xue, C.C. Chemistry and bioactivity of Flos Magnoliae, a Chinese herb for rhinitis and sinusitis. Curr. Med. Chem. 2008, 15, 1616–1627. [Google Scholar] [CrossRef]
- Sharma, U.; Bala, M.; Kumar, N.; Singh, B.; Munshi, R.K.; Bhalerao, S. Immunomodulatory active compounds from Tinospora cordifolia. J. Ethnopharmacol. 2012, 141, 918–926. [Google Scholar] [CrossRef]
- Wei, T.; Xiaojun, X.; Peilong, C. Magnoflorine improves sensitivity to doxorubicin (DOX) of breast cancer cells via inducing apoptosis and autophagy through AKT/mTOR and p38 signaling pathways. Biomed. Pharm. 2020, 121, 109139. [Google Scholar] [CrossRef]
- Sotníková, R.; Kettmann, V.; Kostálová, D.; Táborská, E. Relaxant properties of some aporphine alkaloids from Mahonia aquifolium. Methods Find. Exp. Clin. Pharm. 1997, 19, 589–597. [Google Scholar]
- Lee, J.H.; Stein, B.D. Antimicrobial activity of a combination of Mume fructus, Schizandrae fructus, and Coptidis rhizoma on enterohemorrhagic Escherichia coli O26, O111, and O157 and its effect on Shiga toxin releases. Foodborne Pathog. Dis. 2011, 8, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Du, Z.Z.; Shen, Y.M.; Yang, Y.P. Aporphine alkaloids from Clematis parviloba and their antifungal activity. Arch. Pharm. Res. 2009, 32, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.M.; Hassan, E.M.; Ibrahim, N.A. Cytotoxic and antiviral activities of aporphine alkaloids of Magnolia grandiflora L. Nat. Prod. Res. 2010, 24, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Yamahara, J. Behavioral pharmacology of berberine-type alkaloids. (1) Central depressive action of Coptidis rhizoma and its constituents. Nihon Yakurigaku Zasshi 1976, 72, 899–908. [Google Scholar] [CrossRef]
- Patel, M.B.; Mishra, S.M. Hypoglycemic activity of alkaloidal fraction of Tinospora cordifolia. Phytomedicine 2011, 18, 1045–1052. [Google Scholar] [CrossRef]
- Yan, R.; Wang, W.; Guo, J.; Liu, H.; Zhang, J.; Yang, B. Studies on the alkaloids of the bark of Magnolia officinalis: Isolation and on-line analysis by HPLC-ESI-MS(n). Molecules 2013, 18, 7739–7750. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.; Yadav, A.; Dabur, R. Interactions of a medicinal climber Tinospora cordifolia with supportive interspecific plants trigger the modulation in its secondary metabolic profiles. Sci. Rep. 2019, 9, 14327. [Google Scholar] [CrossRef]
- Alper, J. Biomedicine. New insights into type 2 diabetes. Science 2000, 289, 37–39. [Google Scholar] [CrossRef]
- Baron, A.D. Postprandial hyperglycaemia and alpha-glucosidase inhibitors. Diabetes Res. Clin. Pract 1998, 40 (Suppl. S51–S55). [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Q.; Yin, X. Synthesis of α-glucosidase-immobilized nanoparticles and their application in screening for α-glucosidase inhibitors. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1022, 75–80. [Google Scholar] [CrossRef]
- Patel, M.B.; Mishra, S.M. Magnoflorine from Tinospora cordifolia stem inhibits α-glucosidase and is antiglycemic in rats. J. Funct. Foods 2012, 4, 79–86. [Google Scholar] [CrossRef]
- Bhatta, A.; Yao, L.; Xu, Z.; Toque, H.A.; Chen, J.; Atawia, R.T.; Fouda, A.Y.; Bagi, Z.; Lucas, R.; Caldwell, R.B.; et al. Obesity-Induced vascular dysfunction and arterial stiffening requires endothelial cell arginase 1. Cardiovasc. Res. 2017, 113, 1664–1676. [Google Scholar] [CrossRef]
- Patel, M.B.; Mishra, S.M. Aldose reductase inhibitory activity and anti catraract potential of some traditionally acclaimed antidiabetic medicinal plants Orient Pharm. Exp. Med. 2009, 9, 245–251. [Google Scholar]
- Chen, H.Y.; Ye, X.L.; Cui, X.L.; He, K.; Jin, Y.N.; Chen, Z.; Li, X.G. Cytotoxicity and antihyperglycemic effect of minor constituents from Rhizoma coptis in HepG2 cells. Fitoterapia 2012, 83, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Chen, Y.Y.; Bei, W.J.; Wang, L.Y.; Chen, B.T.; Guo, J. In Vitro screening for antihepatic steatosis active components within Coptidis rhizoma alkaloids extract using liver cell extraction with HPLC analysis and a free fatty acid-induced hepatic steatosis HepG2 cell assay. Evid. Based Complement Altern. Med. 2013, 459390. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.S.; Ali, M.Y.; Jung, H.A.; Oh, S.H.; Choi, R.J.; Kim, E.J. Protein tyrosine phosphatase 1B inhibitory activity of alkaloids from Rhizoma coptidis and their molecular docking studies. J. Ethnopharmacol. 2015, 171, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Kim, J.H.; Ali, M.Y.; Min, B.S.; Kim, G.D.; Jung, H.A. Coptis chinensis alkaloids exert anti-adipogenic activity on 3T3-L1 adipocytes by downregulating C/EBP-α and PPAR-γ. Fitoterapia 2014, 98, 199–208. [Google Scholar] [CrossRef]
- Qian, Q.; Liu, X.; He, W.; An, Y.; Chen, Q.; Wu, J.; Deng, Y.; Guo, L.; Zhang, Y.; Wang, T. TG accumulation inhibitory effects of Jinqi formula by AMPK signaling pathway. J. Ethnopharmacol. 2012, 143, 41–48. [Google Scholar] [CrossRef]
- Cao, Y.; Bei, W.; Hu, Y.; Cao, L.; Huang, L.; Wang, L.; Luo, D.; Chen, Y.; Yao, X.; He, W.; et al. Hypocholesterolemia of Rhizoma coptidis alkaloids is related to the bile acid by up-regulated CYP7A1 in hyperlipidemic rats. Phytomedicine 2012, 19, 686–692. [Google Scholar] [CrossRef]
- He, K.; Hu, Y.; Ma, H.; Zou, Z.; Xiao, Y.; Yang, Y.; Feng, M.; Li, X.; Ye, X. Rhizoma coptidis alkaloids alleviate hyperlipidemia in B6 mice by modulating gut microbiota and bile acid pathways. Biochim. Biophys. Acta 2016, 1862, 1696–1709. [Google Scholar] [CrossRef]
- Yokozawa, T.; Ishida, A.; Cho, E.J.; Nakagawa, T. The effects of Coptidis rhizoma extract on a hypercholesterolemic animal model. Phytomedicine 2003, 10, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharm. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasa, U.S.; Tana, B.W.Q.; Vellayappanb, B.A.; Jeyasekharana, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef] [PubMed]
- Racková, L.; Májeková, M.; Kost’álová, D.; Stefek, M. Antiradical and antioxidant activities of alkaloids isolated from Mahonia aquifolium. Structural aspects. Bioorg. Med. Chem. 2004, 12, 4709–4715. [Google Scholar] [CrossRef] [PubMed]
- Naseer, S.; Lone, S.H.; Lone, J.A.; Khuroo, M.A.; Bhat, K.A. LC-MS guided isolation, quantification and antioxidant evaluation of bioactive principles from Epimedium elatum. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 989, 62–70. [Google Scholar] [CrossRef]
- Kim, J.P.; Jung, M.Y.; Kim, J.P.; Kim, S.Y. Antiphotooxidative activity of protoberberines derived from Coptis japonica makino in the chlorophyll-sensitized photooxidation of oil. J. Agric. Food Chem. 2000, 48, 1058–1063. [Google Scholar] [CrossRef]
- Jung, H.A.; Min, B.S.; Yokozawa, T.; Lee, J.H.; Kim, Y.S.; Choi, J.S. Anti-Alzheimer and antioxidant activities of Coptidis rhizoma alkaloids. Biol. Pharm. Bull. 2009, 32, 1433–1438. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Teng, S.; He, J.; Sun, X.; Du, M.; Kou, L.; Wang, X. Pharmacological basis for application of scutellarin in Alzheimer’s disease: Antioxidation and antiapoptosis. Mol. Med. Rep. 2018, 18, 4289–4296. [Google Scholar] [CrossRef] [Green Version]
- Sattler, S. The role of the immune system beyond the fight against infection. Adv. Exp. Med. Biol. 2017, 1003, 3–14. [Google Scholar] [CrossRef]
- Furman, D.; Davis, M.M. New approaches to understanding the immune response to vaccination and infection. Vaccine 2015, 33, 5271–5281. [Google Scholar] [CrossRef] [Green Version]
- Ortuño-Sahagún, D.; Zänker, K.; Rawat, A.K.S.; Kaveri, S.V.; Hegde, P. Natural immunomodulators. J. Immunol. Res. 2017, 7529408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, W.; Jantan, I.; Kumolosasi, E.; Haque, M.A.; Bukhari, S.N.A. Immunomodulatory effects of Tinospora crispa extract and its major compounds on the immune functions of RAW 264.7 macrophages. Int. Immunopharmacol. 2018, 60, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Jantan, I.; Kumolosasi, E.; Bukhari, S.N. Standardized extract of Tinospora crispa stimulates innate and adaptive immune responses in Balb/c mice. Food Funct. 2016, 7, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Jantan, I.; Harikrishnan, H.; Abdul Wahab, S.M. Magnoflorine enhances LPS-activated pro-inflammatory responses via MyD88-dependent pathways in U937 macrophages. Planta Med. 2018, 84, 1255–1264. [Google Scholar] [CrossRef] [Green Version]
- Nagai, Y.; Takatsu, K. Role of the immune system in obesity-associated inflammation and insulin resistance. In Nutrition in the Prevention and Treatment of Abdominal Obesity; Watson, R., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 281–293. [Google Scholar]
- Deguine, J.; Barton, G.M. MyD88: A central player in innate immune signaling. F1000Prime Rep. 2014, 6, 97. [Google Scholar] [CrossRef]
- Guo, S.; Jiang, K.; Wu, H.; Yang, C.; Yang, Y.; Yang, J.; Zhao, G.; Deng, G. Magnoflorine ameliorates lipopolysaccharide-induced acute lung injury via suppressing NF-κB and MAPK activation. Front. Pharm. 2018, 9, 982. [Google Scholar] [CrossRef]
- Sun, D.; Han, Y.; Wang, W.; Wang, Z.; Ma, X.; Hou, Y.; Bai, G. Screening and identification of Caulis sinomenii bioactive ingredients with dual-target NF-κB inhibition and β2-AR agonizing activities. Biomed. Chromatogr. 2016, 30, 1843–1853. [Google Scholar] [CrossRef]
- Han, Y.L.; Yu, H.L.; Li, D.; Meng, X.L.; Zhou, Z.Y.; Yu, Q.; Zhang, X.Y.; Wang, F.J.; Guo, C. In Vitro inhibition of Huanglian [Rhizoma coptidis (L.)] and its six active alkaloids on six cytochrome P450 isoforms in human liver microsomes. Phytother. Res. 2011, 25, 1660–1665. [Google Scholar] [CrossRef]
- Lynch, T. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am. Fam. Physician 2007, 76, 391–396. [Google Scholar]
- McDonnell, A.M.; Dang, C.H. Basic review of the cytochrome P450 system. J. Adv. Pr. Oncol. 2013, 4, 263. [Google Scholar]
- Lü, S.; Dong, S.; Xu, D.; Duan, J.; Li, G.; Guo, Y.; Kuang, H.; Wang, Q. Spectrum-Effect relationships between fingerprints of Caulophyllum robustum maxim and inhabited pro-inflammation cytokine effects. Molecules 2017, 22, 1826. [Google Scholar] [CrossRef]
- Tan, H.Y.; Wang, N.; Tsao, S.-W.; Zhang, Z.; Feng, Y. Suppression of vascular endothelial growth factor via inactivation of eukaryotic elongation factor 2 by alkaloids in Coptidis rhizome in hepatocellular carcinoma. Integr. Cancer 2014, 13, 425–434. [Google Scholar] [CrossRef] [Green Version]
- Bala, M.; Pratap, K.; Verma, P.K.; Singh, B.; Padwad, Y. Validation of ethnomedicinal potential of Tinospora cordifolia for anticancer and immunomodulatory activities and quantification of bioactive molecules by HPTLC. J. Ethnopharmacol. 2015, 175, 131–137. [Google Scholar] [CrossRef]
- Yao, Z.H.; Qin, Z.F.; Cheng, H.; Wu, X.M.; Dai, Y.; Wang, X.L.; Qin, L.; Ye, W.C.; Yao, X.S. Simultaneous quantification of multiple representative components in the Xian-Ling-Gu-Bao capsule by ultra-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry. Molecules 2017, 22, 927. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; He, Y.; Guo, B.; Tsang, M.C.; Tu, F.; Dai, Y.; Yao, Z.; Zheng, L.; Xie, X.; Wang, N.; et al. In vivo screening for anti-osteoporotic fraction from extract of herbal formula Xianlinggubao in ovariectomized mice. PLoS ONE 2015, 10, e0118184. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Zhong, Q.; Wang, J.; Wang, M.; Fang, F.; Xia, Z.; Zhong, R.; Huang, H.; Ke, Z.; Wei, Y.; et al. Beneficial effects and toxicity studies of Xian-ling-gu-bao on bone metabolism in ovariectomized rats. Front. Pharm. 2017, 8, 273. [Google Scholar] [CrossRef] [Green Version]
- Satoh, H. Electropharmacological actions of the constituents of Sinomeni caulis et Rhizome and Mokuboi-to in guinea pig heart. Am. J. Chin. Med. 2005, 33, 967–979. [Google Scholar] [CrossRef]
- Chandra, G.; Mukherjee, D.; Ray, A.S.; Chatterjee, S.; Bhattacharjee, I. Phytoextracts as antibacterials—A review. Curr. Drug Discov. Technol. 2019. [Google Scholar] [CrossRef]
- Fan, S.; Yu, D.; Gu, Y.; Zhang, M.; Zhang, L.; Li, G. Determination of magnoflorine in Coptidis rhizoma and Phellodendri chinensis cortex by LC-MS. Zhongguo Zhong Yao Za Zhi 2010, 35, 3322–3324. [Google Scholar]
- Feng, X.; Yan, D.; Zhao, K.J.; Luo, J.Y.; Ren, Y.S.; Kong, W.J.; Han, Y.M.; Xiao, X.H. Applications of microcalorimetry in the antibacterial activity evaluation of various Rhizoma coptidis. Pharm. Biol. 2011, 49, 348–353. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.A.; Kwon, Y.J.; Kwon, D.Y.; Lee, J.H. Evaluation of antibacterial effects of a combination of Coptidis rhizoma, Mume Fructus, and Schizandrae Fructus against Salmonella. Int. J. Food Microbiol. 2008, 127, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.J.; Zhao, Y.L.; Xiao, X.H.; Jin, C.; Li, Z.L. Quantitative and chemical fingerprint analysis for quality control of rhizoma Coptidis chinensis based on UPLC-PAD combined with chemometrics methods. Phytomedicine 2009, 16, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ha, T.; Bao, Q.; Shin, Y.-K.; Kim, K.-Y. Antifungal activity of magnoflorine against Candida strains World J. Microbiol. Biotechnol. 2018, 34, 167. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lv, X.-L.; Sun, L. Studies on antifungal activity of extracts from six traditional Chinese medicines against Dermatophyte genus. Chin. J. Dermatovenerol. 2008, 8. Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-ZBFX200808032.htm (accessed on 8 January 2020).
- Ahn, T.G.; Lee, J.Y.; Cheon, S.Y.; An, H.J.; Kook, Y.B. Protective effect of Sam-Hwang-Sa-Sim-Tang against hepatic steatosis in mice fed a high-cholesterol diet. BMC Complement. Altern. Med. 2013, 13, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Botanical Family | Gender Name | Selected Species | References |
|---|---|---|---|
| Annonaceae | Annona | A. glabra | [5] |
| Asimina | A. triloba | [5] | |
| Enantia | E. chlorantha | [6] | |
| Aristolochiaceae | Aristolochia | A. contoria | [7] |
| Berberidaceae | Berberis | B. vulgaris, B. cretica, B. siberica | [4,8] |
| Caulophyllum | C. robustum | [9] | |
| Mahonia | M. napaulensis, M. leschenaultia | [10,11] | |
| Epimedium | E. grandiflorum | [12] | |
| Nandina | N. domestica | [13] | |
| Menispermaceae | Cissampelos | C. pareira | [14] |
| Sinomenium | S. acutum | [15] | |
| Stephania | S. kwangsinensis; S. glabra | [16,17] | |
| Papaveraceae | Argemone | A. mexicana, A. grandiflora | [4,8] |
| Corydalis | C. hendersonii, | [18] | |
| Glaucium | G. flavum | [19] | |
| Papaver | P. orientale, P. rhoeas, P. nudicaule, P. crocetum | [8] | |
| Ranunculacae | Coptis | C. chinensis | [20,21] |
| Adonis | A. aestivalis | [22] | |
| Nigella | N. sativa, N. glandulifera | [23] | |
| Helleborus | H. viridis | [23] | |
| Aquilegia | A. fragrans | [24] | |
| Clematis | C. recta, C. parviloba | [23,25] | |
| Thalictrum | T. foetidum | [26] | |
| Tinospora | T. crispa, T. sinensis, T. cordifolia | [27] | |
| Zanthoxylum | Z. nitidum | [28] | |
| Rutaceae | Phellodendron | P. chinense, P. amurensis | [29,30] |
| Toddalia | T. asiatica | [31] | |
| Rhamnaceae | Ziziphus | Z spinosa | [32] |
| Euphorbiaceae | Croton | C. urucurana | [33] |
| Magnoliaceae | Magnolia | M. officinalis | [34] |
| Olacaceae | Ptychopetalum | P. olacoides | [35] |
| Activity | Pure Substance/Extract | Model | Mechanism of Action | References |
|---|---|---|---|---|
| The Effect on Carbohydrate–Lipid Metabolism | Tinospora cordifolia extract | In vitro in rats and sheep lenses | Inhibition of aldose reductase activity. | [67,70] |
| MGN | In vivo in rats | Inhibition of α- glucosidase competitive activity. | [67] | |
| Isoquinoline alkaloid rich fraction from the stem of T. cordifolia | In vivo in rat hepatocytes and in vitro in Rattus norvegicus RINm5F cell line | Decrease gluconeogenesis in rat hepatocytes and increase insulin secretion in Rattus norvegicus RINm5F cell line. | [50] | |
| Alkaloid extract from Coptidis rhizoma | In vitro in hepatic steatosis HepG2 cell | Reducing effect of triglycerides. | [71] | |
| MGN | In vitro enzyme kinetics and in silico molecular docking simulation | Inhibition of activity of PTP1B and ONOO (−)-mediated protein tyrosine nitration. | [72] | |
| Alkaloids from Coptis chinensis roots (N-butanol and dichloromethane sub-fractions) | Skeletal muscles in C2C12 myotubes | Reduction of hyperglycemia in diabetes through promoting glucose uptake in skeletal muscles. | [46] | |
| Methanol extract from Coptidis rhizoma containing MGN | In vitro in Mus musculus 3T3-L1 cell line | Inhibition of adipocyte differentiation and lipid contents in Mus musculus 3T3-L1 cell line. | [73] | |
| Alkaloid isolated from Coptidis rhizoma extract | In vitro in Mus musculus 3T3-L1 cell line | Reduction of the lipid accumulation, adipogenesis and expression ofPPAR-γ and C/EBP-α. | [73] | |
| MGN isolated from the n-butanol fraction of Coptidis rhizoma | In vitro in Mus musculus 3T3-L1 cell line | Inhibition of accumulation of cellular triglyceride and reduction of accumulation of the lipid in the 3T3-L1 adipocytes. | [73] | |
| Jingi formula containing the extract from Coptidis rhizoma, Lonicerae japonicae, and Astragali radix | Mature Mus musculus embryo 3T3-L1 adipocytes | Suppression of the accumulation of triglycerides and free fatty acids increase the expression and tyrosine phosphorylation of AMPK and decrease the expression of enzymes from lipid metabolism: ACC, HSL, and FAS. | [74] | |
| Jingi formula containing the extract from Coptidis rhizoma, Lonicerae japonicae, and Astragali radix | In vivo in mice | Body weight reduction without changing food intake and concentration of serum glucose, triglycerides, and free fatty acids. Increase of expression and tyrosine phosphorylation of AMPK. Reduction of the expression of HSL and ACC and stimulation of the expression of IRS-1 in mice livers. | [74] | |
| Sam-Hwang-Sa-Sim-Tang (SHSST), composed of three herbs: Coptidis rhizoma, Scutellariae radix, and Rhei rhizoma | In vivo in mice | Suppression of the development of hyperlipidemia. Reduction of the level of serum TC, LDL, SREBP, SREBP-2, LXR, LDLR, and HMG-CoA expression. | [112] | |
| Alcohol extract from Coptidis rhizoma | In vivo in rats | Promotion of the conversion of cholesterol into bile acids by increasing CYP7A1 activity, positive regulation of PPARα, and the negative modulation of the nuclear FXR, bile acid receptor. | [75] | |
| Alkaloids isolated from Coptidis rhizoma | In vivo hyperlipidemic B6 mice | Reduction of the body weight gain, total cholesterol, TG, LDL, total bile acids, and LPS in serum. | [76] | |
| Extract from Coptidis rhizoma | In vivo in rats | Reduction of the levels of total cholesterol, LDL, oxidized LDL, pathological damage caused by hypercholesterolemia, serum TBARS level, and lipid peroxidation. | [77] | |
| The Antioxidant Activity | MGN isolated from Mahonia aquifolium | In vitro using DPPH test (antiradical scavenging activity) and AAPH test (antioxidant activity) | Ability to scavenge free stable DPPH radical and inhibition of lipid peroxidation. | [80] |
| MGN isolated from Epimedium elatum | In vitro using DPPH assay | Ability to scavenge free stable DPPH radical. | [81] | |
| MGN isolated from Coptidis rhizoma | In vitro using AAPH and TBARS assay | Inhibition of oxidation high-density lipoprotein HDL exposed to Cu2+-independent form and peroxyl radicals, reduction of TBARS formation. | [52] | |
| MGN isolated from Coptidis rhizoma | In vitro using TBARS assay | Inhibition of oxidation of glycated and glycoxidated LDL induced by Cu2+ and prevention of production of TBARS. | [51] | |
| Alkaloids from Coptis japonica | In vitro using chlorophyll-sensitized photooxidation of linoleic acid. | Prevention of photooxidation of linoleic acid by butanol fraction | [82] | |
| The Anti-Alzheimer Activity | MGN isolated from Berberis cretica root | In vivo study in mice | Raise in the cognitive processes of short-term and long-term memory. | [4] |
| Anti-Inflammatory Activity | MGN isolated from Tinospora crispa | In vitro, mouse macrophages RAW 264.7 cell line | Contribution to the production of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) and stimulation of the PGE2production. | [88] |
| Extract from T. crispa | In vivo study in mice | Establishment in the level of serum immunoglobulins (IgG and IgM). | [89] | |
| MGN isolated from T. crispa | In vitro, in LPS-activated U937 macrophages | Upregulation of the Phosphorylation of p65 and increase of COX-2 and PGE2 expression, promotion the phosphorylation, and ubiquitination of IκBα, upregulation of the phosphorylation of p65 and increase of COX-2 and PGE2 expression, promotion of the phosphorylation and ubiquitination of IκBα, upregulation of the Akt phosphorylation, enhancement of the phosphorylation of JNK1/2, ERK11/2, and p38 MAPKs, support in MyD88 and TLR4 activation. | [90] | |
| MGN isolated from Sinomenii caulis | In vivo study in mice and guinea pigs | Inhibition of NF- κB and inflammatory cytokines (IL-6 and IL-8). | [94] | |
| MGN | In vivo study in LPS-induced ALI in mice | Reduction of pathological changes induced by LPS. | [93] | |
| Anticancer Activity | MGN | In vitro in MCF7, MDA-MB-231, MDA-MB-453, and BT474 breast cancer cells | Promotion of anti-cancer effect of DOX by inducing cellular apoptosis and autophagy in breast cancer cells. Reduction of viability, proliferation, migration, and invasion of breast cancer cells as well as increase expression of epithelial marker E-cadherin and decreased of mesenchymal N-cadherin, vimentin, and α-SMA after MGN and DOX treatment compared to DOX separately. | [56] |
| MGN/DOX | In vivo in MCF7 xenograft model | Reduction of the tumor growth. Expression of p53, LC3-II, cleaved Caspase-3, and induction of phospho-p38, phospho-AKT, and phospho-PI3K and downregulation of phospho-mTOR expression after DOX/MGN combinational treatment. | [56] | |
| MGN from the methanol extract of Magnolia grandiflora L. leaves | In vivo in Hela cervix tumor cell line, U251 brain tumor cell line and HEPG2 hepatocellular carcinoma cell line | Inhibition of cell viability and cytotoxixity. | [59] | |
| MGN isolated from Ziziphus jujube fruit | In vivo in MCF7 breast cancer, A549 lung cancer, HepG2 hepatocellular carcinoma, and HT-29 colon cancer cell lines | Weak cytotoxic effect. | [32] | |
| Coptidis rhizoma aqueous extract | In vivo in MHCC97L and HEP G2 hepatocellular carcinoma cells | Cytotoxic effect; reduction of VEGF protein secretion, inactivation of the elongation factor 2 EF2. | [99] | |
| Water extract from Coptidis rhizoma | In vivo in mice model | Reduction of neovascularization level and tumor size. | [99] | |
| T. cordifolia extract | KB human oral squamous carcinoma, HT-29 human colon cancer, CHOK-1 hamster ovary, and SiHa human cervical cancer | Cytotoxicity effect. | [100] | |
| Effect on the Nervous System | Methanol extract from Coptis root | In vivo in mice | No activity demonstrated. | [61] |
| Antidepressant Effect | MGN and MGN-phospholipid complex | Chronic unpredictable mild stress animal model | Significant improvement in the antidepressant effect, drug properties, and liposolubility of MGN induced by MGN–phospholipid complex. | [60] |
| Antiosteoporosis Effect | Xian-Ling-Gu-Bao (XLGB) capsule containing MGN | In vivo in ovariectomized mice and rats | Positive effect on the bone health. | [47] |
| MGN | Isolated aorta from rat | Weak relaxation effect in noradrenaline- and KCl-induced contractions. | [57] | |
| Cardiovascular Effects | MGN isolated from Mokuboi-to drug | Ventricular cardimyocytes from Guinea Pig heart | Prolongation of the APD75; weak or no effect on the action potential parameters in the papillary muscles. | [104] |
| Antibacterial Activity | Methanolic extract isolated from herbal combination containing MGN | In vivo in mice after initial enterohemorrhagic E. coli infection (EHEC) | Reduction of the release of Shiga toxin from EHEC O26, EHEC O111, and EHEC O157 strains. | [58] |
| Methanolic extract isolated from herbal combination containing MGN | S. Gallinarum infection chicken model | Reduction of the clinical signs inter alia congestion and necrotic changes in the kidney, liver, and spleen. | [107] | |
| Extract from R. coptidis | In vitro in Staphylococcus aureus | Inhibition of the growth of bacteria. | [108] | |
| Anti-Fungal Activity | MGN-α and -β isolated from the aerial parts of Clematis parviloba | In vitro Penicillium avellaneum and Penicillium oryzae | Inhibition of the growth of Penicillium avellaneum and Penicillium oryzae. | [25] |
| MGN | Candida strains | Inhibition of the activity of α-glucosidase, which is required for virulence and cell-wall composition of Candida albicans. Reduction of the formation of C. albicans’ biofilm. | [110] | |
| Aqueous and ethanol extracts isolated from Coptidis rhizoma | Dermatophyte strains | Inhibition of the growth. | [111] | |
| Antiviral Activity | The methanol extract isolated form Magnnolia grandiflora | In vitro HSV-1 and PV1 | Antiviral activity in a plaque reduction bioassay. | [59] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okon, E.; Kukula-Koch, W.; Jarzab, A.; Halasa, M.; Stepulak, A.; Wawruszak, A. Advances in Chemistry and Bioactivity of Magnoflorine and Magnoflorine-Containing Extracts. Int. J. Mol. Sci. 2020, 21, 1330. https://doi.org/10.3390/ijms21041330
Okon E, Kukula-Koch W, Jarzab A, Halasa M, Stepulak A, Wawruszak A. Advances in Chemistry and Bioactivity of Magnoflorine and Magnoflorine-Containing Extracts. International Journal of Molecular Sciences. 2020; 21(4):1330. https://doi.org/10.3390/ijms21041330
Chicago/Turabian StyleOkon, Estera, Wirginia Kukula-Koch, Agata Jarzab, Marta Halasa, Andrzej Stepulak, and Anna Wawruszak. 2020. "Advances in Chemistry and Bioactivity of Magnoflorine and Magnoflorine-Containing Extracts" International Journal of Molecular Sciences 21, no. 4: 1330. https://doi.org/10.3390/ijms21041330
APA StyleOkon, E., Kukula-Koch, W., Jarzab, A., Halasa, M., Stepulak, A., & Wawruszak, A. (2020). Advances in Chemistry and Bioactivity of Magnoflorine and Magnoflorine-Containing Extracts. International Journal of Molecular Sciences, 21(4), 1330. https://doi.org/10.3390/ijms21041330






