Efficient Prodrug Activator Gene Therapy by Retroviral Replicating Vectors Prolongs Survival in an Immune-Competent Intracerebral Glioma Model
Abstract
1. Introduction
2. Results
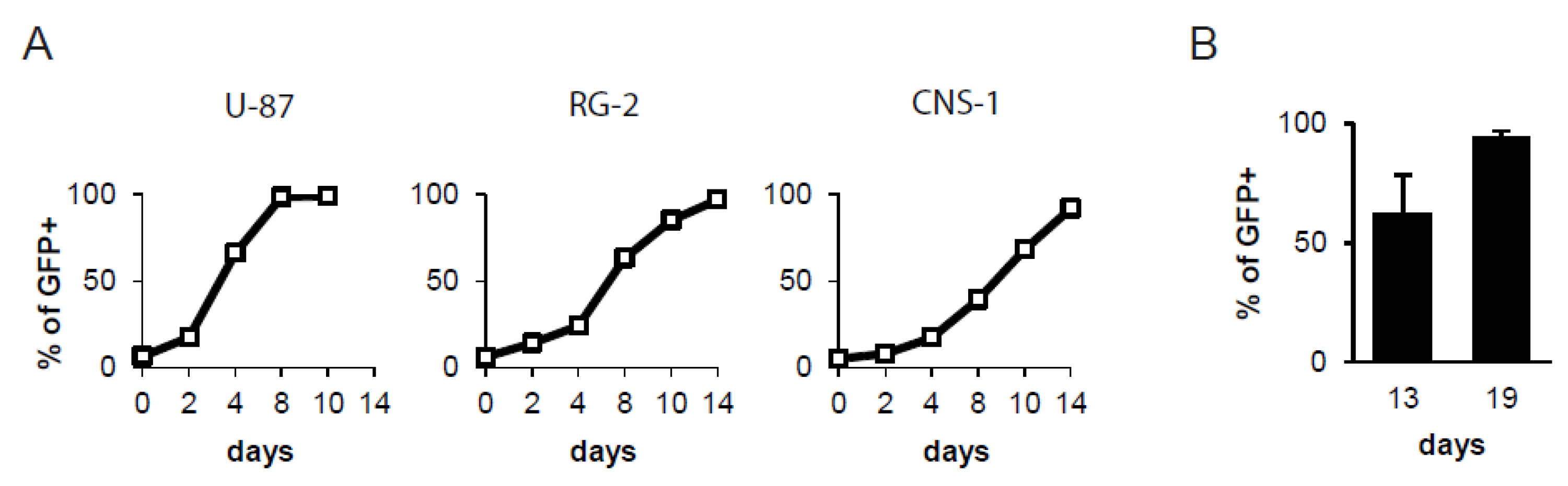
2.1. GALV-RRV Achieves Highly Efficient Transgene Delivery to Glioma Cells
2.2. Efficient and Progressive Spread of GALV-RRV in an Intracerebral Glioma Model
2.3. No Detectable Spread of GALV-RRV to Extratumoral Tissues
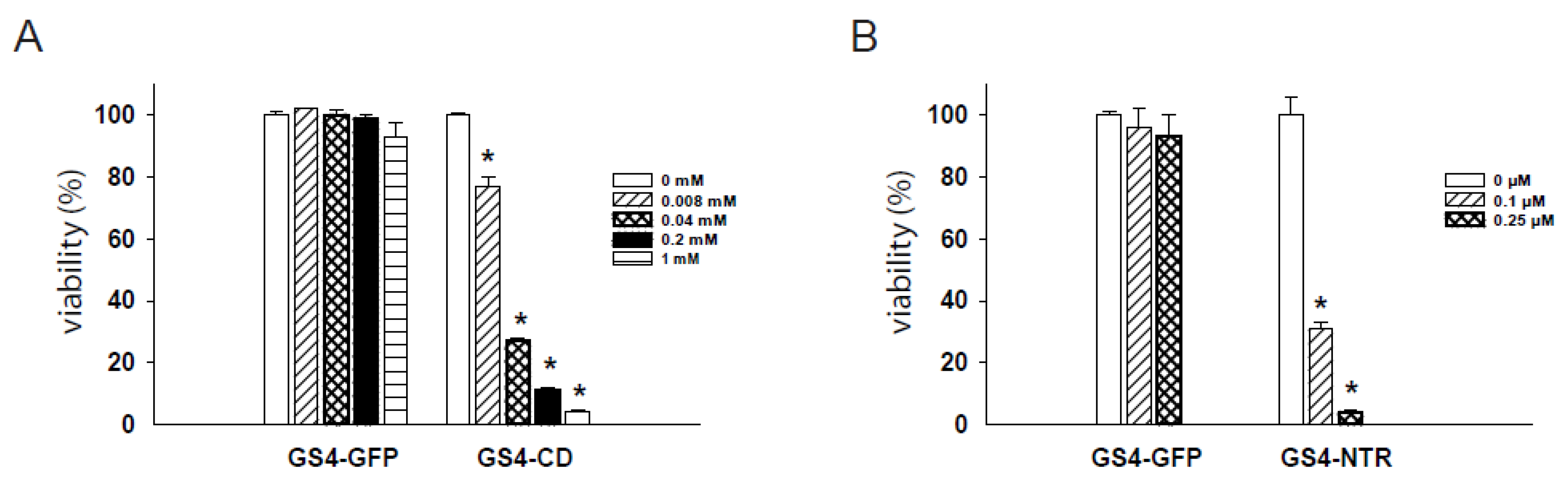
2.4. Dose-Dependent Cytotoxicity of RRV-Transduced Cells after Prodrug Administration
2.5. RRV-Mediated Prodrug Activator Gene Delivery Significantly Improves Survival of Immune-Competent Rats Bearing Gliomas
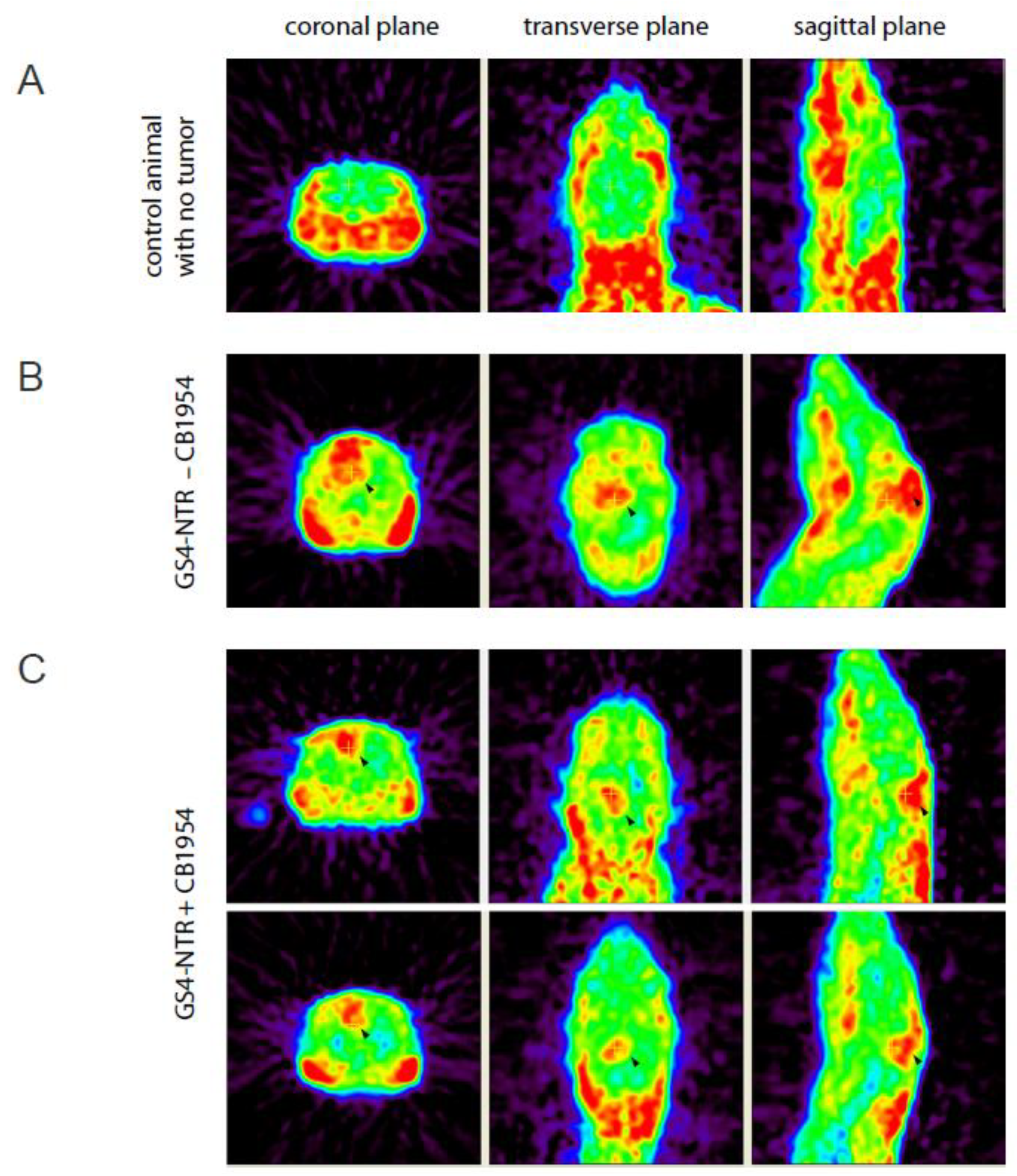
2.6. MicroPET Imaging of RG-2 Glioma-Bearing Rats after Prodrug Activator Gene Therapy
3. Discussion
4. Materials and Methods
4.1. Viral Vectors and Cell Lines
4.2. Viral Vector Replication Assays in Glioma Cells
4.3. Viral Vector Replication Assay in Intracerebral Glioma Model
4.4. Real-Time PCR Analysis
4.5. In Vitro Cytotoxicity Assay
4.6. Survival Assay Using Intracerebral Glioma Models
4.7. MicroPET Imaging
4.8. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fisher, J.L.; Langer, C.E.; Pekmezci, M.; Schwartzbaum, J.A.; Turner, M.C.; Walsh, K.M.; et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro-oncology 2014, 16, 896–913. [Google Scholar] [CrossRef] [PubMed]
- Rainov, N.G. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum. Gene Ther. 2000, 11, 2389–2401. [Google Scholar] [CrossRef] [PubMed]
- Galanis, E.; Vile, R.; Russell, S.J. Delivery systems intended for in vivo gene therapy of cancer: targeting and replication competent viral vectors. Crit. Rev. Oncol. Hematol. 2001, 38, 177–192. [Google Scholar] [CrossRef]
- Kirn, D.; Martuza, R.L.; Zwiebel, J. Replication-selective virotherapy for cancer: Biological principles, risk management and future directions. Nat. Med. 2001, 7, 781–787. [Google Scholar] [CrossRef]
- Alemany, R.; Balague, C.; Curiel, D.T. Replicative adenoviruses for cancer therapy. Nat. Biotechnol. 2000, 18, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Sinkovics, J.G.; Horvath, J.C. Newcastle disease virus (NDV): brief history of its oncolytic strains. J. Clin. Virol. 2000, 16, 1–15. [Google Scholar] [CrossRef]
- Walker, J.R.; McGeagh, K.G.; Sundaresan, P.; Jorgensen, T.J.; Rabkin, S.D.; Martuza, R.L. Local and systemic therapy of human prostate adenocarcinoma with the conditionally replicating herpes simplex virus vector G207. Hum. Gene Ther. 1999, 10, 2237–2243. [Google Scholar] [CrossRef]
- Norman, K.L.; Lee, P.W. Reovirus as a novel oncolytic agent. J. Clin. Investig. 2000, 105, 1035–1038. [Google Scholar] [CrossRef]
- Wang, W.J.; Tai, C.K.; Kasahara, N.; Chen, T.C. Highly efficient and tumor-restricted gene transfer to malignant gliomas by replication-competent retroviral vectors. Hum. Gene Ther. 2003, 14, 117–127. [Google Scholar] [CrossRef]
- Solly, S.K.; Trajcevski, S.; Frisen, C.; Holzer, G.W.; Nelson, E.; Clerc, B.; Abordo-Adesida, E.; Castro, M.; Lowenstein, P.; Klatzmann, D. Replicative retroviral vectors for cancer gene therapy. Cancer Gene Ther. 2003, 10, 30–39. [Google Scholar] [CrossRef]
- Tai, C.K.; Wang, W.; Lai, Y.H.; Logg, C.R.; Parker, W.B.; Li, Y.F.; Hong, J.S.; Sorscher, E.J.; Chen, T.C.; Kasahara, N. Enhanced efficiency of prodrug activation therapy by tumor-selective replicating retrovirus vectors armed with the Escherichia coli purine nucleoside phosphorylase gene. Cancer Gene Ther. 2010, 17, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.K.; Wang, W.J.; Chen, T.C.; Kasahara, N. Single-shot, multicycle suicide gene therapy by replication-competent retrovirus vectors achieves long-term survival benefit in experimental glioma. Mol. Ther. 2005, 12, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tai, C.K.; Kershaw, A.D.; Solly, S.K.; Klatzmann, D.; Kasahara, N.; Chen, T.C. Use of replication-competent retroviral vectors in an immunocompetent intracranial glioma model. Neurosurg. Focus. 2006, 20, E25. [Google Scholar] [PubMed]
- Huang, T.T.; Parab, S.; Burnett, R.; Diago, O.; Ostertag, D.; Hofman, F.M.; Espinoza, F.L.; Martin, B.; Ibanez, C.E.; Kasahara, N.; et al. Intravenous administration of retroviral replicating vector, Toca 511, demonstrates therapeutic efficacy in orthotopic immune-competent mouse glioma model. Hum. Gene Ther. 2015, 26, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Cloughesy, T.F.; Landolfi, J.; Vogelbaum, M.A.; Ostertag, D.; Elder, J.B.; Bloomfield, S.; Carter, B.; Chen, C.C.; Kalkanis, S.N.; Kesari, S.; et al. Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC. Neuro-oncology 2018, 20, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Cloughesy, T.F.; Landolfi, J.; Hogan, D.J.; Bloomfield, S.; Carter, B.; Chen, C.C.; Elder, J.B.; Kalkanis, S.N.; Kesari, S.; Lai, A.; et al. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci. Transl. Med. 2016, 8, 341ra375. [Google Scholar] [CrossRef]
- Overbaugh, J.; Miller, A.D.; Eiden, M.V. Receptors and entry cofactors for retroviruses include single and multiple transmembrane-spanning proteins as well as newly described glycophosphatidylinositol-anchored and secreted proteins. Microbiol. Mol. Biol. Rev. 2001, 65, 371–389. [Google Scholar] [CrossRef]
- Lu, Y.C.; Luo, Y.P.; Wang, Y.W.; Tai, C.K. Highly efficient gene transfer to solid tumors in vivo by tumor-selective replicating retrovirus vectors. Int. J. Mol. Med. 2010, 25, 769–775. [Google Scholar]
- Lu, Y.C.; Chen, Y.J.; Yu, Y.R.; Lai, Y.H.; Cheng, J.C.; Li, Y.F.; Shen, C.H.; Tai, C.K. Replicating retroviral vectors for oncolytic virotherapy of experimental hepatocellular carcinoma. Oncol. Rep. 2012, 28, 21–26. [Google Scholar] [CrossRef]
- Kubo, S.; Takagi-Kimura, M.; Kasahara, N. Efficient tumor transduction and antitumor efficacy in experimental human osteosarcoma using retroviral replicating vectors. Cancer Gene Ther. 2019, 26, 41–47. [Google Scholar] [CrossRef]
- Kubo, S.; Takagi-Kimura, M.; Logg, C.R.; Kasahara, N. Highly efficient tumor transduction and antitumor efficacy in experimental human malignant mesothelioma using replicating gibbon ape leukemia virus. Cancer Gene Ther. 2013, 20, 671–677. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kubo, S.; Takagi-Kimura, M.; Tagawa, M.; Kasahara, N. Dual-vector prodrug activator gene therapy using retroviral replicating vectors. Cancer Gene Ther. 2019, 26, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Kievit, E.; Bershad, E.; Ng, E.; Sethna, P.; Dev, I.; Lawrence, T.S.; Rehemtulla, A. Superiority of yeast over bacterial cytosine deaminase for enzyme/prodrug gene therapy in colon cancer xenografts. Cancer Res. 1999, 59, 1417–1421. [Google Scholar] [PubMed]
- Anlezark, G.M.; Melton, R.G.; Sherwood, R.F.; Coles, B.; Friedlos, F.; Knox, R.J. The bioactivation of 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB1954)--I. Purification and properties of a nitroreductase enzyme from Escherichia coli--a potential enzyme for antibody-directed enzyme prodrug therapy (ADEPT). Biochem. Pharmacol. 1992, 44, 2289–2295. [Google Scholar] [CrossRef]
- McNeish, I.A.; Green, N.K.; Gilligan, M.G.; Ford, M.J.; Mautner, V.; Young, L.S.; Kerr, D.J.; Searle, P.F. Virus directed enzyme prodrug therapy for ovarian and pancreatic cancer using retrovirally delivered E. coli nitroreductase and CB1954. Gene Ther. 1998, 5, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Mullen, J.T.; Chandrasekhar, S.; Pawlik, T.M.; Yoon, S.S.; Tanabe, K.K. Multimodality therapy with a replication-conditional herpes simplex virus 1 mutant that expresses yeast cytosine deaminase for intratumoral conversion of 5-fluorocytosine to 5-fluorouracil. Cancer Res. 2001, 61, 5447–5452. [Google Scholar]
- Knox, R.J.; Friedlos, F.; Jarman, M.; Roberts, J.J. A new cytotoxic, DNA interstrand crosslinking agent, 5-(aziridin-1-yl)-4-hydroxylamino-2-nitrobenzamide, is formed from 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB 1954) by a nitroreductase enzyme in Walker carcinoma cells. Biochem. Pharmacol. 1988, 37, 4661–4669. [Google Scholar] [CrossRef]
- Logg, C.R.; Baranick, B.T.; Lemp, N.A.; Kasahara, N. Adaptive evolution of a tagged chimeric gammaretrovirus: identification of novel cis-acting elements that modulate splicing. J. Mol. Biol. 2007, 369, 1214–1229. [Google Scholar] [CrossRef]
- Wang, H.E.; Wu, S.Y.; Chang, C.W.; Liu, R.S.; Hwang, L.C.; Lee, T.W.; Chen, J.C.; Hwang, J.J. Evaluation of F-18-labeled amino acid derivatives and [18F]FDG as PET probes in a brain tumor-bearing animal model. Nucl. Med. Biol. 2005, 32, 367–375. [Google Scholar] [CrossRef]
- Bridgewater, J.A.; Springer, C.J.; Knox, R.J.; Minton, N.P.; Michael, N.P.; Collins, M.K. Expression of the bacterial nitroreductase enzyme in mammalian cells renders them selectively sensitive to killing by the prodrug CB1954. Eur. J. Cancer 1995, 31A, 2362–2370. [Google Scholar] [CrossRef]
- Naik, S.; Russell, S.J. Engineering oncolytic viruses to exploit tumor specific defects in innate immune signaling pathways. Expert Opin. Biol. Ther. 2009, 9, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.Q.; Mills, K.H. Immunosuppressive networks and checkpoints controlling antitumor immunity and their blockade in the development of cancer immunotherapeutics and vaccines. Oncogene 2014, 33, 4623–4631. [Google Scholar] [CrossRef] [PubMed]
- Marshall, E. Clinical research. Gene therapy a suspect in leukemia-like disease. Science 2002, 298, 34–35. [Google Scholar] [CrossRef] [PubMed]
- Hacein-Bey-Abina, S.; Von Kalle, C.; Schmidt, M.; McCormack, M.P.; Wulffraat, N.; Leboulch, P.; Lim, A.; Osborne, C.S.; Pawliuk, R.; Morillon, E.; et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 2003, 302, 415–419. [Google Scholar] [CrossRef]
- McCormack, M.P.; Rabbitts, T.H. Activation of the T-cell oncogene LMO2 after gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2004, 350, 913–922. [Google Scholar] [CrossRef]
- Whiteway, J.; Koziarz, P.; Veall, J.; Sandhu, N.; Kumar, P.; Hoecher, B.; Lambert, I.B. Oxygen-insensitive nitroreductases: analysis of the roles of nfsA and nfsB in development of resistance to 5-nitrofuran derivatives in Escherichia coli. J. Bacteriol. 1998, 180, 5529–5539. [Google Scholar] [CrossRef]
- DuBridge, R.B.; Tang, P.; Hsia, H.C.; Leong, P.M.; Miller, J.H.; Calos, M.P. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol. Cell. Biol. 1987, 7, 379–387. [Google Scholar] [CrossRef]
- Tai, C.K.; Logg, C.R.; Park, J.M.; Anderson, W.F.; Press, M.F.; Kasahara, N. Antibody-mediated targeting of replication-competent retroviral vectors. Hum. Gene Ther. 2003, 14, 789–802. [Google Scholar] [CrossRef]
- Hiraoka, K.; Kimura, T.; Logg, C.R.; Tai, C.K.; Haga, K.; Lawson, G.W.; Kasahara, N. Therapeutic efficacy of replication-competent retrovirus vector-mediated suicide gene therapy in a multifocal colorectal cancer metastasis model. Cancer Res. 2007, 67, 5345–5353. [Google Scholar] [CrossRef]


| Copies of GALV env Per Cell | |
|---|---|
| tumor | 1.46 ± 0.23 |
| contralateral normal brain | − |
| bone marrow | − |
| spleen | − |
| intestine | − |
| liver | − |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-H.; Sun, J.-M.; Chen, B.-M.; Lin, S.-C.; Chang, H.-F.; Collins, S.; Chang, D.; Wu, S.-F.; Lu, Y.-C.; Wang, W.; et al. Efficient Prodrug Activator Gene Therapy by Retroviral Replicating Vectors Prolongs Survival in an Immune-Competent Intracerebral Glioma Model. Int. J. Mol. Sci. 2020, 21, 1433. https://doi.org/10.3390/ijms21041433
Chen S-H, Sun J-M, Chen B-M, Lin S-C, Chang H-F, Collins S, Chang D, Wu S-F, Lu Y-C, Wang W, et al. Efficient Prodrug Activator Gene Therapy by Retroviral Replicating Vectors Prolongs Survival in an Immune-Competent Intracerebral Glioma Model. International Journal of Molecular Sciences. 2020; 21(4):1433. https://doi.org/10.3390/ijms21041433
Chicago/Turabian StyleChen, Shih-Han, Jui-Ming Sun, Bing-Mao Chen, Sheng-Che Lin, Hao-Fang Chang, Sara Collins, Deching Chang, Shu-Fen Wu, Yin-Che Lu, Weijun Wang, and et al. 2020. "Efficient Prodrug Activator Gene Therapy by Retroviral Replicating Vectors Prolongs Survival in an Immune-Competent Intracerebral Glioma Model" International Journal of Molecular Sciences 21, no. 4: 1433. https://doi.org/10.3390/ijms21041433
APA StyleChen, S.-H., Sun, J.-M., Chen, B.-M., Lin, S.-C., Chang, H.-F., Collins, S., Chang, D., Wu, S.-F., Lu, Y.-C., Wang, W., Chen, T. C., Kasahara, N., Wang, H.-E., & Tai, C.-K. (2020). Efficient Prodrug Activator Gene Therapy by Retroviral Replicating Vectors Prolongs Survival in an Immune-Competent Intracerebral Glioma Model. International Journal of Molecular Sciences, 21(4), 1433. https://doi.org/10.3390/ijms21041433






