NADPH Oxidase Overactivity Underlies Telomere Shortening in Human Atherosclerosis
Abstract
:1. Introduction
2. Results
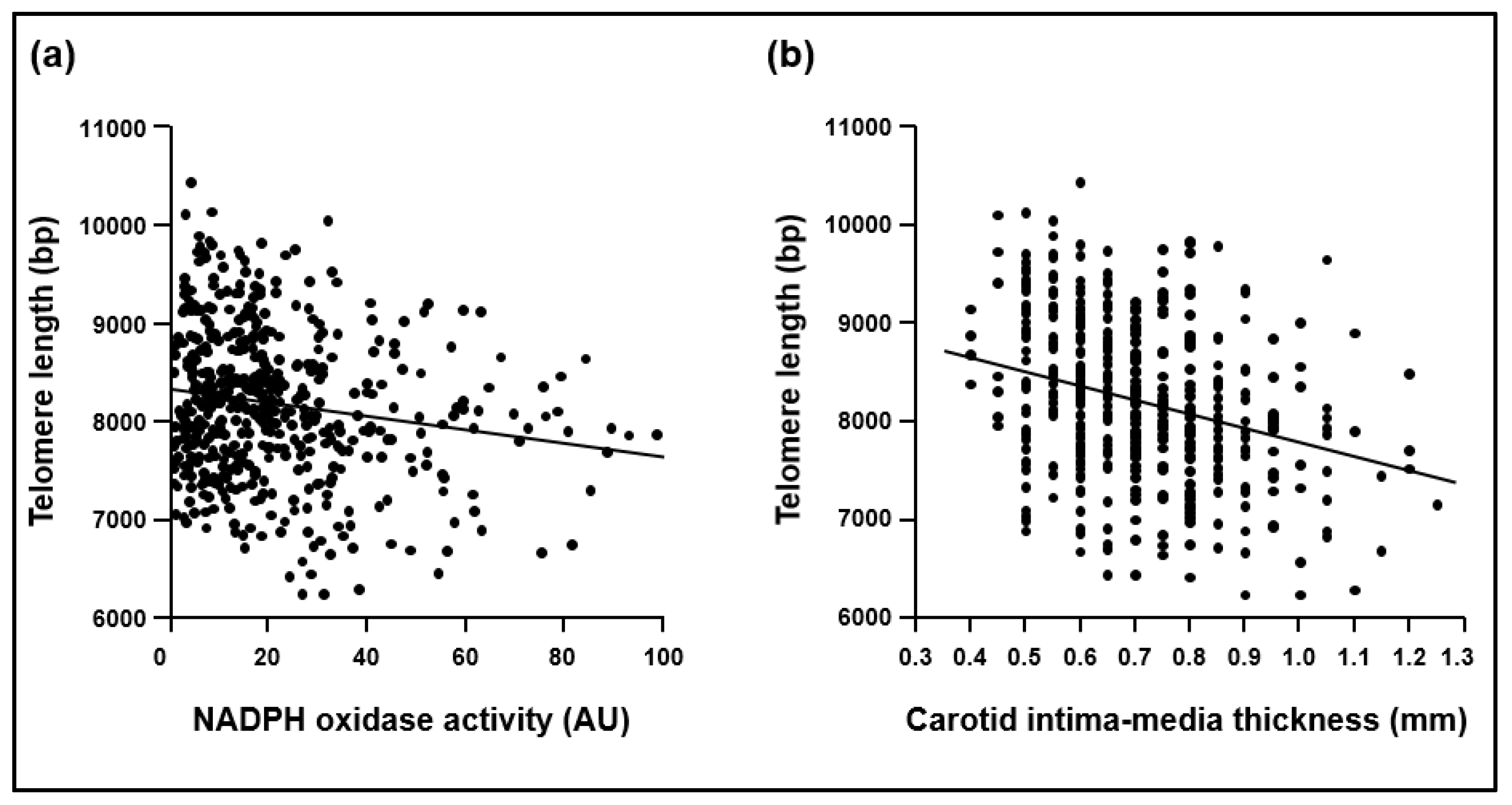
2.1. Cohort 1
2.2. Cohort 2
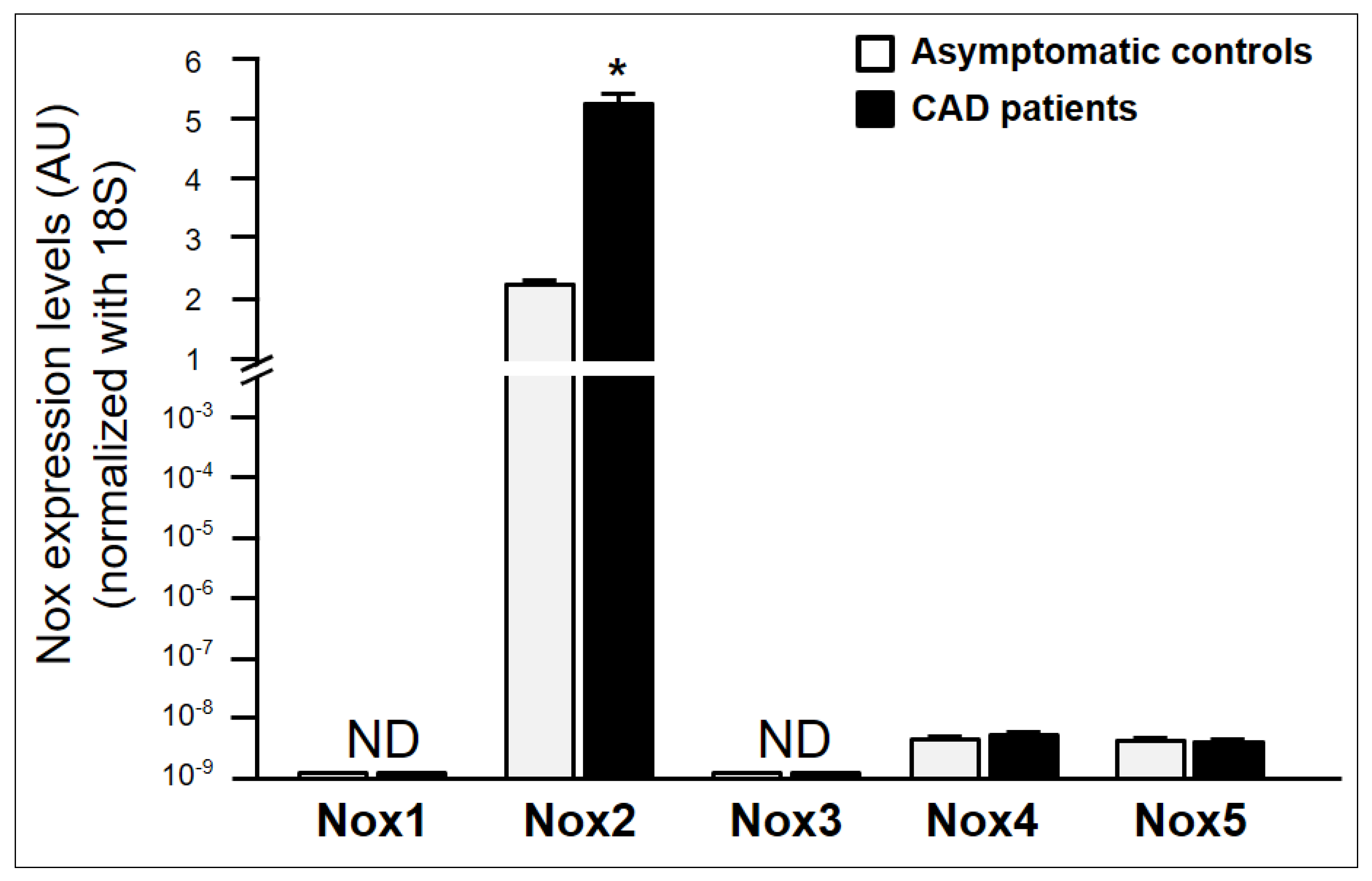
2.3. Characterization of NADPH Oxidase Isoforms Involved in NADPH Oxidase Activity in Asymptomatic Individuals and CAD Patients
3. Discussion
4. Materials and Methods
4.1. Human Studies
4.2. Length of Telomeres
4.3. Determination of Superoxide Production in Human Peripheral Blood Cells
4.4. RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction
4.5. Measurement of 8-hydroxy-2-deoxyguanosine Levels
4.6. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lopez, A.D.; Mathers, C.D.; Ezzati, M.; Jamison, D.T.; Murray, C.J. Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data. Lancet 2006, 367, 1747–1757. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Ventura, J.L.; Rodrigues-Diez, R.; Martinez-Lopez, D.; Salaices, M.; Blanco-Colio, L.M.; Briones, A.M. Oxidative stress in human atherothrombosis: Sources, markers and therapeutic targets. Int. J. Mol. Sci. 2017, 18, 2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, F.; Tengattini, S.; Fabiano, A.; Bianchi, R.; Rezzani, R. Atherosclerosis and oxidative stress. Histol. Histopathol. 2008, 23, 381–390. [Google Scholar] [PubMed]
- García-Redondo, A.B.; Aguado, A.; Briones, A.M.; Salaices, M. NADPH oxidases and vascular remodeling in cardiovascular diseases. Pharmacol. Res. 2016, 114, 110–120. [Google Scholar] [CrossRef]
- Drummond, G.R.; Selemidis, S.; Griendling, K.K.; Sobey, C.G. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 453–471. [Google Scholar] [CrossRef] [Green Version]
- Azumi, H.; Inoue, N.; Ohashi, Y.; Terashima, M.; Mori, T.; Fujita, H.; Awano, K.; Kobayashi, K.; Maeda, K.; Hata, K.; et al. Superoxide generation in directional coronary atherectomy specimens of patients with angina pectoris. Important role of NAD(P)H oxidase. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1838–1844. [Google Scholar] [CrossRef]
- Guzik, T.J.; Chen, W.; Gongora, M.C.; Guzik, B.; Lob, H.E.; Mangalat, D.; Hoch, N.; Dikalov, S.; Rudzinski, P.; Kapelak, B.; et al. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J. Am. Coll. Cardiol. 2008, 52, 1803–1809. [Google Scholar] [CrossRef] [Green Version]
- Konior, A.; Schramm, A.; Czesnikiewicz-Guzik, M.; Guzik, T.J. NADPH oxidases in vascular pathology. Antioxid. Redox Signal. 2014, 20, 2794–2814. [Google Scholar] [CrossRef] [Green Version]
- Sorescu, D.; Weiss, D.; Lassegue, B.; Clempus, R.E.; Szöcs, K.; Sorescu, G.P.; Valppu, L.; Quinn, M.T.; Lambeth, J.D.; Vega, J.D.; et al. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 2002, 105, 1429–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalinina, N.; Agrotis, A.; Tararak, E.; Antropova, Y.; Kanellakis, P.; Ilyinskaya, O.; Quinn, M.T.; Smirnov, V.; Bobik, A. Cytochrome b558-dependent NAD(P)H oxidase-phox units in smooth muscle and macrophages of atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 2037–2043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madrigal-Matute, J.; Fernandez-Laso, V.; Sastre, C.; Llamas-Granda, P.; Egido, J.; Martin-Ventura, J.L.; Zalba, G.; Blanco-Colio, L.M. TWEAK/Fn14 interaction promotes oxidative stress through NADPH oxidase activation in macrophages. Cardiovasc. Res. 2015, 108, 139–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zalba, G.; Beloqui, O.; San José, G.; Moreno, M.U.; Fortuño, A.; Díez, J. NADPH oxidase-dependent superoxide production is associated with carotid intima-media thickness in subjects free of clinical atherosclerotic disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1452–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zalba, G.; Fortuño, A.; Orbe, J.; San José, G.; Moreno, M.U.; Belzunce, M.; Rodríguez, J.A.; Beloqui, O.; Páramo, J.A.; Díez, J. Phagocytic NADPH oxidase-dependent superoxide production stimulates matrix metalloproteinase-9: Implications for human atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 587–593. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, E.H. Structure and function of telomeres. Nature 1991, 350, 569–573. [Google Scholar] [CrossRef]
- Houben, J.M.; Moonen, H.J.; van Schooten, F.J.; Hageman, G.J. Telomere length assessment: Biomarker of chronic oxidative stress? Free Radic. Biol. Med. 2008, 44, 235–246. [Google Scholar] [CrossRef]
- Matthews, C.; Gorenne, I.; Scott, S.; Figg, N.; Kirkpatrick, P.; Ritchie, A.; Goddard, M.; Bennett, M. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: Effects of telomerase and oxidative stress. Circ. Res. 2006, 99, 156–164. [Google Scholar] [CrossRef] [Green Version]
- Benetos, A.; Gardner, J.P.; Zureik, M.; Labat, C.; Xiaobin, L.; Adamopoulos, C.; Temmar, M.; Bean, K.E.; Thomas, F.; Aviv, A. Short telomeres are associated with increased carotid atherosclerosis in hypertensive subjects. Hypertension 2004, 43, 182–185. [Google Scholar] [CrossRef] [Green Version]
- Sampson, M.J.; Winterbone, M.S.; Hughes, J.C.; Dozio, N.; Hughes, D.A. Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care 2006, 29, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Adaikalakoteswari, A.; Balasubramanyam, M.; Ravikumar, R.; Deepa, R.; Mohan, V. Association of telomere shortening with impaired glucose tolerance and diabetic macroangiopathy. Atherosclerosis 2007, 195, 83–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benetos, A.; Toupance, S.; Gautier, S.; Labat, C.; Kimura, M.; Rossi, P.M.; Settembre, N.; Hubert, J.; Frimat, L.; Bertrand, B.; et al. Short leukocyte telomere length precedes clinical expression of atherosclerosis: The blood-and-muscle model. Circ. Res. 2018, 122, 616–623. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, T.; Nawrot, T.; Bekaert, S.; De Buyzere, M.L.; Rietzschel, E.R.; Andrés, V. Telomere length as cardiovascular aging biomarker: JACC review topic of the week. J. Am. Coll. Cardiol. 2018, 72, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Cui, N.H.; Zhang, S.; Liu, Z.J.; Ma, J.F.; Ming, L. Leukocyte telomere length, mitochondrial DNA copy number, and coronary artery disease risk and severity: A two-stage case-control study of 3064 Chinese subjects. Atherosclerosis 2019, 284, 165–172. [Google Scholar] [CrossRef]
- Tian, R.; Zhang, L.N.; Zhang, T.T.; Pang, H.Y.; Chen, L.F.; Shen, Z.J.; Liu, Z.; Fang, Q.; Zhang, S.Y. Association between oxidative stress and peripheral leukocyte telomere length in patients with premature coronary artery disease. Med. Sci. Monit. 2017, 23, 4382–4390. [Google Scholar] [CrossRef] [Green Version]
- Kurz, D.J.; Decary, S.; Hong, Y.; Trivier, E.; Akhmedov, A.; Erusalimsky, J.D. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J. Cell Sci. 2004, 117, 2417–2426. [Google Scholar] [CrossRef] [Green Version]
- Herbert, K.E.; Mistry, Y.; Hastings, R.; Poolman, T.; Niklason, L.; Williams, B. Angiotensin II-mediated oxidative DNA damage accelerates cellular senescence in cultured human vascular smooth muscle cells via telomere-dependent and independent pathways. Circ. Res. 2008, 102, 201–208. [Google Scholar] [CrossRef]
- Mistry, Y.; Poolman, T.; Williams, B.; Herbert, K.E. A role for mitochondrial oxidants in stress-induced premature senescence of human vascular smooth muscle cells. Redox Biol. 2013, 1, 411–417. [Google Scholar] [CrossRef] [Green Version]
- Voghel, G.; Thorin-Trescases, N.; Farhat, N.; Mamarbachi, A.M.; Villeneuve, L.; Fortier, A.; Perrault, L.P.; Carrier, M.; Thorin, E. Chronic treatment with N-acetyl-cystein delays cellular senescence in endothelial cells isolated from a subgroup of atherosclerotic patients. Mech. Ageing Dev. 2008, 129, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Cafueri, G.; Parodi, F.; Pistorio, A.; Bertolotto, M.; Ventura, F.; Gambini, C.; Bianco, P.; Dallegri, F.; Pistoia, V.; Pezzolo, A.; et al. Endothelial and smooth muscle cells from abdominal aortic aneurysm have increased oxidative stress and telomere attrition. PLoS ONE 2012, 7, e35312. [Google Scholar] [CrossRef] [Green Version]
- Voghel, G.; Thorin-Trescases, N.; Farhat, N.; Nguyen, A.; Villeneuve, L.; Mamarbachi, A.M.; Fortier, A.; Perrault, L.P.; Carrier, M.; Thorin, E. Cellular senescence in endothelial cells from atherosclerotic patients is accelerated by oxidative stress associated with cardiovascular risk factors. Mech. Ageing Dev. 2007, 128, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Salazar, G. NADPH oxidases and mitochondria in vascular senescence. Int. J. Mol. Sci. 2018, 19, 1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lener, B.; Kozieł, R.; Pircher, H.; Hütter, E.; Greussing, R.; Herndler-Brandstetter, D.; Hermann, M.; Unterluggauer, H.; Jansen-Dürr, P. The NADPH oxidase Nox4 restricts the replicative lifespan of human endothelial cells. Biochem. J. 2009, 423, 363–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezende, F.; Schürmann, C.; Schütz, S.; Harenkamp, S.; Herrmann, E.; Seimetz, M.; Weißmann, N.; Schröder, K. Knock out of the NADPH oxidase Nox4 has no impact on life span in mice. Redox Biol. 2017, 11, 312–314. [Google Scholar] [CrossRef]
- Childs, B.G.; Baker, D.J.; Wijshake, T.; Conover, C.A.; Campisi, J.; van Deursen, J.M. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 2016, 354, 472–477. [Google Scholar] [CrossRef]
- Beloqui, O.; Moreno, M.U.; San José, G.; Pejenaute, Á.; Cortés, A.; Landecho, M.F.; Díez, J.; Fortuño, A.; Zalba, G. Increased phagocytic NADPH oxidase activity associates with coronary artery calcification in asymptomatic men. Free Radic. Res. 2017, 51, 389–396. [Google Scholar] [CrossRef]
- Margaritis, M.; Sanna, F.; Lazaros, G.; Akoumianakis, I.; Patel, S.; Antonopoulos, A.S.; Duke, C.; Herdman, L.; Psarros, C.; Oikonomou, E.K.; et al. Predictive value of telomere length on outcome following acute myocardial infarction: Evidence for contrasting effects of vascular vs. blood oxidative stress. Eur. Heart J. 2017, 38, 3094–3104. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Murugesan, P.; Huang, K.; Cai, H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: Novel therapeutic targets. Nat. Rev. Cardiol. 2019. [Google Scholar] [CrossRef]
- Violi, F.; Carnevale, R.; Loffredo, L.; Pignatelli, P.; Gallin, J.I. NADPH oxidase-2 and atherothrombosis: Insight from chronic granulomatous disease. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.F.; Qiao, M.; Schröder, K.; Zhao, Q.; Asmis, R. Nox4 is a novel inducible source of reactive oxygen species in monocytes and macrophages and mediates oxidized low density lipoprotein-induced macrophage death. Circ. Res. 2010, 106, 1489–1497. [Google Scholar] [CrossRef] [Green Version]
- Manea, A.; Manea, S.A.; Gan, A.M.; Constantin, A.; Fenyo, I.M.; Raicu, M.; Muresian, H.; Simionescu, M. Human monocytes and macrophages express NADPH oxidase 5; a potential source of reactive oxygen species in atherosclerosis. Biochem. Biophys. Res. Commun. 2015, 461, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Vecoli, C.; Borghini, A.; Pulignani, S.; Mercuri, A.; Turchi, S.; Picano, E.; Andreassi, M.G. Independent and combined effects of telomere shortening and mtDNA4977 deletion on long-term outcomes of patients with coronary artery disease. Int. J. Mol. Sci. 2019, 20, 5508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín-Ventura, J.L.; Madrigal-Matute, J.; Muñoz-Garcia, B.; Blanco-Colio, L.M.; Van Oostrom, M.; Zalba, G.; Fortuño, A.; Gomez-Guerrero, C.; Ortega, L.; Ortiz, A.; et al. Increased CD74 expression in human atherosclerotic plaques: Contribution to inflammatory responses in vascular cells. Cardiovasc. Res. 2009, 83, 586–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef]
- Garcia-Calzon, S.; Moleres, A.; Martinez-Gonzalez, M.A.; Martinez, J.A.; Zalba, G.; Marti, A.; GENOI members. Dietary total antioxidant capacity is associated with leukocyte telomere length in a children and adolescent population. Clin. Nutr. 2015, 34, 694–699. [Google Scholar] [CrossRef]
- Moreno, M.U.; San José, G.; Fortuño, A.; Beloqui, O.; Redón, J.; Chaves, F.J.; Corella, D.; Díez, J.; Zalba, G. A novel CYBA variant, the -675A/T polymorphism, is associated with essential hypertension. J. Hypertens. 2007, 25, 1620–1626. [Google Scholar] [CrossRef]
- Moreno, M.U.; San José, G.; Pejenaute, Á.; Landecho, M.F.; Díez, J.; Beloqui, Ó.; Fortuño, A.; Zalba, G. Association of phagocytic NADPH oxidase activity with hypertensive heart disease: A role for cardiotrophin-1? Hypertension 2014, 63, 468–474. [Google Scholar] [CrossRef] [Green Version]


| Parameters | Atherosclerotic Plaques in Carotid Arteries | |
|---|---|---|
| Absence (n = 389) | Presence (n = 116) | |
| Age, year | 54 ± 1 | 61 ± 1 * |
| Male Gender, % | 80 | 88* |
| BMI, kg/m2 | 28.8 ± 0.2 | 28.9 ± 0.5 |
| SBP, mmHg | 127 ± 1 | 137 ± 2 * |
| DBP, mmHg | 82 ± 1 | 83 ± 2 |
| Glucose, mg/dL | 98 ± 1 | 107 ± 2 * |
| HDL-cholesterol, mg/dL | 49 ± 1 | 51 ± 2 |
| LDL-cholesterol, mg/dL | 145 ± 3 | 139 ± 5 |
| Total cholesterol, mg/dL | 219 ± 4 | 212 ± 3 |
| Triglycerides, mg/dL | 117 ± 3 | 113 ± 5 |
| Carotid IMT, mm | 0.67 ± 0.01 | 0.79 ± 0.02 * |
| Medication | ||
| Antihypertensives, % | 15 | 37 * |
| Statins, % | 16 | 31 * |
| Oral hypoglycemics, % | 8 | 16 * |
| Parameters | Control Subjects (n = 25) | CAD Patients (n = 25) |
|---|---|---|
| Age, year | 59 ± 2 | 62 ± 3 |
| Male Gender, % | 80 | 80 |
| BMI, kg/m2 | 28.7 ± 1.6 | 28.3 ± 1.7 |
| SBP, mmHg | 128 ± 2 | 138 ± 2 * |
| Glucose, mg/dL | 99 ± 2 | 111 ± 2 * |
| HDL-cholesterol, mg/dL | 51 ± 3 | 53 ± 3 |
| LDL-cholesterol, mg/dL | 124 ± 14 | 98 ± 12 |
| Total cholesterol, mg/dL | 205 ± 18 | 178 ± 16 |
| Triglycerides, mg/dL | 115 ± 12 | 110 ± 13 |
| Arterial Hypertension, % | 24 | 68* |
| Hyperlipidemia, % | 20 | 24 |
| Diabetes, % | 12 | 44* |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pejenaute, Á.; Cortés, A.; Marqués, J.; Montero, L.; Beloqui, Ó.; Fortuño, A.; Martí, A.; Orbe, J.; Zalba, G. NADPH Oxidase Overactivity Underlies Telomere Shortening in Human Atherosclerosis. Int. J. Mol. Sci. 2020, 21, 1434. https://doi.org/10.3390/ijms21041434
Pejenaute Á, Cortés A, Marqués J, Montero L, Beloqui Ó, Fortuño A, Martí A, Orbe J, Zalba G. NADPH Oxidase Overactivity Underlies Telomere Shortening in Human Atherosclerosis. International Journal of Molecular Sciences. 2020; 21(4):1434. https://doi.org/10.3390/ijms21041434
Chicago/Turabian StylePejenaute, Álvaro, Adriana Cortés, Javier Marqués, Laura Montero, Óscar Beloqui, Ana Fortuño, Amelia Martí, Josune Orbe, and Guillermo Zalba. 2020. "NADPH Oxidase Overactivity Underlies Telomere Shortening in Human Atherosclerosis" International Journal of Molecular Sciences 21, no. 4: 1434. https://doi.org/10.3390/ijms21041434





