Root Response to Drought Stress in Rice (Oryza sativa L.)
Abstract
1. Introduction
2. Global Status of Drought Stress
3. Drought Stress
3.1. Root Function for Water Uptake
3.2. Root System Architecture under Drought Stress
4. Physiological Responses of Roots to Drought Stress
4.1. Phytohormones on Stress
4.2. Osmoregulation
5. Genetic Mechanism of Drought Stress
5.1. Genetics of Root Traits under Drought Stress
5.2. Genetic Mechanisms Governing Drought Tolerance
5.3. Molecular Level Responses to Drought Stress
6. Effects of Drought on Plant–Soil Microbe Interactions
7. Screening Methods for Identifying Root Traits Associated with Drought Stress
7.1. Process of Two-Dimensional (2D) Imaging Phenotyping
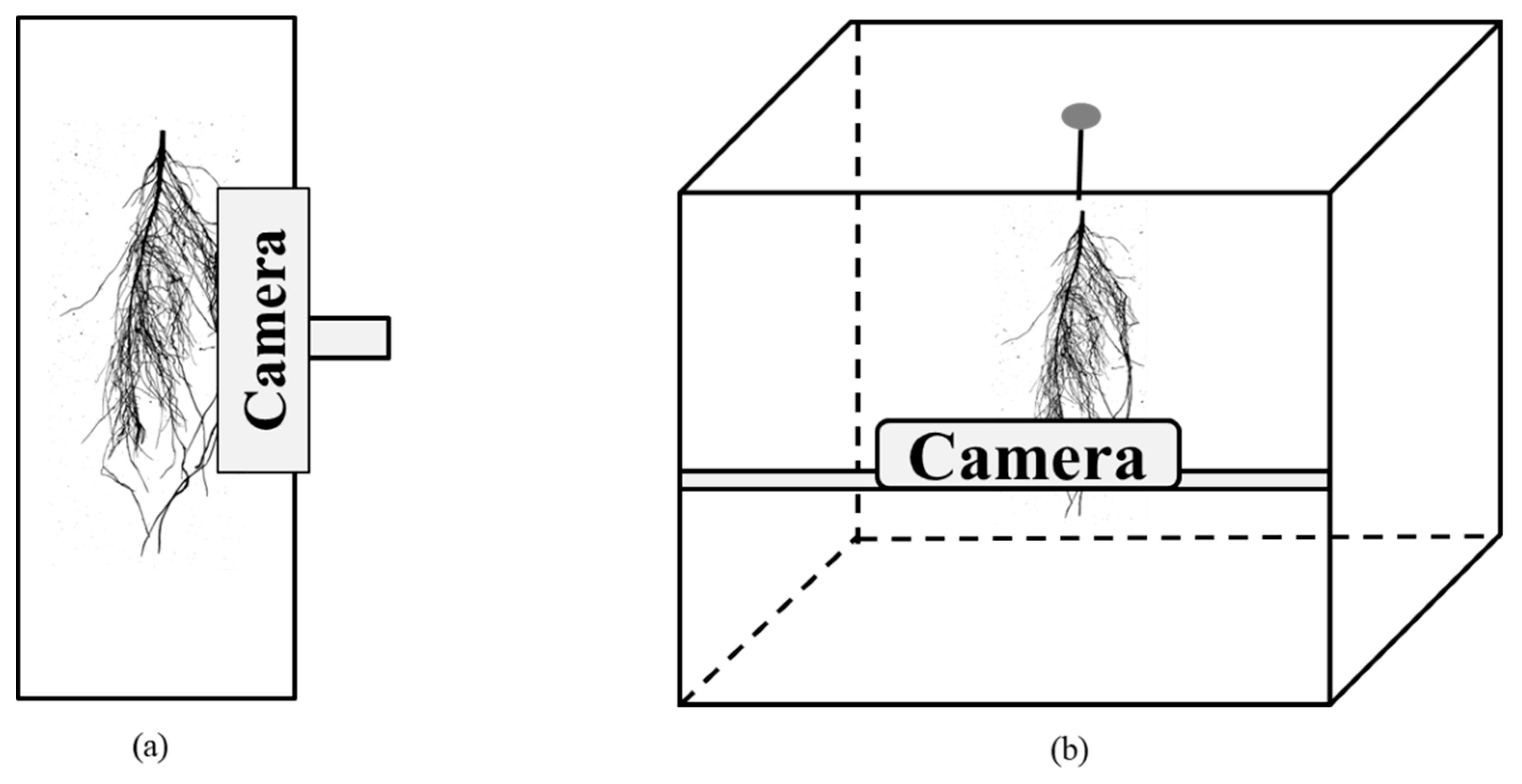
7.1.1. Selection of Imaging Platform
7.1.2. Digging the Root Samples, Image Collection, and Analysis
7.2. Various Root Phenotyping Methods in Crops
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmadi, N.; Audebert, A.; Bennett, M.J.; Bishopp, A.; de Oliveira, A.C.; Courtois, B.; Diedhiou, A.; Diévart, A.; Gantet, P.; Ghesquière, A. The roots of future rice harvests. Rice 2014, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Lafitte, H.; Ismail, A.; Bennett, J. Abiotic stress tolerance in rice for Asia: Progress and the future. Proceedings of 4th International Crop Sci. Congress, Brisbane, Australia, 26 September–1 October 2004. [Google Scholar]
- Tuong, T.; Bouman, B. Rice production in water-scarce environments. In Water Productivity in Agriculture: Limits and Opportunities for Improvement; CABI: Wallingford, UK; International Water Management Institute (IWMI): Colombo, Sri Lanka, 2003; Volume 1, pp. 13–42. [Google Scholar]
- Pandey, S.; Bhandari, H.; Ding, S.; Prapertchob, P.; Sharan, R.; Naik, D.; Taunk, S.K.; Sastri, A. Coping with drought in rice farming in Asia: Insights from a cross-country comparative study. Agric. Econ.-Blackwell 2007, 37, 213–224. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and drought stresses in crops and approaches for their mitigation. Front. Chem. 2018, 6, 26. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Hwang, S.-J.; Waqas, M.; Khan, A.L.; Lee, J.-H.; Lee, J.-D.; Nguyen, H.T.; Lee, I.-J. Comparative analysis of endogenous hormones level in two soybean (Glycine max L.) lines differing in waterlogging tolerance. Front. Plant Sci. 2015, 6, 714. [Google Scholar] [CrossRef]
- Kim, Y.; Seo, C.-W.; Khan, A.L.; Mun, B.-G.; Shahzad, R.; Ko, J.-W.; Yun, B.-W.; Park, S.-K.; Lee, I.-J. Exo-ethylene application mitigates waterlogging stress in soybean (Glycine max L.). BMC Plant Biol. 2018, 18, 254. [Google Scholar] [CrossRef]
- Bielach, A.; Hrtyan, M.; Tognetti, V.B. Plants under stress: Involvement of auxin and cytokinin. Int. J. Mol. Sci. 2017, 18, 1427. [Google Scholar] [CrossRef]
- de Zélicourt, A.; Synek, L.; Saad, M.M.; Alzubaidy, H.; Jalal, R.; Xie, Y.; Andrés-Barrao, C.; Rolli, E.; Guerard, F.; Mariappan, K.G. Ethylene induced plant stress tolerance by Enterobacter sp. SA187 is mediated by 2-keto-4-methylthiobutyric acid production. PLoS Genet. 2018, 14, e1007273. [Google Scholar] [CrossRef]
- Nadarajah, K.; Kumar, I.S. Drought Response in Rice: The miRNA Story. Int. J. Mol. Sci. 2019, 20, 3766. [Google Scholar] [CrossRef]
- Koevoets, I.T.; Venema, J.H.; Elzenga, J.T.; Testerink, C. Roots withstanding their environment: Exploiting root system architecture responses to abiotic stress to improve crop tolerance. Front. Plant Sci. 2016, 7, 1335. [Google Scholar] [CrossRef]
- Zhu, Y.-X.; Gong, H.-J.; Yin, J.-L. Role of silicon in mediating salt tolerance in plants: A review. Plants 2019, 8, 147. [Google Scholar] [CrossRef] [PubMed]
- Kaashyap, M.; Ford, R.; Kudapa, H.; Jain, M.; Edwards, D.; Varshney, R.; Mantri, N. Differential regulation of genes involved in root morphogenesis and cell wall modification is associated with salinity tolerance in chickpea. Sci. Rep. 2018, 8, 4855. [Google Scholar] [CrossRef] [PubMed]
- Kallis, G. Droughts. Annu. Rev. Environ. Resour. 2008, 33, 85–118. [Google Scholar] [CrossRef]
- Zhao, T.; Dai, A. The Magnitude and Causes of Global Drought Changes in the Twenty-First Century under a Low–Moderate Emissions Scenario. J. Clim. 2015, 28, 4490–4512. [Google Scholar] [CrossRef]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Chang. 2013, 3, 52. [Google Scholar] [CrossRef]
- Trenberth, K.E.; Dai, A.; van der Schrier, G.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global warming and changes in drought. Nat. Clim. Chang. 2013, 4, 17–22. [Google Scholar] [CrossRef]
- Naumann, G.; Alfieri, L.; Wyser, K.; Mentaschi, L.; Betts, R.A.; Carrao, H.; Spinoni, J.; Vogt, J.; Feyen, L. Global Changes in Drought Conditions Under Different Levels of Warming. Geophys. Res. Lett. 2018, 45, 3285–3296. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Lai, C.; Zeng, Z.; Zhong, R.; Chen, X.; Zhou, X.; Wang, M. Does drought in China show a significant decreasing trend from 1961 to 2009? Sci. Total Environ. 2017, 579, 314–324. [Google Scholar] [CrossRef]
- Masih, I.; Maskey, S.; Mussá, F.E.F.; Trambauer, P. A review of droughts on the African continent: A geospatial and long-term perspective. Hydrol. Earth Syst. Sci. 2014, 18, 3635–3649. [Google Scholar] [CrossRef]
- Barnabas, B.; Jager, K.; Feher, A. The effect of drought and heat stress on reproductive processes in cereals. Plant. Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef]
- Kumar, A.; Sandhu, N.; Dixit, S.; Yadav, S.; Swamy, B.P.M.; Shamsudin, N.A.A. Marker-assisted selection strategy to pyramid two or more QTLs for quantitative trait-grain yield under drought. Rice (N Y) 2018, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Cairns, J.; Impa, S.; O’Toole, J.; Jagadish, S.; Price, A. Influence of the soil physical environment on rice (Oryza sativa L.) response to drought stress and its implications for drought research. Field Crops Res. 2011, 121, 303–310. [Google Scholar] [CrossRef]
- Fukai, S.; Cooper, M. Development of drought-resistant cultivars using physiomorphological traits in rice. Field Crops Res. 1995, 40, 67–86. [Google Scholar] [CrossRef]
- Tripathy, J.; Zhang, J.; Robin, S.; Nguyen, T.T.; Nguyen, H. QTLs for cell-membrane stability mapped in rice (Oryza sativa L.) under drought stress. Theor. Appl. Genet. 2000, 100, 1197–1202. [Google Scholar] [CrossRef]
- Yoshida, S.; Hasegawa, S. The rice root system: Its development and function. In Drought Resistance in Crops with Emphasis on Rice; Paddyfield: Manila, Philippines, 1982; Volume 10, pp. 97–134. [Google Scholar]
- Tahere, A.-S.; Yamauchi, A.; Kamoshita, A.; Wade, L.J. Genotypic variation in response of rainfed lowland rice to drought and rewatering: II. Root growth. Plant Prod. Sci. 2000, 3, 180–188. [Google Scholar]
- Xu, W.; Cui, K.; Xu, A.; Nie, L.; Huang, J.; Peng, S. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol. Plant. 2015, 37, 1760. [Google Scholar] [CrossRef]
- Wopereis, M.; Kropff, M.; Maligaya, A.; Tuong, T. Drought-stress responses of two lowland rice cultivars to soil water status. Field Crops Res. 1996, 46, 21–39. [Google Scholar] [CrossRef]
- Ghosh, D.; Xu, J. Abiotic stress responses in plant roots: A proteomics perspective. Front. Plant. Sci. 2014, 5, 6. [Google Scholar] [CrossRef]
- Nardini, A.; Salleo, S.; Tyree, M.T. Ecological aspects of water permeability of roots. In Plant Roots; CRC Press: Boca Raton, FL, USA, 2002; pp. 1069–1093. [Google Scholar]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef]
- Fitter, A. Characteristics and functions of root systems. In Plant Roots; CRC Press: Boca Raton, FL, USA, 2002; pp. 49–78. [Google Scholar]
- Henry, A.; Gowda, V.R.P.; Torres, R.O.; McNally, K.L.; Serraj, R. Variation in root system architecture and drought response in rice (Oryza sativa): Phenotyping of the OryzaSNP panel in rainfed lowland fields. Field Crops Res. 2011, 120, 205–214. [Google Scholar] [CrossRef]
- Maseda, P.H.; Fernandez, R.J. Stay wet or else: Three ways in which plants can adjust hydraulically to their environment. J. Exp. Bot. 2006, 57, 3963–3977. [Google Scholar] [CrossRef] [PubMed]
- Bloom, A.J.; Chapin, F.S., III; Mooney, H.A. Resource limitation in plants-an economic analogy. Annu. Rev. Ecol. Evol. Syst. 1985, 16, 363–392. [Google Scholar] [CrossRef]
- Shipley, B.; Meziane, D. The balanced-growth hypothesis and the allometry of leaf and root biomass allocation. Funct. Ecol. 2002, 16, 326–331. [Google Scholar] [CrossRef]
- Comas, L.H.; Mueller, K.E.; Taylor, L.L.; Midford, P.E.; Callahan, H.S.; Beerling, D.J. Evolutionary Patterns and Biogeochemical Significance of Angiosperm Root Traits. Int. J. Plant Sci. 2012, 173, 584–595. [Google Scholar] [CrossRef]
- Hernández, E.; Vilagrosa, A.; Pausas, J.; Bellot, J. Morphological traits and water use strategies in seedlings of Mediterranean coexisting species. Plant Ecol. 2010, 207, 233–244. [Google Scholar] [CrossRef]
- Wasson, A.P.; Richards, R.A.; Chatrath, R.; Misra, S.C.; Prasad, S.V.; Rebetzke, G.J.; Kirkegaard, J.A.; Christopher, J.; Watt, M. Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J. Exp. Bot. 2012, 63, 3485–3498. [Google Scholar] [CrossRef]
- Robinson, D.; Hodge, A.; Griffiths, B.S.; Fitter, A.H. Plant root proliferation in nitrogen–rich patches confers competitive advantage. Proc. R. Soc. Lond. Ser. B: Biol. Sci. 1999, 266, 431–435. [Google Scholar] [CrossRef]
- Bates, T.R.; Lynch, J.P. Root hairs confer a competitive advantage under low phosphorus availability. Plant Soil 2001, 236, 243–250. [Google Scholar] [CrossRef]
- Suzuki, N.; Taketa, S.; Ichii, M. Morphological and physiological characteristics of a root-hairless mutant in rice (Oryza sativa L.). In Roots: The Dynamic Interface between Plants and the Earth; Springer: Dordrecht, The Netherlands, 2003; pp. 9–17. [Google Scholar]
- Wen, T.J.; Schnable, P.S. Analyses of mutants of three genes that influence root hair development in Zea mays (Gramineae) suggest that root hairs are dispensable. Am. J. Bot. 1994, 81, 833–842. [Google Scholar] [CrossRef]
- Lian, H.-L.; Yu, X.; Ye, Q.; Ding, X.-S.; Kitagawa, Y.; Kwak, S.-S.; Su, W.-A.; Tang, Z.-C. The role of aquaporin RWC3 in drought avoidance in rice. Plant Cell Physiol. 2004, 45, 481–489. [Google Scholar] [CrossRef]
- Sakurai, J.; Ishikawa, F.; Yamaguchi, T.; Uemura, M.; Maeshima, M. Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant Cell Physiol. 2005, 46, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Vandeleur, R.K.; Mayo, G.; Shelden, M.C.; Gilliham, M.; Kaiser, B.N.; Tyerman, S.D. The role of plasma membrane intrinsic protein aquaporins in water transport through roots: Diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiol. 2009, 149, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Laur, J.; Hacke, U.G. Transpirational demand affects aquaporin expression in poplar roots. J. Exp. Bot. 2013, 64, 2283–2293. [Google Scholar] [CrossRef] [PubMed]
- Comas, L.H.; Anderson, L.J.; Dunst, R.M.; Lakso, A.N.; Eissenstat, D.M. Canopy and environmental control of root dynamics in a long-term study of Concord grape. New Phytol. 2005, 167, 829–840. [Google Scholar] [CrossRef]
- Robinson, D.; Linehan, D.; Caul, S. What limits nitrate uptake from soil? Plant Cell Environ. 1991, 14, 77–85. [Google Scholar] [CrossRef]
- Newman, E.; Andrews, R.E. Uptake of phosphorus and potassium in relation to root growth and root density. Plant Soil 1973, 38, 49–69. [Google Scholar] [CrossRef]
- Kramer, P.J.; Boyer, J.S. Water Relations of Plants and Soils; Academic press: San Diego, CA, USA, 1995. [Google Scholar]
- Tyree, M.T.; Kolb, K.J.; Rood, S.B.; Patiño, S. Vulnerability to drought-induced cavitation of riparian cottonwoods in Alberta: A possible factor in the decline of the ecosystem? Tree Physiol. 1994, 14, 455–466. [Google Scholar] [CrossRef]
- Alder, N.; Sperry, J.; Pockman, W. Root and stem xylem embolism, stomatal conductance, and leaf turgor in Acer grandidentatum populations along a soil moisture gradient. Oecologia 1996, 105, 293–301. [Google Scholar] [CrossRef]
- Pockman, W.T.; Sperry, J.S. Vulnerability to xylem cavitation and the distribution of Sonoran desert vegetation. Am. J. Bot. 2000, 87, 1287–1299. [Google Scholar] [CrossRef]
- Passioura, J.B. Roots and drought resistance. Agric. Water Manag. 1983, 7, 1983. [Google Scholar] [CrossRef]
- Lynch, J.P.; Chimungu, J.G.; Brown, K.M. Root anatomical phenes associated with water acquisition from drying soil: Targets for crop improvement. J. Exp. Bot. 2014, 65, 6155–6166. [Google Scholar] [CrossRef]
- Champoux, M.; Wang, G.; Sarkarung, S.; Mackill, D.; O’Toole, J.; Huang, N.; McCouch, S. Locating genes associated with root morphology and drought avoidance in rice via linkage to molecular markers. Theor. Appl. Genet. 1995, 90, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.X.; Ray, J.D. Use of wax-petrolatum layers for screening rice root penetration. Crop. Sci. 1995, 35, 684–687. [Google Scholar] [CrossRef]
- Inthapan, P.; Fukai, S. Growth and yield of rice cultivars under sprinkler irrigation in south-eastern Queensland. Aust. J. Exp. Agric. 1988, 28, 243–248. [Google Scholar] [CrossRef]
- Bañoc, D.M.; Yamauchi, A.; Kamoshita, A.; Wade, L.J.; Pardales, J.R. Dry matter production and root system development of rice cultivars under fluctuating soil moisture. Plant Prod. Sci. 2000, 3, 197–207. [Google Scholar] [CrossRef]
- O’toole, J.C. Adaptation of rice environments. In Drought Resistance in Crops with Emphasis on Rice; Paddyfield: Manila, Philippines, 1982; pp. 195–213. [Google Scholar]
- MAMBANI, B.; Lal, R. Response of upland rice varieties to drought stress: I. Relation between root system development and leaf water potential. Plant Soil 1983, 59–72. [Google Scholar] [CrossRef]
- De Willigen, P.; Nielsen, N.; Claassen, N.; Castrignanò, A. Modelling water and nutrient uptake. In Root Methods; Springer: Morris, TX, USA, 2000; pp. 509–543. [Google Scholar]
- Ryser, P. The mysterious root length. Plant Soil 2006, 286, 1–6. [Google Scholar] [CrossRef]
- Eapen, D.; Barroso, M.L.; Ponce, G.; Campos, M.E.; Cassab, G.I. Hydrotropism: Root growth responses to water. Trends Plant Sci. 2005, 10, 44–50. [Google Scholar] [CrossRef]
- Brown, L.K.; George, T.S.; Thompson, J.A.; Wright, G.; Lyon, J.; Dupuy, L.; Hubbard, S.F.; White, P.J. What are the implications of variation in root hair length on tolerance to phosphorus deficiency in combination with water stress in barley (Hordeum vulgare)? Ann. Bot. 2012, 110, 319–328. [Google Scholar] [CrossRef]
- Barlow, P.W. The Root Cap: Cell Dynamics, Cell Differentiation and Cap Function. J. Plant Growth Regul. 2003, 21, 261–286. [Google Scholar] [CrossRef]
- Robbins, N.E., 2nd; Dinneny, J.R. The divining root: Moisture-driven responses of roots at the micro- and macro-scale. J. Exp. Bot. 2015, 66, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J.K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Basnayake, J.; Fukai, S.; Ouk, M. Contribution of potential yield, drought tolerance and escape to adaptation of 15 rice varieties in rainfed lowlands in Cambodia. In Proceedings of the Australian Agronomy Conference; Australian Society of Agronomy: Brisbane, Australia, 2006; pp. 10–16. [Google Scholar]
- Lynch, J.P. Steep, cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. 2013, 112, 347–357. [Google Scholar] [CrossRef]
- Doussan, C.; Pierret, A.; Garrigues, E.; Pagès, L. Water Uptake by Plant Roots: II—Modelling of Water Transfer in the Soil Root-system with Explicit Account of Flow within the Root System— Comparison with Experiments. Plant Soil 2006, 283, 99–117. [Google Scholar] [CrossRef]
- Uga, Y.; Sugimoto, K.; Ogawa, S.; Rane, J.; Ishitani, M.; Hara, N.; Kitomi, Y.; Inukai, Y.; Ono, K.; Kanno, N.; et al. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat. Genet. 2013, 45, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, T.C.; Xu, L.K. Sensitivity of growth of roots versus leaves to water stress: Biophysical analysis and relation to water transport. J. Exp. Bot. 2000, 51, 1595–1616. [Google Scholar] [CrossRef] [PubMed]
- Price, A. QTLs for root growth and drought resistance in rice. In Molecular Techniques in Crop Improvement; Springer: New York, NY, USA, 2002; pp. 563–584. [Google Scholar]
- Medrano, H.; Escalona, J.M.; Bota, J.; Gulias, J.; Flexas, J. Regulation of photosynthesis of C3 plants in response to progressive drought: Stomatal conductance as a reference parameter. Ann. Bot. 2002, 89, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Henry, A.; Cal, A.J.; Batoto, T.C.; Torres, R.O.; Serraj, R. Root attributes affecting water uptake of rice (Oryza sativa) under drought. J. Exp. Bot. 2012, 63, 4751–4763. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, F.; Li, D.; Zhang, H.; Huang, R. Expression of ethylene response factor JERF1 in rice improves tolerance to drought. Planta 2010, 232, 765–774. [Google Scholar] [CrossRef]
- Abe, H.; Urao, T.; Ito, T.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 2003, 15, 63–78. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, J.; Gao, X.; Tong, J.; Xiao, L.; Li, W.; Zhang, H. The Arabidopsis AP2/ERF transcription factor RAP2.6 participates in ABA, salt and osmotic stress responses. Gene 2010, 457, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Peleg, Z.; Blumwald, E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Argueso, C.T.; Ferreira, F.J.; Kieber, J.J. Environmental perception avenues: The interaction of cytokinin and environmental response pathways. Plant. Cell Environ. 2009, 32, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Bandurska, H.; Stroiński, A.; Kubiś, J. The effect of jasmonic acid on the accumulation of ABA, proline and spermidine and its influence on membrane injury under water deficit in two barley genotypes. Acta Physiol. Plant. 2003, 25, 279–285. [Google Scholar] [CrossRef]
- Ren, D.; Rao, Y.; Wu, L.; Xu, Q.; Li, Z.; Yu, H.; Zhang, Y.; Leng, Y.; Hu, J.; Zhu, L. The pleiotropic ABNORMAL FLOWER AND DWARF1 affects plant height, floral development and grain yield in rice. J. Integr. Plant Biol. 2016, 58, 529–539. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, T.; Wang, X.; Deng, Y.; Ma, H.; Zhang, R.; Zhao, J. OsAUX 1 controls lateral root initiation in rice (O ryza sativa L.). Plant Cell Environ. 2015, 38, 2208–2222. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Zhang, W.; Yan, S.; Wang, R.; Zhao, J.; Li, Y.; Qi, Z.; Sun, Z.; Zhu, Z. The putative auxin efflux carrier OsPIN3t is involved in the drought stress response and drought tolerance. Plant J. 2012, 72, 805–816. [Google Scholar] [CrossRef]
- Du, H.; Wu, N.; Fu, J.; Wang, S.; Li, X.; Xiao, J.; Xiong, L. A GH3 family member, OsGH3-2, modulates auxin and abscisic acid levels and differentially affects drought and cold tolerance in rice. J. Exp. Bot. 2012, 63, 6467–6480. [Google Scholar] [CrossRef]
- Zhang, S.-W.; Li, C.-H.; Cao, J.; Zhang, Y.-C.; Zhang, S.-Q.; Xia, Y.-F.; Sun, D.-Y.; Sun, Y. Altered architecture and enhanced drought tolerance in rice via the down-regulation of indole-3-acetic acid by TLD1/OsGH3. 13 activation. Plant Physiol. 2009, 151, 1889–1901. [Google Scholar] [CrossRef]
- Ljung, K. Auxin metabolism and homeostasis during plant development. Development 2013, 140, 943–950. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kamiya, N.; Morinaka, Y.; Matsuoka, M.; Sazuka, T. Auxin biosynthesis by the YUCCA genes in rice. Plant Physiol. 2007, 143, 1362–1371. [Google Scholar] [CrossRef] [PubMed]
- Bais, H.P.; Vepachedu, R.; Gilroy, S.; Callaway, R.M.; Vivanco, J.M. Allelopathy and exotic plant invasion: From molecules and genes to species interactions. Science 2003, 301, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Saad, R.B.; Zouari, N.; Ramdhan, W.B.; Azaza, J.; Meynard, D.; Guiderdoni, E.; Hassairi, A. Improved drought and salt stress tolerance in transgenic tobacco overexpressing a novel A20/AN1 zinc-finger “AlSAP” gene isolated from the halophyte grass Aeluropus littoralis. Plant Mol. Biol. 2010, 72, 171. [Google Scholar] [CrossRef] [PubMed]
- Kemble, A.; Macpherson, H.T. Liberation of amino acids in perennial rye grass during wilting. Biochem. J. 1954, 58, 46. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.-J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef]
- Kishor, P.K.; Sangam, S.; Amrutha, R.; Laxmi, P.S.; Naidu, K.; Rao, K.S.; Rao, S.; Reddy, K.; Theriappan, P.; Sreenivasulu, N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 424–438. [Google Scholar]
- Hirayama, T.; Shinozaki, K. Research on plant abiotic stress responses in the post-genome era: Past, present and future. Plant J. 2010, 61, 1041–1052. [Google Scholar] [CrossRef]
- Messina, C.D.; Sinclair, T.R.; Hammer, G.L.; Curan, D.; Thompson, J.; Oler, Z.; Gho, C.; Cooper, M. Limited-Transpiration Trait May Increase Maize Drought Tolerance in the US Corn Belt. Agron. J. 2015, 107. [Google Scholar] [CrossRef]
- Gamuyao, R.; Chin, J.H.; Pariasca-Tanaka, J.; Pesaresi, P.; Catausan, S.; Dalid, C.; Slamet-Loedin, I.; Tecson-Mendoza, E.M.; Wissuwa, M.; Heuer, S. The protein kinase Pstol1 from traditional rice confers tolerance of phosphorus deficiency. Nature 2012, 488, 535–539. [Google Scholar] [CrossRef]
- Courtois, B.; Ahmadi, N.; Khowaja, F.; Price, A.H.; Rami, J.-F.; Frouin, J.; Hamelin, C.; Ruiz, M. Rice Root Genetic Architecture: Meta-analysis from a Drought QTL Database. Rice 2009, 2, 115–128. [Google Scholar] [CrossRef]
- Paez-Garcia, A.; Motes, C.M.; Scheible, W.R.; Chen, R.; Blancaflor, E.B.; Monteros, M.J. Root Traits and Phenotyping Strategies for Plant Improvement. Plants 2015, 4, 334–355. [Google Scholar] [CrossRef] [PubMed]
- de Dorlodot, S.; Forster, B.; Pages, L.; Price, A.; Tuberosa, R.; Draye, X. Root system architecture: Opportunities and constraints for genetic improvement of crops. Trends Plant Sci. 2007, 12, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.; van Eeuwijk, F.A.; Hammer, G.L.; Podlich, D.W.; Messina, C. Modeling QTL for complex traits: Detection and context for plant breeding. Curr. Opin. Plant Biol. 2009, 12, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Den Herder, G.; Van Isterdael, G.; Beeckman, T.; De Smet, I. The roots of a new green revolution. Trends Plant Sci. 2010, 15, 600–607. [Google Scholar] [CrossRef]
- Hamelin, C.; Sempere, G.; Jouffe, V.; Ruiz, M. TropGeneDB, the multi-tropical crop information system updated and extended. Nucleic Acids Res. 2013, 41, D1172–D1175. [Google Scholar] [CrossRef]
- Collins, N.C.; Tardieu, F.; Tuberosa, R. Quantitative trait loci and crop performance under abiotic stress: Where do we stand? Plant Physiol. 2008, 147, 469–486. [Google Scholar] [CrossRef]
- Wojciechowski, T.; Gooding, M.J.; Ramsay, L.; Gregory, P.J. The effects of dwarfing genes on seedling root growth of wheat. J. Exp. Bot. 2009, 60, 2565–2573. [Google Scholar] [CrossRef]
- Hargreaves, C.E.; Gregory, P.J.; Bengough, A.G. Measuring root traits in barley (Hordeum vulgare ssp. vulgare and ssp. spontaneum) seedlings using gel chambers, soil sacs and X-ray microtomography. Plant Soil 2008, 316, 285–297. [Google Scholar] [CrossRef]
- Fitz Gerald, J.N.; Lehti-Shiu, M.D.; Ingram, P.A.; Deak, K.I.; Biesiada, T.; Malamy, J.E. Identification of quantitative trait loci that regulate Arabidopsis root system size and plasticity. Genetics 2006, 172, 485–498. [Google Scholar] [CrossRef]
- Xiong, L.; Wang, R.G.; Mao, G.; Koczan, J.M. Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic Acid. Plant Physiol. 2006, 142, 1065–1074. [Google Scholar] [CrossRef]
- Bernier, J.; Serraj, R.; Kumar, A.; Venuprasad, R.; Impa, S.; Veeresh Gowda, R.P.; Oane, R.; Spaner, D.; Atlin, G. The large-effect drought-resistance QTL qtl12.1 increases water uptake in upland rice. Field Crops Res. 2009, 110, 139–146. [Google Scholar] [CrossRef]
- Steele, K.; Price, A.H.; Shashidhar, H.; Witcombe, J. Marker-assisted selection to introgress rice QTLs controlling root traits into an Indian upland rice variety. Theor. Appl. Genet. 2006, 112, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Tracy, S.R.; Roberts, J.A.; Black, C.R.; McNeill, A.; Davidson, R.; Mooney, S.J. The X-factor: Visualizing undisturbed root architecture in soils using X-ray computed tomography. J. Exp. Bot. 2010, 61, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Courtois, B.; Audebert, A.; Dardou, A.; Roques, S.; Ghneim-Herrera, T.; Droc, G.; Frouin, J.; Rouan, L.; Goze, E.; Kilian, A.; et al. Genome-wide association mapping of root traits in a japonica rice panel. PLoS ONE 2013, 8, e78037. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.J.; Droc, G.; Guiderdoni, E.; Hsing, Y.I. International Consortium of Rice Mutagenesis: Resources and beyond. Rice (N Y) 2013, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Sezen, U.; Paterson, A.H. Domestication and plant genomes. Curr. Opin. Plant Biol. 2010, 13, 160–166. [Google Scholar] [CrossRef]
- Kantar, M.; Lucas, S.J.; Budak, H. Drought Stress. In Plant Responses to Drought and Salinity Stress—Developments in a Post-Genomic Era; Academic Press: Cambridge, MA, USA, 2011; pp. 445–493. [Google Scholar] [CrossRef]
- Rahman, H.; Ramanathan, V.; Nallathambi, J.; Duraialagaraja, S.; Muthurajan, R. Over-expression of a NAC 67 transcription factor from finger millet (Eleusine coracana L.) confers tolerance against salinity and drought stress in rice. BMC Biotechnol. 2016, 16 (Suppl. 1), 35. [Google Scholar] [CrossRef]
- Ning, J.; Li, X.; Hicks, L.M.; Xiong, L. Raf-like MAPKKK gene DSM1 mediates drought resistance through reactive oxygen species scavenging in rice. Plant Physiol. 2010, 152, 876–890. [Google Scholar] [CrossRef]
- Ramanathan, V.; Rahman, H.; Subramanian, S.; Nallathambi, J.; Kaliyaperumal, A.; Manickam, S.; Ranganathan, C.; Muthurajan, R. OsARD4 encoding an acireductone dioxygenase improves root architecture in rice by promoting development of secondary roots. Sci. Rep. 2018, 8, 15713. [Google Scholar] [CrossRef]
- Jones, L.; McQueen-Mason, S. A role for expansins in dehydration and rehydration of the resurrection plantCraterostigma plantagineum. FEBS Lett. 2004, 559, 61–65. [Google Scholar] [CrossRef]
- Wu, Y.; Thorne, E.T.; Sharp, R.E.; Cosgrove, D.J. Modification of expansin transcript levels in the maize primary root at low water potentials. Plant Physiol. 2001, 126, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Todaka, D.; Mizoi, J.; Yoshida, T.; Kidokoro, S.; Matsukura, S.; Takasaki, H.; Sakurai, T.; Yamamoto, Y.Y.; Yoshiwara, K.; et al. Identification of cis-acting promoter elements in cold- and dehydration-induced transcriptional pathways in Arabidopsis, rice, and soybean. DNA Res. 2012, 19, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, K.; Hwang, H.; Bhatnagar, N.; Kim, D.-Y.; Yoon, I.S.; Byun, M.-O.; Kim, S.T.; Jung, K.-H.; Kim, B.-G. Overexpression of PYL5 in rice enhances drought tolerance, inhibits growth, and modulates gene expression. J. Exp. Bot. 2014, 65, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Matsukura, S.; Mizoi, J.; Yoshida, T.; Todaka, D.; Ito, Y.; Maruyama, K.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Comprehensive analysis of rice DREB2-type genes that encode transcription factors involved in the expression of abiotic stress-responsive genes. Mol. Genet. Genom. 2010, 283, 185–196. [Google Scholar] [CrossRef]
- Maruyama, K.; Urano, K.; Yoshiwara, K.; Morishita, Y.; Sakurai, N.; Suzuki, H.; Kojima, M.; Sakakibara, H.; Shibata, D.; Saito, K.; et al. Integrated analysis of the effects of cold and dehydration on rice metabolites, phytohormones, and gene transcripts. Plant Physiol. 2014, 164, 1759–1771. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, Y.S.; Redillas, M.C.; Jang, G.; Jung, H.; Bang, S.W.; Choi, Y.D.; Ha, S.H.; Reuzeau, C.; Kim, J.K. OsNAC5 overexpression enlarges root diameter in rice plants leading to enhanced drought tolerance and increased grain yield in the field. Plant Biotechnol. J. 2013, 11, 101–114. [Google Scholar] [CrossRef]
- Degenkolbe, T.; Do, P.T.; Kopka, J.; Zuther, E.; Hincha, D.K.; Kohl, K.I. Identification of drought tolerance markers in a diverse population of rice cultivars by expression and metabolite profiling. PLoS ONE 2013, 8, e63637. [Google Scholar] [CrossRef]
- Wang, D.; Pan, Y.; Zhao, X.; Zhu, L.; Fu, B.; Li, Z. Genome-wide temporal-spatial gene expression profiling of drought responsiveness in rice. BMC Genom. 2011, 12, 149. [Google Scholar] [CrossRef]
- Lenka, S.K.; Katiyar, A.; Chinnusamy, V.; Bansal, K.C. Comparative analysis of drought-responsive transcriptome in Indica rice genotypes with contrasting drought tolerance. Plant Biotechnol. J. 2011, 9, 315–327. [Google Scholar] [CrossRef]
- Zong, W.; Zhong, X.; You, J.; Xiong, L. Genome-wide profiling of histone H3K4-tri-methylation and gene expression in rice under drought stress. Plant Mol. Biol. 2013, 81, 175–188. [Google Scholar] [CrossRef]
- MacMillan, K.; Emrich, K.; Piepho, H.P.; Mullins, C.E.; Price, A.H. Assessing the importance of genotype x environment interaction for root traits in rice using a mapping population II: Conventional QTL analysis. Theor. Appl. Genet. 2006, 113, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; De Smet, I. Root system architecture: Insights from Arabidopsis and cereal crops. Philos. Trans. R. Soc. B 2012, 367, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Xiong, L. Genetic mechanisms conferring adaptation to submergence and drought in rice: Simple or complex? Curr. Opin. Plant Biol. 2013, 16, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Virmani, S.S.; Ilyas-Ahmed, M. Rice breeding for sustainable production. In Breeding Major Food Staples; Blackwell Pub.: Hoboken, NJ, USA, 2007; pp. 141–191. [Google Scholar]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, P.U.; Bennett, A.E.; Tack, A.J.M.; Singh, B. The impact of elevated temperature and drought on the ecology and evolution of plant–soil microbe interactions. J. Ecol. 2019. [Google Scholar] [CrossRef]
- Valyi, K.; Mardhiah, U.; Rillig, M.C.; Hempel, S. Community assembly and coexistence in communities of arbuscular mycorrhizal fungi. ISME J. 2016, 10, 2341–2351. [Google Scholar] [CrossRef]
- Brady, K.U.; Kruckeberg, A.R.; Bradshaw, H.D., Jr. Evolutionary Ecology of Plant Adaptation to Serpentine Soils. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 243–266. [Google Scholar] [CrossRef]
- Johnson, N.C.; Wilson, G.W.; Bowker, M.A.; Wilson, J.A.; Miller, R.M. Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proc. Natl. Acad. Sci. USA 2010, 107, 2093–2098. [Google Scholar] [CrossRef]
- van der Putten, W.H.; Bardgett, R.D.; Bever, J.D.; Bezemer, T.M.; Casper, B.B.; Fukami, T.; Kardol, P.; Klironomos, J.N.; Kulmatiski, A.; Schweitzer, J.A.; et al. Plant-soil feedbacks: The past, the present and future challenges. J. Ecol. 2013, 101, 265–276. [Google Scholar] [CrossRef]
- Kulmatiski, A.; Beard, K.H.; Stevens, J.R.; Cobbold, S.M. Plant-soil feedbacks: A meta-analytical review. Ecol. Lett. 2008, 11, 980–992. [Google Scholar] [CrossRef]
- Berg, M.P.; Kiers, E.T.; Driessen, G.; van der Heijden, M.; Kooi, B.W.; Kuenen, F.; Liefting, M.; Verhoef, H.A.; Ellers, J. Adapt or disperse: Understanding species persistence in a changing world. Glob. Chang. Biol. 2010, 16, 587–598. [Google Scholar] [CrossRef]
- Deveautour, C.; Donn, S.; Power, S.A.; Bennett, A.E.; Powell, J.R. Experimentally altered rainfall regimes and host root traits affect grassland arbuscular mycorrhizal fungal communities. Mol. Ecol. 2018, 27, 2152–2163. [Google Scholar] [CrossRef] [PubMed]
- Jayne, B.; Quigley, M. Influence of arbuscular mycorrhiza on growth and reproductive response of plants under water deficit: A meta-analysis. Mycorrhiza 2014, 24, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Mohan, J.E.; Cowden, C.C.; Baas, P.; Dawadi, A.; Frankson, P.T.; Helmick, K.; Hughes, E.; Khan, S.; Lang, A.; Machmuller, M.; et al. Mycorrhizal fungi mediation of terrestrial ecosystem responses to global change: Mini-review. Fungal Ecol. 2014, 10, 3–19. [Google Scholar] [CrossRef]
- Hawkes, C.V.; Kivlin, S.N.; Rocca, J.D.; Huguet, V.; Thomsen, M.A.; Suttle, K.B. Fungal community responses to precipitation. Glob. Chang. Biol. 2011, 17, 1637–1645. [Google Scholar] [CrossRef]
- Barnes, C.J.; van der Gast, C.J.; McNamara, N.P.; Rowe, R.; Bending, G.D. Extreme rainfall affects assembly of the root-associated fungal community. New Phytol. 2018, 220, 1172–1184. [Google Scholar] [CrossRef]
- Griffiths, R.I.; Whiteley, A.S.; O’Donnell, A.G.; Bailey, M.J. Physiological and community responses of established grassland bacterial populations to water stress. Appl. Environ. Microbiol. 2003, 69, 6961–6968. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Antibus, R.; Linkins, A.; McClaugherty, C.; Rayburn, L.; Repert, D.; Weiland, T. Wood decomposition: Nitrogen and phosphorus dynamics in relation to extracellular enzyme activity. Ecology 1993, 74, 1586–1593. [Google Scholar] [CrossRef]
- Hueso, S.; García, C.; Hernández, T. Severe drought conditions modify the microbial community structure, size and activity in amended and unamended soils. Soil Biol. Biochem. 2012, 50, 167–173. [Google Scholar] [CrossRef]
- Birch, H.F. The effect of soil drying on humus decomposition and nitrogen availability. Plant Soil 1958, 10, 9–31. [Google Scholar] [CrossRef]
- Yuste, J.C.; Janssens, I.A.; Ceulemans, R. Calibration and validation of an empirical approach to model soil CO2 efflux in a deciduous forest. Biogeochemistry 2005, 73, 209–230. [Google Scholar] [CrossRef]
- Meisner, A.; Rousk, J.; Bååth, E. Prolonged drought changes the bacterial growth response to rewetting. Soil Biol. Biochem. 2015, 88, 314–322. [Google Scholar] [CrossRef]
- Moyano, F.E.; Manzoni, S.; Chenu, C. Responses of soil heterotrophic respiration to moisture availability: An exploration of processes and models. Soil Biol. Biochem. 2013, 59, 72–85. [Google Scholar] [CrossRef]
- Davidson, E.A.; Samanta, S.; Caramori, S.S.; Savage, K. The D ual A rrhenius and M ichaelis–M enten kinetics model for decomposition of soil organic matter at hourly to seasonal time scales. Glob. Chang. Biol. 2012, 18, 371–384. [Google Scholar] [CrossRef]
- Li, X.; Sarah, P. Arylsulfatase activity of soil microbial biomass along a Mediterranean-arid transect. Soil Biol. Biochem. 2003, 35, 925–934. [Google Scholar] [CrossRef]
- Fageria, N.K.; Moreira, A. The Role of Mineral Nutrition on Root Growth of Crop Plants. Adv. Agron. 2011, 251–331. [Google Scholar] [CrossRef]
- Lafitte, H.R.; Yongsheng, G.; Yan, S.; Li, Z.K. Whole plant responses, key processes, and adaptation to drought stress: The case of rice. J. Exp. Bot. 2007, 58, 169–175. [Google Scholar] [CrossRef]
- Abd Allah, A.; Badawy, S.A.; Zayed, B.; El-Gohary, A. The role of root system traits in the drought tolerance of rice (Oryza sativa L.). World Acad. Sci. Eng. Technol. 2010, 68, 1378–1382. [Google Scholar]
- Bashar, M. Xylem vessel variability at three positions of rice root (Oryza sativa L.). Bangl, J. Bot. 1990. Available online: http://agris.fao.org/agris-search/search.do?recordID=BD9225086 (accessed on 17 September 2019).
- Ristova, D.; Barbez, E. Root Development: Methods and Protocols; Springer: Dordrecht, The Netherlands, 2018. [Google Scholar]
- Morita, S.; Nemoto, K. Morphology and anatomy of rice roots with special reference to coordination in organo-and histogenesis. In Structure and Function of Roots; Springer: Dordrecht, The Netherlands, 1995; pp. 75–86. [Google Scholar]
- Venuprasad, R.; Impa, S.; Gowda, R.V.; Atlin, G.; Serraj, R. Rice near-isogenic-lines (NILs) contrasting for grain yield under lowland drought stress. Field Crops Res. 2011, 123, 38–46. [Google Scholar] [CrossRef]
- Joshi, D.C.; Singh, V.; Hunt, C.; Mace, E.; van Oosterom, E.; Sulman, R.; Jordan, D.; Hammer, G. Development of a phenotyping platform for high throughput screening of nodal root angle in sorghum. Plant Methods 2017, 13, 56. [Google Scholar] [CrossRef]
- Uga, Y.; Okuno, K.; Yano, M. Dro1, a major QTL involved in deep rooting of rice under upland field conditions. J. Exp. Bot. 2011, 62, 2485–2494. [Google Scholar] [CrossRef] [PubMed]
- Metzner, R.; Eggert, A.; van Dusschoten, D.; Pflugfelder, D.; Gerth, S.; Schurr, U.; Uhlmann, N.; Jahnke, S. Direct comparison of MRI and X-ray CT technologies for 3D imaging of root systems in soil: Potential and challenges for root trait quantification. Plant Methods 2015, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zeng, Z.; Connor, J.N.; Huang, C.Y.; Melino, V.; Kumar, P.; Miklavcic, S.J. RootGraph: A graphic optimization tool for automated image analysis of plant roots. J. Exp. Bot. 2015, 66, 6551–6562. [Google Scholar] [CrossRef] [PubMed]
- Trachsel, S.; Kaeppler, S.M.; Brown, K.M.; Lynch, J.P. Shovelomics: High throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant Soil 2011, 341, 75–87. [Google Scholar] [CrossRef]
- French, A.; Ubeda-Tomás, S.; Holman, T.J.; Bennett, M.J.; Pridmore, T. High-throughput quantification of root growth using a novel image-analysis tool. Plant Physiol. 2009, 150, 1784–1795. [Google Scholar] [CrossRef]
- Cai, G.; Vanderborght, J.; Klotzsche, A.; van der Kruk, J.; Neumann, J.; Hermes, N.; Vereecken, H. Construction of minirhizotron facilities for investigating root zone processes. Vadose Zone J. 2016, 15. [Google Scholar] [CrossRef]
- Hund, A.; Trachsel, S.; Stamp, P. Growth of axile and lateral roots of maize: I development of a phenotying platform. Plant Soil 2009, 325, 335–349. [Google Scholar] [CrossRef]
- Clark, R.T.; MacCurdy, R.B.; Jung, J.K.; Shaff, J.E.; McCouch, S.R.; Aneshansley, D.J.; Kochian, L.V. Three-dimensional root phenotyping with a novel imaging and software platform. Plant Physiol. 2011, 156, 455–465. [Google Scholar] [CrossRef]
- Aravena, J.E.; Berli, M.; Ghezzehei, T.A.; Tyler, S.W. Effects of root-induced compaction on rhizosphere hydraulic properties-X-ray microtomography imaging and numerical simulations. Environ. Sci. Technol. 2011, 45, 425–431. [Google Scholar] [CrossRef]
- Mattupalli, C.; Seethepalli, A.; York, L.M.; Young, C.A. Digital imaging to evaluate root system architectural changes associated with soil biotic factors. Phytobiomes J. 2019, 3, 102–111. [Google Scholar] [CrossRef]
- Martins, S.M.; de Brito, G.G.; da Conceição Gonçalves, W.; Tripode, B.M.D.; Lartaud, M.; Duarte, J.B.; de Lelis Morello, C.; Giband, M. PhenoRoots: An inexpensive non-invasive phenotyping system to assess the variability of the root system architecture. Sci. Agric. 2020, 77, e20180420. [Google Scholar] [CrossRef]
- Ma, L.; Shi, Y.; Siemianowski, O.; Yuan, B.; Egner, T.K.; Mirnezami, S.V.; Lind, K.R.; Ganapathysubramanian, B.; Venditti, V.; Cademartiri, L. Hydrogel-based transparent soils for root phenotyping in vivo. Proc. Natl. Acad. Sci. USA 2019, 116, 11063–11068. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Bodner, G.; Rewald, B.; Leitner, D.; Nagel, K.A.; Nakhforoosh, A. Root architecture simulation improves the inference from seedling root phenotyping towards mature root systems. J. Exp. Bot. 2017, 68, 965–982. [Google Scholar] [CrossRef] [PubMed]
- Slota, M.; Maluszynski, M.; Szarejko, I. An automated, cost-effective and scalable, flood-and-drain based root phenotyping system for cereals. Plant Methods 2016, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.J.; Meng, P.; Zhang, J.S.; Wan, X. Variation in soil water uptake and its effect on plant water status in Juglans regia L. during dry and wet seasons. Tree Physiol. 2011, 31, 1378–1389. [Google Scholar] [CrossRef]
- Davies, W.J.; Wilkinson, S.; Loveys, B. Stomatal control by chemical signalling and the exploitation of this mechanism to increase water use efficiency in agriculture. New Phytol. 2002, 153, 449–460. [Google Scholar] [CrossRef]
- Fischer, D.; Glaser, B. Synergisms between compost and biochar for sustainable soil amelioration. In Management of Organic Waste; InTech: Rijeka, Croatia, 2012; Volume 1. [Google Scholar]
- Pennisi, E. Sowing the seeds for the ideal crop. Science 2010, 327, 802–803. [Google Scholar] [CrossRef]
| Gene | Expression Analysis | Location of Expression | Function in Drought Tolerance | Reference |
|---|---|---|---|---|
| DRO1 | Upregulated | Root apical meristem in the root tip and crown root primordia | Influences root growth angle, induces root elongation and deeper rooting | [75] |
| EcNAC67 | Upregulated | Leaves and roots | Increases relative water content in leaves, delays leaf rolling symptoms, ensures better stomatal regulation during dehydration, and maintains higher root and shoot biomass | [119] |
| DsM1 | Downregulated | Stamen, pistil, mature leaves and roots | Increases dehydration tolerance in the seedling stage, regulates scavenging of reactive oxygen species | [120] |
| OsPYL/RCAR5 | Upregulated | Leaf blade | Stomatal closure, maintains the fresh weight of leaves | [125] |
| OsDREB1F | Upregulated | Almost all tissues, but higher in callus and panicle | Regulates the ABA-dependent signaling pathway and provides osmotic-stress tolerance | [126] |
| OsDREB2B | Upregulated | Leaf sheath, root tissues | Increases root number and length | [126] |
| CYP735A | Downregulated | Shoot | Regulates cytokinin levels | [127] |
| OsNAC5 | Upregulated | Roots | Increases root diameter | [128] |
| Crop | Trait | Method | Reference |
|---|---|---|---|
| Maize (Zea mays) | Root architectural traits of root crown - brace roots, number of brace roots, branching density of brace roots - number, angles, and branching density of crown root | At harvest, roots were excavated by removing a soil cylinder of 40 cm in diameter and 25-cm depth, with the plant base as the horizontal center of the soil cylinder. After root washing, clean roots were visually scored. | Trachsel et al. [170] |
| Arabidopsis (Arabidopsis thaliana) | Root system architecture - length, curvature, and stimulus-response parameters | Images were captured of Arabidopsis grown in the agarose gel condition contained in vertically-arranged plates to permit roots to grow on the surface of the medium. | French et al. [171] |
| Winter wheat (Triticum aestivum) | Root development and distribution - Number of total roots at different soil levels - Number of roots per observation depths | Field mini-rhizotrons were set up. Detailed images are available in the attached reference. Transparent rhizotubes were inserted into soil. Then, images were captured by the camera, which was located on both sides of the rhizotubes. The camera was positioned using an indexing handle at 20 observation locations in the tubes. | Cai et al. [172] |
| Maize (Zea mays) | Root morphology - axile - lateral root | Germinated seeds were transferred to moistened blotting paper in pouches. Root images were acquired by the scanner and then analyzed by WinRHIZO software. | Hund et al. [173] |
| Rice (Oryza sativa) | Root morphology - length - width - initiation angle - root tip | Rice seeds germinated in Petri plates were transplanted into glass growth cylinders containing 1.3 L of growth medium. The camera was placed in front of the growth cylinder. Image sequences were captured daily for each plant root system grown in the growth medium, consisting of 40 silhouette images taken every 9° for the entire 360° of rotation. RootReader3D software was used for the analysis of the 3D root images. | Clark et al. [174] |
| Sweet pea (Lathyrus odoratus), Sunflower (Helianthus annuus) | Analysis of soil aggregates to anticipate water flow toward the root | Sweet pea and sunflower seeds were planted on the surface and were grown for 30 days. An X-ray microtomography image was measured by high-resolution XMT beamline 8.3.2 at the Advanced Light Source (Lawrence Berkeley National Laboratory, USA). Transmitted X-ray light is converted to visible light using a CdWO4 single crystal scintillator, magnified by a Canon 2X lens, and imaged on a Cooke PCO 4000 CCD camera. | Aravena et al. [175] |
| Alfalfa (Medicago sativa) | Root system architecture - number of root tips - total root length - diameter - root angle orientation frequency | Alfalfa root crowns were separated from the aboveground foliage. Soil was brushed off the roots, which were then imaged in the laboratory using the RhizoVision Crown platform. | Mattupalli et al. [176] |
| Upland cotton (Gossypium hirsutum L.) | Root system architecture - total root length - average diameter of roots - number of root tips - maximum root depth - total explored area - maximum root width | Development of a root phenotyping platform, PhenoRoots, which allows for the non-invasive study of plant root system architecture. Substrate or soil-filled rhizotrons are used to grow plantlets, whose roots are directly visible through a glass plate. Pictures were taken using a digital camera and then analyzed by WinRHIZO and ImageJ software. | Martins et al. [177] |
| Soybean (Glycine max L. Merr.) | Root biomass and morphology - length - area | This study used “transparent soil” formed by the spherification of hydrogels of biopolymers. It is specifically designed to support root growth in the presence of air, water, and nutrients, and allows the time-resolved phenotyping of roots in vivo by both photography and microscopy. The roots developed by soybean plants in this medium were markedly more similar to those developed in real soil than those developed in hydroponic conditions and did not show signs of hypoxia. | Ma et al. [178] |
| Pea (Pisum sativum L.) | Root morphology - length of the tap root and lateral roots Root system architecture - number of lateral branches, branching angle representing the angle between the tap root and branched lateral roots | Measurements of root traits were performed on two phenotyping platforms. One system represented a typical high-throughput phenotyping platform for seedling root screening using agar-filled plates. The other system focused on mature root systems grown under more natural conditions (sand-filled columns) with less potential throughput. Images were analyzed using the software GrowScreen-Root | Zhao et al. [179] |
| Sorghum (Sorghum bicolor L. Moench) | Root system architecture - nodal root angle | The phenotyping platform consisted of 500 soil-filled root chambers (50 × 45 × 0.3 cm in size), made of transparent Perspex sheets that were placed in metal tubs and covered with polycarbonate sheets. Around 3 weeks after sowing, once the first flush of nodal roots was visible, roots were imaged in situ using an imaging box that included two digital cameras that were remotely controlled by two android tablets. Free software (openGelPhoto.tcl) allowed precise measurement of the nodal root angle from the digital images. | Joshi et al. [166] |
| Spring barley (Hordeum vulgare L.) | Destructive methods - total root length - root system surface - root volume - root diameter - number of tips Non‑destructive methods - root system depth - projected root surface - sum of the root lengths | The correspondence between a destructive (WinRHIZO scans) and non-destructive (RGB root imaging) method for root phenotyping using a described system was tested. The root images were analyzed after the staining of roots with powdered active charcoal. Root images were taken in the photographic room using an RGB camera. The images (JPG or TIFF files) of the plants taken in the photographic chamber were analyzed using ImageJ software. Root system scanning was performed using a specialized root scanner (STD4800 scanner) coupled with WinRHIZO Pro software (Regent Instruments, Quebec, Canada). | Slota et al. [180] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Chung, Y.S.; Lee, E.; Tripathi, P.; Heo, S.; Kim, K.-H. Root Response to Drought Stress in Rice (Oryza sativa L.). Int. J. Mol. Sci. 2020, 21, 1513. https://doi.org/10.3390/ijms21041513
Kim Y, Chung YS, Lee E, Tripathi P, Heo S, Kim K-H. Root Response to Drought Stress in Rice (Oryza sativa L.). International Journal of Molecular Sciences. 2020; 21(4):1513. https://doi.org/10.3390/ijms21041513
Chicago/Turabian StyleKim, Yoonha, Yong Suk Chung, Eungyeong Lee, Pooja Tripathi, Seong Heo, and Kyung-Hwan Kim. 2020. "Root Response to Drought Stress in Rice (Oryza sativa L.)" International Journal of Molecular Sciences 21, no. 4: 1513. https://doi.org/10.3390/ijms21041513
APA StyleKim, Y., Chung, Y. S., Lee, E., Tripathi, P., Heo, S., & Kim, K.-H. (2020). Root Response to Drought Stress in Rice (Oryza sativa L.). International Journal of Molecular Sciences, 21(4), 1513. https://doi.org/10.3390/ijms21041513





