Low Doses of Methylmercury Induce the Proliferation of Thyroid Cells In Vitro Through Modulation of ERK Pathway
Abstract
1. Introduction
2. Results
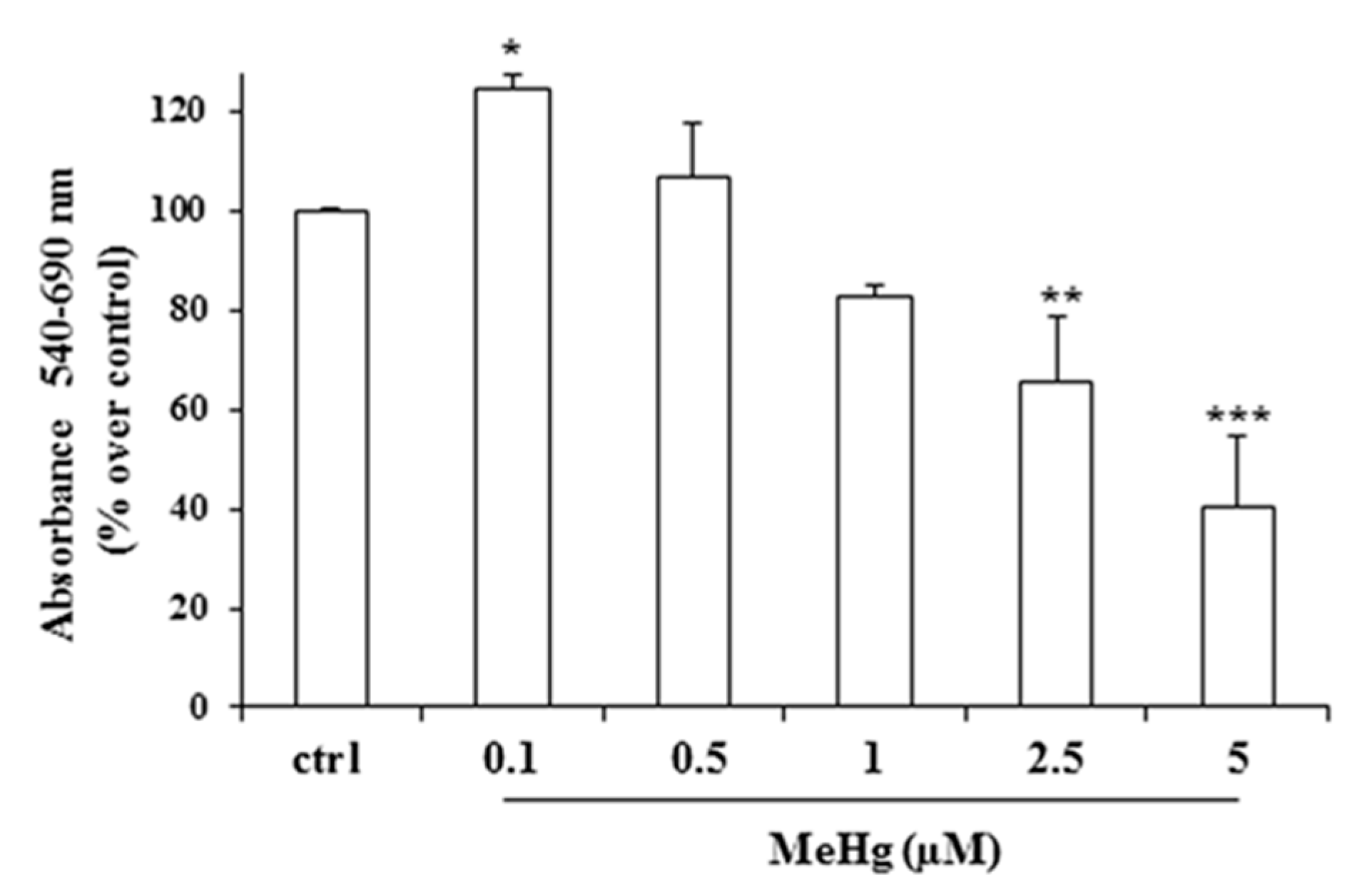
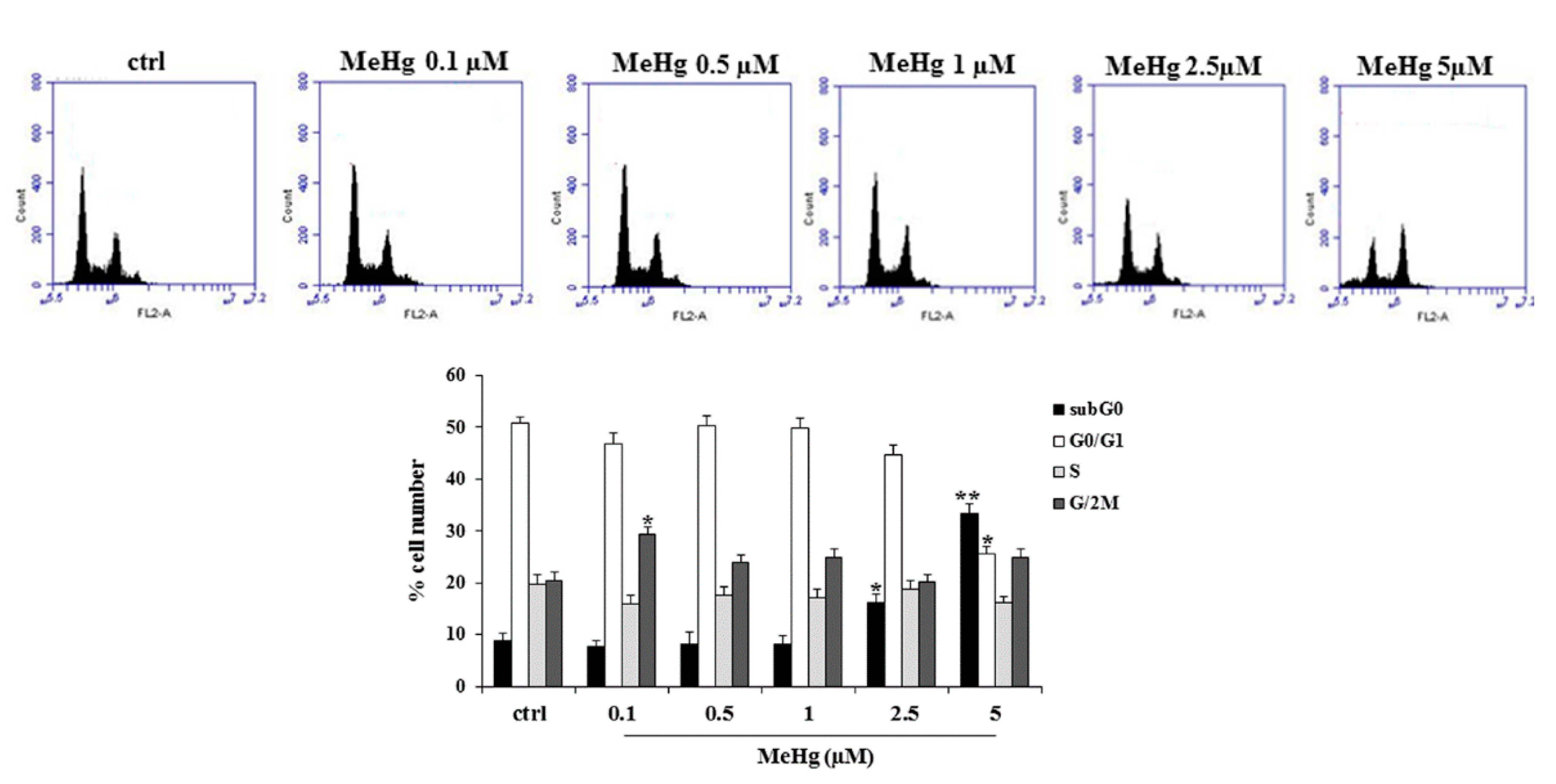
2.1. Effects of Short Treatment of Nthy-ori-3-1 Cells with MeHg on the Growth and Cell Cycle
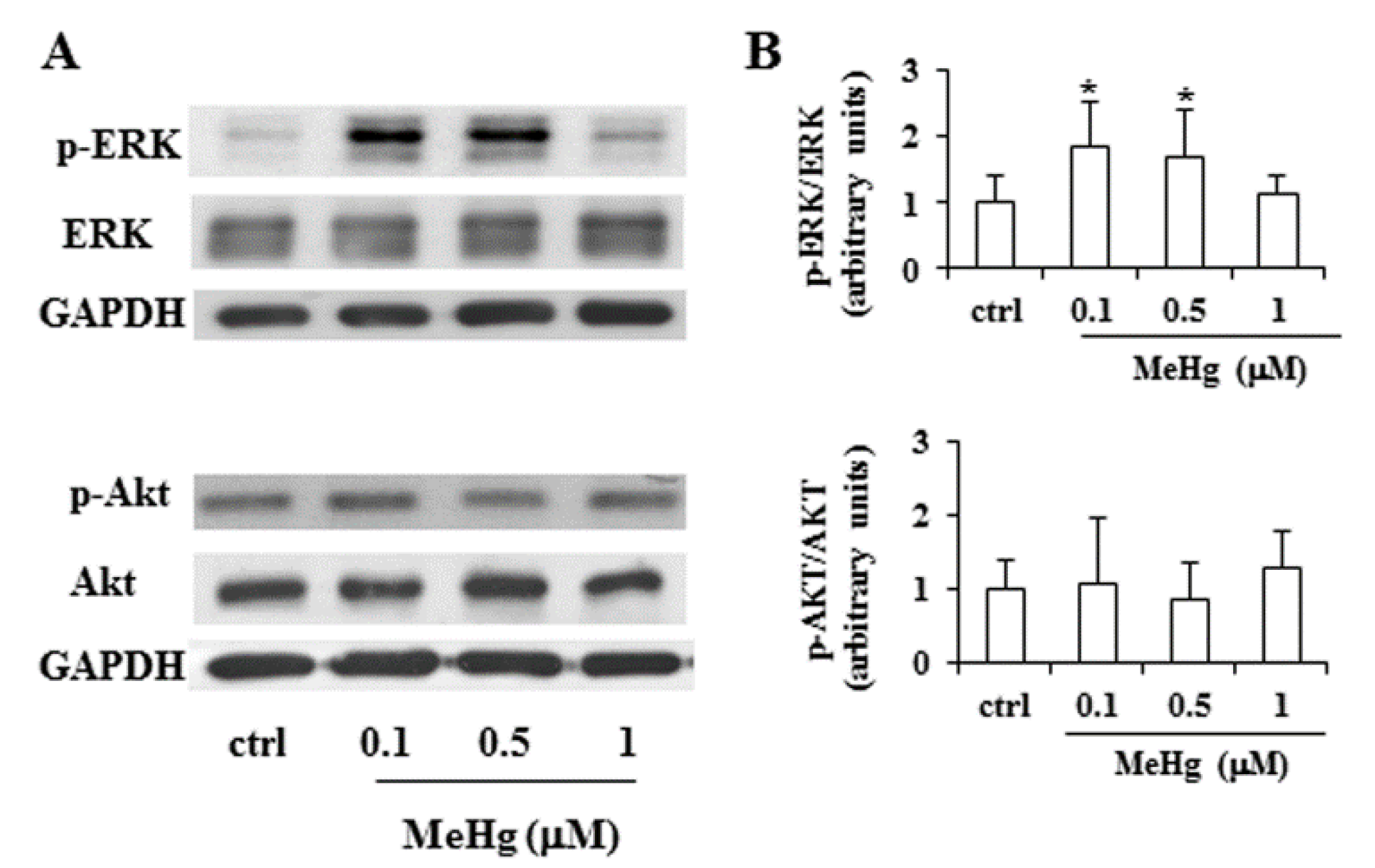
2.2. Effects of Short Treatment with MeHg on ERK and Akt Pathways and Expression of Thyrocyte Differentiation Markers
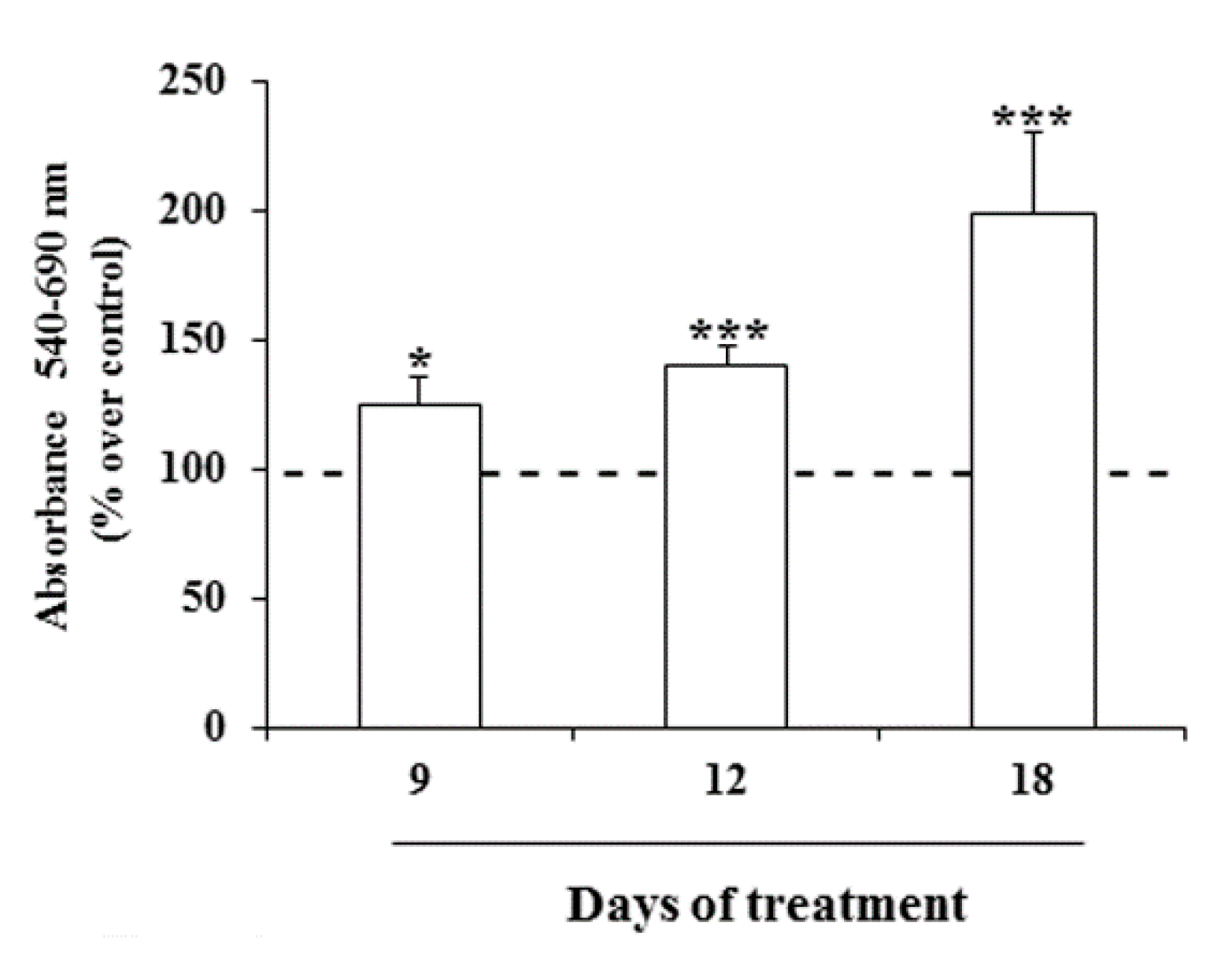
2.3. Effects of Prolonged Treatment with MeHg on the Growth of Nthy-ori-3-1 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Cell Proliferation Assay
4.2. Cell Cycle Assay
4.3. ROS Assay
4.4. Western Blot Analysis
4.5. RNA Extraction and Real-Time PCR
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ito, Y.; Nikiforov, Y.E.; Schlumberger, M.; Vigneri, R. Increasing incidence of thyroid cancer: Controversies explored. Nat. Rev. Endocrinol. 2013, 9, 178–184. [Google Scholar] [CrossRef]
- Nettore, I.C.; Colao, A.; Macchia, P.E. Nutritional and Environmental Factors in Thyroid Carcinogenesis. Int. J. Environ. Res. Public Health 2018, 15, E1735. [Google Scholar] [CrossRef]
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a global pollutant: Sources, pathways, and effects. Environ. Sci. Technol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef]
- Pirrone, N.; Cinnirella, S.; Feng, X.; Finkelman, R.B.; Friedli, H.R.; Leaner, J.; Mason, R.; Mukherjee, A.B.; Stracher, G.B.; Streets, D.G.; et al. Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmos. Chem. Phys. 2010, 10, 5951–5964. [Google Scholar] [CrossRef]
- Ursinyova, M.; Uhnakova, I.; Serbin, R.; Masanova, V.; Husekova, Z.; Wsolova, L. The relation between human exposure to mercury and thyroid hormone status. Biol. Trace Elem. Res. 2012, 148, 281–291. [Google Scholar] [CrossRef]
- Chen, A.; Kim, S.S.; Chung, E.; Dietrich, K.N. Thyroid hormones in relation to lead, mercury, and cadmium exposure in the National Health and Nutrition Examination Survey, 2007–2008. Environ. Health Perspect. 2013, 121, 181–186. [Google Scholar] [CrossRef]
- Malandrino, P.; Russo, M.; Ronchi, A.; Minoia, C.; Cataldo, D.; Regalbuto, C.; Giordano, C.; Attard, M.; Squatrito, S.; Trimarchi, F.; et al. Increased thyroid cancer incidence in a basaltic volcanic area is associated with non-anthropogenic pollution and biocontamination. Endocrine 2016, 53, 471–479. [Google Scholar] [CrossRef]
- Zidane, M.; Ren, Y.; Xhaard, C.; Leufroy, A.; Côte, S.; Dewailly, E.; Noël, L.; Guérin, T.; Bouisset, P.; Bernagout, S.; et al. Non-Essential Trace Elements Dietary Exposure in French Polynesia: Intake Assessment, Nail Bio Monitoring and Thyroid Cancer Risk. Asian Pac. J. Cancer Prev. 2019, 20, 355–367. [Google Scholar] [CrossRef]
- Zaichick, V.Y.; Tsyb, A.F.; Vtyurin, B.M. Trace elements and thyroid cancer. Analyst 1995, 120, 817–821. [Google Scholar] [CrossRef]
- Vigneri, R.; Malandrino, P.; Gianì, F.; Russo, M.; Vigneri, P. Heavy metals in the volcanic environment and thyroid cancer. Mol. Cell. Endocrinol. 2017, 457, 73–80. [Google Scholar] [CrossRef]
- Duntas, L.H. Chemical contamination and the thyroid. Endocrine 2015, 48, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Patrick, L. Thyroid disruption: Mechanism and clinical implications in human health. Altern. Med. Rev. 2009, 14, 326–346. [Google Scholar] [PubMed]
- Pellegriti, G.; De Vathaire, F.; Scollo, C.; Attard, M.; Giordano, C.; Arena, S.; Dardanoni, G.; Frasca, F.; Malandrino, P.; Vermiglio, F.; et al. Papillary thyroid cancer incidence in the volcanic area of Sicily. J. Natl. Cancer Inst. 2009, 101, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Leux, C.; Truong, T.; Petit, C.; Baron-Dubourdieu, D.; Guénel, P. Family history of malignant and benign thyroid diseases and risk of thyroid cancer: A population-based case-control study in New Caledonia. Cancer Causes Control. 2012, 23, 745–755. [Google Scholar] [CrossRef]
- Jakimska, A.; Konieczka, P.; Skóra, K.; Namieśnik, J. Bioaccumulation of Metals in Tissues of Marine Animals, Part I: The Role and Impact of Heavy Metals on Organisms. Pol. J. Environ. Stud. 2011, 20, 1117–1125. [Google Scholar]
- Sukocheva, O.A.; Yang, Y.; Gierthy, J.F.; Seegal, R.F. Methyl Mercury Influences Growth-Related Signaling in MCF-7 Breast Cancer Cells. Environ. Toxicol. 2005, 20, 32–44. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef]
- Fagin, J.A.; Wells, S.A., Jr. Biologic and Clinical Perspectives on Thyroid Cancer. N. Engl. J. Med. 2016, 375, 1054–1067. [Google Scholar] [CrossRef]
- Zaballos, M.A.; Santisteban, P. Key signaling pathways in thyroid cancer. J. Endocrinol. 2017, 235, R43–R61. [Google Scholar] [CrossRef]
- Maggisano, V.; Celano, M.; Lombardo, G.E.; Lepore, S.M.; Sponziello, M.; Rosignolo, F.; Verrienti, A.; Baldan, F.; Puxeddu, E.; Durante, C.; et al. Silencing of hTERT blocks growth and migration of anaplastic thyroid cancer cells. Mol. Cell. Endocrinol. 2017, 448, 34–40. [Google Scholar] [CrossRef]
- Celano, M.; Maggisano, V.; De Rose, R.F.; Bulotta, S.; Maiuolo, J.; Navarra, M.; Russo, D. Flavonoid fraction of Citrus reticulata juice reduces proliferation and migration of anaplastic thyroid carcinoma cells. Nutr. Cancer 2015, 67, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Bulotta, S.; Corradino, R.; Celano, M.; Maiuolo, J.; D’Agostino, M.; Oliverio, M.; Procopio, A.; Filetti, S.; Russo, D. Antioxidant and antigrowth action of peracetylated oleuropein in thyroid cancer cells. J. Mol. Endocrinol. 2013, 51, 181–189. [Google Scholar] [CrossRef]
- Sponziello, M.; Verrienti, A.; Rosignolo, F.; De Rose, R.F.; Pecce, V.; Maggisano, V.; Durante, C.; Bulotta, S.; Damante, G.; Giacomelli, L.; et al. PDE5 expression in human thyroid tumors and effects of PDE5 inhibitors on growth and migration of cancer cells. Endocrine 2015, 50, 434–441. [Google Scholar] [CrossRef]
- D’Agostino, M.; Voce, P.; Celano, M.; Sponziello, M.; Moretti, S.; Maggisano, V.; Verrienti, A.; Durante, C.; Filetti, S.; Puxeddu, E.; et al. Sunitinib Exerts Only Limited Effects on the Proliferation and Differentiation of Anaplastic Thyroid Cancer Cells. Thyroid 2012, 22, 138–144. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maggisano, V.; Bulotta, S.; Celano, M.; Maiuolo, J.; Lepore, S.M.; Abballe, L.; Iannone, M.; Russo, D. Low Doses of Methylmercury Induce the Proliferation of Thyroid Cells In Vitro Through Modulation of ERK Pathway. Int. J. Mol. Sci. 2020, 21, 1556. https://doi.org/10.3390/ijms21051556
Maggisano V, Bulotta S, Celano M, Maiuolo J, Lepore SM, Abballe L, Iannone M, Russo D. Low Doses of Methylmercury Induce the Proliferation of Thyroid Cells In Vitro Through Modulation of ERK Pathway. International Journal of Molecular Sciences. 2020; 21(5):1556. https://doi.org/10.3390/ijms21051556
Chicago/Turabian StyleMaggisano, Valentina, Stefania Bulotta, Marilena Celano, Jessica Maiuolo, Saverio Massimo Lepore, Luana Abballe, Michelangelo Iannone, and Diego Russo. 2020. "Low Doses of Methylmercury Induce the Proliferation of Thyroid Cells In Vitro Through Modulation of ERK Pathway" International Journal of Molecular Sciences 21, no. 5: 1556. https://doi.org/10.3390/ijms21051556
APA StyleMaggisano, V., Bulotta, S., Celano, M., Maiuolo, J., Lepore, S. M., Abballe, L., Iannone, M., & Russo, D. (2020). Low Doses of Methylmercury Induce the Proliferation of Thyroid Cells In Vitro Through Modulation of ERK Pathway. International Journal of Molecular Sciences, 21(5), 1556. https://doi.org/10.3390/ijms21051556






