miR-9-5p Inhibits Skeletal Muscle Satellite Cell Proliferation and Differentiation by Targeting IGF2BP3 through the IGF2-PI3K/Akt Signaling Pathway
Abstract
:1. Introduction
2. Results
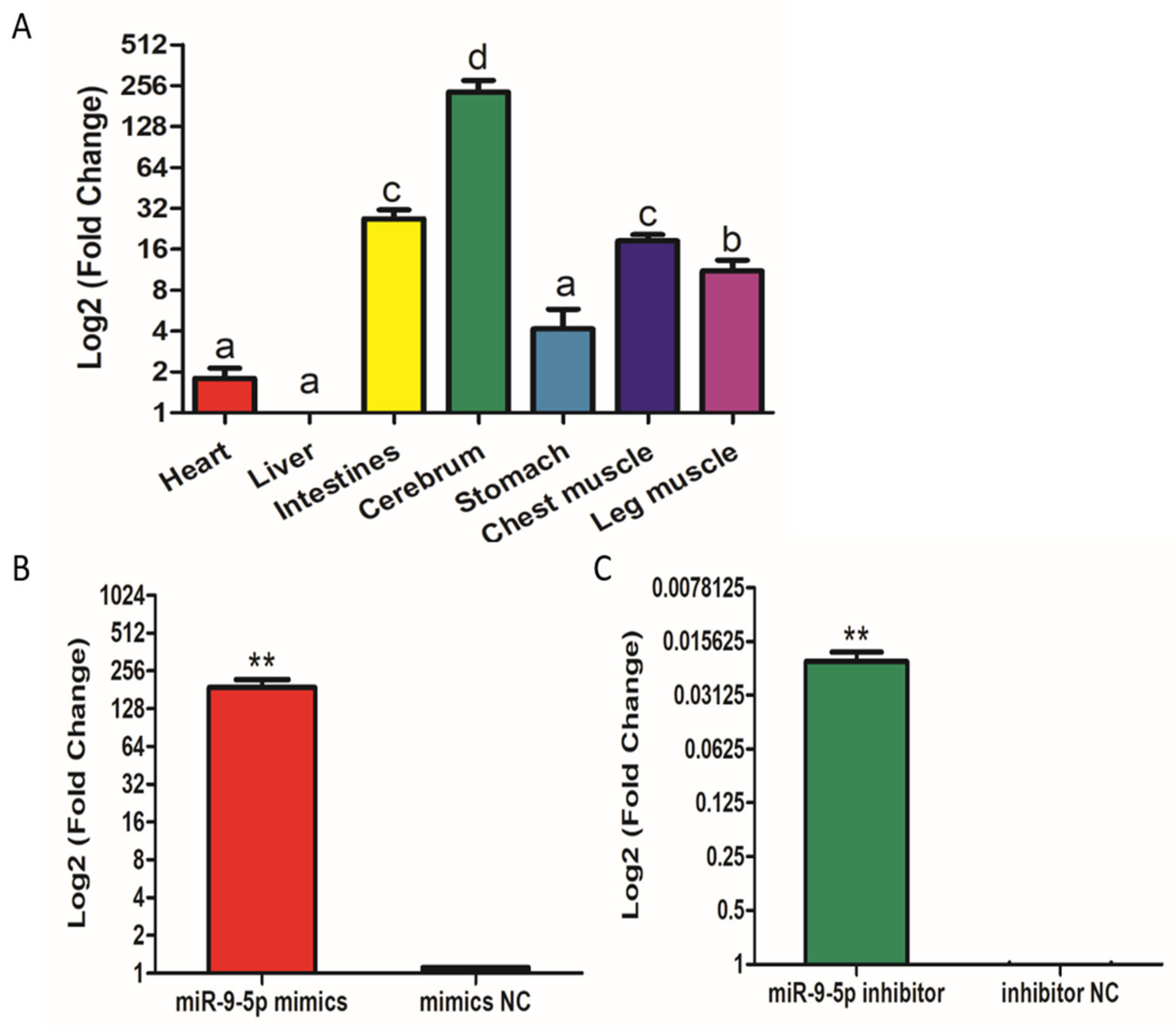
2.1. Expression of miR-9-5p in Skeletal Muscle Satellite Cells (SMSCs)
2.2. MiR-9-5p Inhibits the Proliferation of SMSCs
2.3. MiR-9-5p Inhibits the Differentiation of SMSCs
2.4. MiR-9-5p Targets the IGF2BP3 Gene Directly
2.5. IGF2BP3 Promotes the Proliferation of SMSCs
2.6. IGF2BP3 Promotes the Differentiation of SMSCs
2.7. IGF2BP3 is Involved in the IGF2-PI3k/AKt Pathway.
3. Discussion
4. Materials and Methods
4.1. Ethics Standards
4.2. Animals
4.3. Cells Cultures
4.4. RNA Oligonucleotides and Plasmids Construction
4.5. Cell Transfection
4.6. Extraction of RNA and Real-Time PCR (RT-PCR)
4.7. CCK-8 Assay
4.8. EdU Assay
4.9. Immunofluorescence
4.10. Western Blot
4.11. Luciferase Reporter Assay
4.12. Prediction of Target Genes
4.13. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Han, S.; Cui, C.; Wang, Y.; He, H.; Shen, X.; Chen, Y.; Liu, Z.; Zhu, Q.; Li, D.; Yin, H. FHL3 negatively regulates the differentiation of skeletal muscle satellite cells in chicken. 3 Biotech. 2019, 9, 206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-W.; Cai, H.-F.; Wei, X.-F.; Sun, J.-J.; Lan, X.-Y.; Lei, C.-Z.; Lin, F.-P.; Qi, X.-L.; Plath, M.; Chen, H. miR-30-5p regulates muscle differentiation and alternative splicing of muscle-related genes by targeting MBNL. Int. J. Mol. Sci. 2016, 17, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.D.; Kim, S.; Zhu, H.Y.; Jin, L.; Guo, Q.; Li, X.C.; Zhang, Y.C.; Xing, X.X.; Xuan, M.F.; Zhang, G.L. Generation of cloned adult muscular pigs with myostatin gene mutation by genetic engineering. RSC Adv. 2017, 7, 12541–12549. [Google Scholar] [CrossRef] [Green Version]
- Dumont, N.A.; Bentzinger, C.F.; Sincennes, M.C.; Rudnicki, M.A. Satellite cells and skeletal muscle regeneration. Compr. Physiol. 2011, 5, 1027–1059. [Google Scholar]
- Roberts, T.C.; Etxaniz, U.; Dall’Agnese, A.; Wu, S.-Y.; Chiang, C.-M.; Brennan, P.E.; Wood, M.J.; Puri, P.L. BRD3 and BRD4 BET bromodomain proteins differentially regulate skeletal myogenesis. Sci. Rep. 2017, 7, 6153. [Google Scholar] [CrossRef]
- Buckingham, M.; Rigby, P.W. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev. Cell 2014, 28, 225–238. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.; Ma, R.; Yang, L.; Hu, G.; Chen, X.; Duan, M.; Kook, Y.; Niu, F.; Liao, K.; Fu, M. MiR-9 promotes microglial activation by targeting MCPIP1. Nature Commun. 2014, 5, 4386. [Google Scholar] [CrossRef] [Green Version]
- Ropka-Molik, K.; Pawlina-Tyszko, K.K.Ż.; Piórkowska, K.G.Ż.; Gurgul, A.; Derebecka, N.; Wesoły, J. Examining the Genetic Background of Porcine Muscle Growth and Development Based on Transcriptome and miRNAome Data. Int. J. Mol. Sci. 2018, 19, 1208. [Google Scholar] [CrossRef] [Green Version]
- Ambros, V. microRNAs: Tiny regulators with great potential. Cell 2001, 107, 823–826. [Google Scholar] [CrossRef] [Green Version]
- Callis, T.E.; Chen, J.-F.; Wang, D.-Z. MicroRNAs in skeletal and cardiac muscle development. DNA Cell Biol. 2007, 26, 219–225. [Google Scholar] [CrossRef]
- Krichevsky, A.M.; Sonntag, K.C.; Isacson, O.; Kosik, K.S. Specific microRNAs modulate embryonic stem cell–derived neurogenesis. Stem. Cells 2006, 24, 857–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Zhang, W.; Zuo, Y.; Ding, M.; Ke, C.; Yan, R.; Zhan, H.; Liu, J.; Wang, J. miR-9 promotes cell proliferation and inhibits apoptosis by targeting LASS2 in bladder cancer. Tumor Biol. 2015, 36, 9631–9640. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Luo, H.; Liu, X.; Peng, Y.; Zhang, B.; Wang, L.; Xu, X.; Peng, X.; Li, G.; Tian, W. miR-9 targets CXCR4 and functions as a potential tumor suppressor in nasopharyngeal carcinoma. Carcinogenesis 2013, 35, 554–563. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, H.; He, X.; Li, G.; Xu, H.; Jia, X.; Nie, Q.; Zhang, X. Deep sequencing analysis of miRNA expression in breast muscle of fast-growing and slow-growing broilers. Int. J. Mol. Sci. 2015, 16, 16242–16262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ennajdaoui, H.; Howard, J.M.; Sterne-Weiler, T.; Jahanbani, F.; Coyne, D.J.; Uren, P.J.; Dargyte, M.; Katzman, S.; Draper, J.M.; Wallace, A. IGF2BP3 modulates the interaction of invasion-associated transcripts with RISC. Cell Rep. 2016, 15, 1876–1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.; Luo, W.; Ye, Y.; Bekele, E.J.; Nie, Q.; Li, Y.; Zhang, X. Let-7b regulates myoblast proliferation by inhibiting IGF2BP3 expression in dwarf and normal chicken. Front. Physiol. 2017, 8, 477. [Google Scholar] [CrossRef] [Green Version]
- Javed, R.; Jing, L.; Yang, J.; Li, X.; Cao, J.; Zhao, S. miRNA transcriptome of hypertrophic skeletal muscle with overexpressed myostatin propeptide. Biomed. Res. Int. 2014, 2014, 328935. [Google Scholar] [CrossRef]
- Frontera, W.R.; Julien, O. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef]
- Federica, B.; Júlia Meireles, N.; Svenja, W.H.; Débora Rodrigues, S.; Katarzyna, H.; Susanne, D. Time course and side-by-side analysis of mesodermal, pre-myogenic, myogenic and differentiated cell markers in the chicken model for skeletal muscle formation. J. Anat. 2015, 227, 361–382. [Google Scholar]
- Sabourin, L.A.; Rudnicki, M.A. The molecular regulation of myogenesis. Clin. Genet. 2000, 57, 16–25. [Google Scholar] [CrossRef]
- Selcuklu, S.D.; Donoghue, M.T.; Rehmet, K.; de Souza Gomes, M.; Fort, A.; Kovvuru, P.; Muniyappa, M.K.; Kerin, M.J.; Enright, A.J.; Spillane, C. MicroRNA-9 inhibition of cell proliferation and identification of novel miR-9 targets by transcriptome profiling in breast cancer cells. J. Biol. Chem. 2012, 287, 29516–29528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, S.; Jin, L.; Zhang, F.; Sarnow, P.; Kay, M.A. Biological basis for restriction of microRNA targets to the 3’ untranslated region in mammalian mRNAs. Nat. Struct. Mol. Biol. 2009, 16, 144–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Hu, W.; Li, G.; Guo, Y.; Wan, Z.; Yu, J. Inhibition of miR-9-5p suppresses prostate cancer progress by targeting StarD13. Cell. Mol. Biol. Lett. 2019, 24, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pichayanoot, R.; Yoshimitsu, A.; Yutaka, H.; Takeshi, O.; Yasuhito, Y. MiR-9 downregulates CDX2 expression in gastric cancer cells. Int. J. Cancer 2011, 129, 2611–2620. [Google Scholar]
- Simona, U.; Giorgio, Z.; Alberto, M.; Marco, V.; James, A.; Silvano, C.; Luca, M. Influence of physical exercise on microRNAs in skeletal muscle regeneration, aging and diseases. Oncotarget 2018, 9, 17220–17237. [Google Scholar]
- Nielsen, F.C.; Nielsen, J.; Christiansen, J. A family of IGF-II mRNA binding proteins (IMP) involved in RNA trafficking. Scand. J. Clin. Lab. Investig. 2001, 61, 93–99. [Google Scholar] [CrossRef]
- Suvasini, R.; Shruti, B.; Thota, B.; Shinde, S.V.; Friedmann-Morvinski, D.; Nawaz, Z.; Prasanna, K.V.; Thennarasu, K.; Hegde, A.S.; Arivazhagan, A.; et al. Insulin growth factor-2 binding protein 3 (IGF2BP3) is a glioblastoma-specific marker that activates phosphatidylinositol 3-kinase/mitogen-activated protein kinase (PI3K/MAPK) pathways by modulating IGF-2. J. Biol. Chem. 2011, 286, 25882–25890. [Google Scholar] [CrossRef] [Green Version]
- Müeller-Pillasch, F.; Lacher, U.; Wallrapp, C.; Micha, A.; Zimmerhackl, F.; Hameister, H.; Varga, G.; Friess, H.; Büchler, M.; Beger, H.G. Cloning of a gene highly overexpressed in cancer coding for a novel KH-domain containing protein. Oncogene 1997, 14, 2729–2733. [Google Scholar] [CrossRef] [Green Version]
- Mueller, F.; Bommer, M.; Lacher, U.; Ruhland, C.; Stagge, V.; Adler, G.; Gress, T.M.; Seufferlein, T. KOC is a novel molecular indicator of malignancy. Br. J. Cancer 2003, 88, 699–701. [Google Scholar] [CrossRef] [Green Version]
- Pryor, J.G.; Bourne, P.A.; Yang, Q.; Spaulding, B.O.; Scott, G.A.; Xu, H. IMP-3 is a novel progression marker in malignant melanoma. Mod. Pathol. 2008, 21, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Clauditz, T.S.; Gontarewicz, A.; Blessmann, M.; Tennstedt, P.; Borgmann, K.; Tribius, S.; Sauter, G.; Dalchow, C.; Knecht, R. Expression of insulin-like growth factor II mRNA-binding protein 3 in squamous cell carcinomas of the head and neck. J. Oral Pathol. Med. 2013, 42, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Huang, L. Glaucocalyxin A induces G2/M cell cycle arrest and apoptosis through the PI3K/Akt pathway in human bladder cancer cells. Int. J. Biol. Sci. 2018, 14, 418–426. [Google Scholar]
- Sun, Y.; Sun, X.; Liu, S.; Liu, L.; Chen, J. The overlap between regeneration and fibrosis in injured skeletal muscle is regulated by phosphatidylinositol 3-kinase/Akt signaling pathway. Gene 2018, 672, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Guo, L.; Zhao, M.; Zhang, D.; Xu, H.; Nie, Q. The Inhibition on MDFIC and PI3K/AKT Pathway Caused by miR-146b-3p Triggers Suppression of Myoblast Proliferation and Differentiation and Promotion of Apoptosis. Cells 2019, 8, 656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, M.; Wang, X.; Chen, X.; Cai, R.; Chen, F.; Dong, W.; Yang, G.; Pang, W. MicroRNA-432 targeting E2F3 and P55PIK inhibits myogenesis through PI3K/AKT/mTOR signaling pathway. RNA Biol. 2017, 14, 347–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, L.; Ruyi, L.; Yalan, Y.; Xinhua, H.; Zishuai, W.; Shiyun, Z.; Chuduan, W.; Zhonglin, T.; Kui, L. MicroRNA-21 Regulates PI3K/Akt/mTOR Signaling by Targeting TGFβI during Skeletal Muscle Development in Pigs. PLoS ONE 2015, 10, e0119396. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Cui, C.; Wang, Y.; He, H.; Liu, Z.; Shen, X.; Chen, Y.; Li, D.; Zhu, Q.; Yin, H. Knockdown of CSRP3 inhibits differentiation of chicken satellite cells by promoting TGF-β/Smad3 signaling. Gene 2019, 707, 36–43. [Google Scholar] [CrossRef]







| Name | Sequence (5′-3′) |
|---|---|
| miR-9-5p mimic | UCUUUGGUUAUCUAGCUGUAUGA |
| Negative mimic | UUGUACUACACAAAAGUACUG |
| miR-9-5p inhibitor | UCAUACAGCUAGAUAACCAAAGA |
| Negative inhibitor | CAGUACUUUUGUGUAGUACAA |
| Si-IGF2BP3-984 | GCUGCUGAGAAACCAAUUATT UAAUUGGUUUCUCAGCAGCTT |
| Si-IGF2BP3-1214 | GCAGGACUUGACACUCUAUTT AUAGAGUGUCAAGUCCUGCTT |
| Si-IGF2BP3-1519 | CCUUGGCAGUUGGAGCUAUTT AUAGCUCCAACUGCCAAGGTT |
| Si-NC | UUCUUCGAACGUGUCACGUTT ACGUGACACGUUCGGAGAATT |
| Genes | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| β-actin | GTCCACCGCAAATGCTTCTAA | TGCGCATTTATGGGTTTTGTT |
| MyoG | TACAGCGACCAACAGTACGC | TCTGCATTGTTTCCATCCTG |
| MyoD1 | AGCACTACAGTGGCGACTCA | GGCCGCTGTAATCCATCA |
| MYHC | GAAGGAGACCTCAACGAGATGG | ATTCAGGTGTCCCAAGTCATCC |
| IGF2BP3 | GCTGCTGCTGCTTCATATCCAC | CCTGCTTGCCAATAATAGCTCCA |
| IGF2 | AGGATCAACCGTGGCATTGT | TCTGACTTGACGGACTTGGC |
| miR-9-5p | TCTTTGGTTATCTAGCTGTATGA | CAGGTCCAGTTTTTTTTTTTTTT |
| U6 | GGGCCATGCTAATCTTCTCTGTA | CAGGTCCAGTTTTTTTTTTTTTT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, H.; He, H.; Shen, X.; Zhao, J.; Cao, X.; Han, S.; Cui, C.; Chen, Y.; Wei, Y.; Xia, L.; et al. miR-9-5p Inhibits Skeletal Muscle Satellite Cell Proliferation and Differentiation by Targeting IGF2BP3 through the IGF2-PI3K/Akt Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 1655. https://doi.org/10.3390/ijms21051655
Yin H, He H, Shen X, Zhao J, Cao X, Han S, Cui C, Chen Y, Wei Y, Xia L, et al. miR-9-5p Inhibits Skeletal Muscle Satellite Cell Proliferation and Differentiation by Targeting IGF2BP3 through the IGF2-PI3K/Akt Signaling Pathway. International Journal of Molecular Sciences. 2020; 21(5):1655. https://doi.org/10.3390/ijms21051655
Chicago/Turabian StyleYin, Huadong, Haorong He, Xiaoxu Shen, Jing Zhao, Xinao Cao, Shunshun Han, Can Cui, Yuqi Chen, Yuanhang Wei, Lu Xia, and et al. 2020. "miR-9-5p Inhibits Skeletal Muscle Satellite Cell Proliferation and Differentiation by Targeting IGF2BP3 through the IGF2-PI3K/Akt Signaling Pathway" International Journal of Molecular Sciences 21, no. 5: 1655. https://doi.org/10.3390/ijms21051655
APA StyleYin, H., He, H., Shen, X., Zhao, J., Cao, X., Han, S., Cui, C., Chen, Y., Wei, Y., Xia, L., Wang, Y., Li, D., & Zhu, Q. (2020). miR-9-5p Inhibits Skeletal Muscle Satellite Cell Proliferation and Differentiation by Targeting IGF2BP3 through the IGF2-PI3K/Akt Signaling Pathway. International Journal of Molecular Sciences, 21(5), 1655. https://doi.org/10.3390/ijms21051655





