Combined Effect of Light and Nutrients on the Micromorphology of the White rot Fungus Cerrena unicolor
Abstract
:1. Introduction
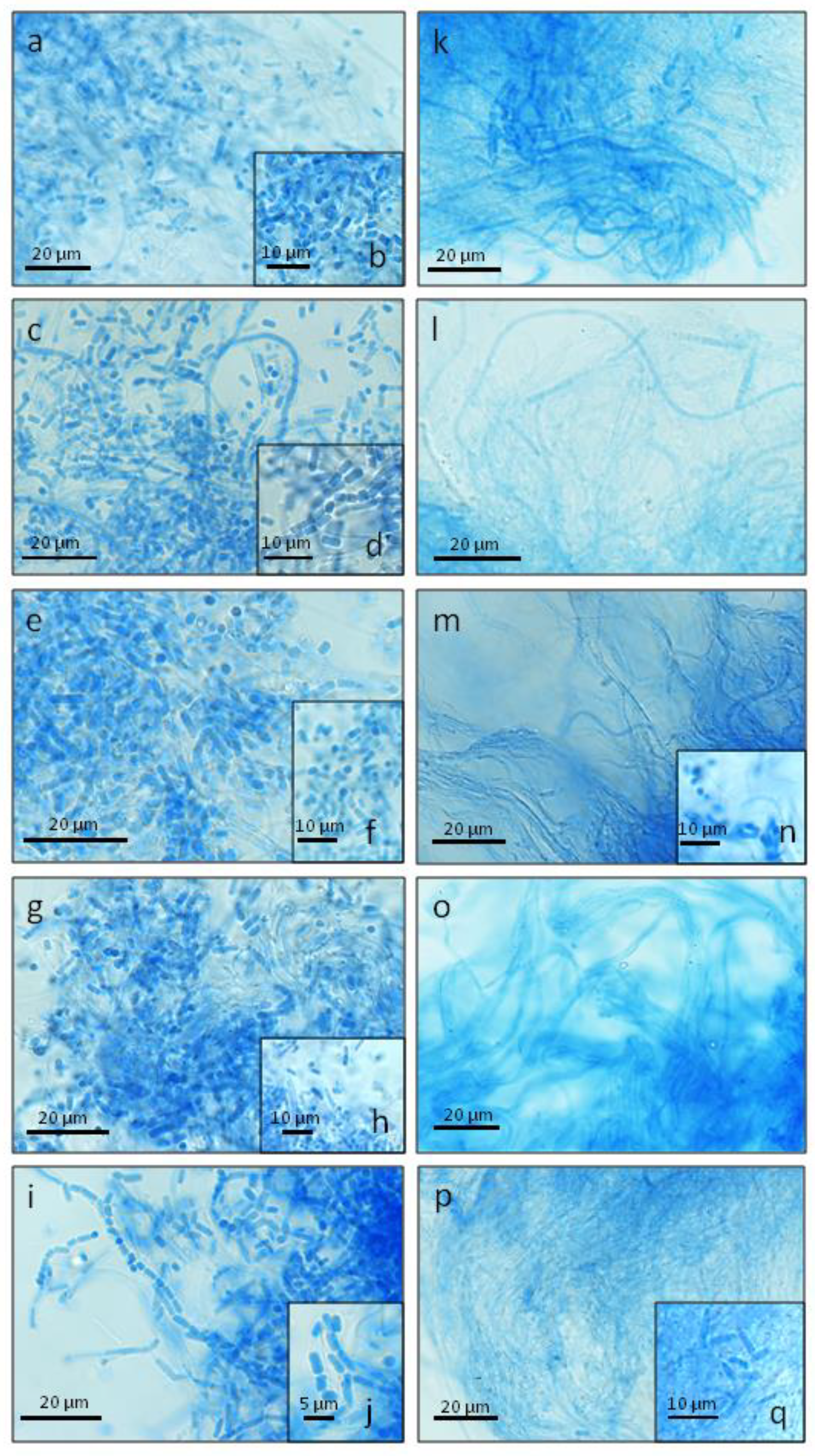
2. Results
3. Discussion
4. Materials and Methods
4.1. Strain, Medium, and Cultivation Conditions
4.2. Visualization of the Micromorphology of Hyphae Using the Confocal Laser Scanning Microscope (CLSM)
4.3. Scanning Electron Microscope (SEM) Analysis of the Structure of Fungal Hyphae
4.4. Data Analysis
4.5. Analysis of the Expression Profile of Genes Engaged in Fungal Growth and Development
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DEGs | Differentially Expressed Genes |
| GO | Gene Ontology |
| CLSM | Confocal Laser Scanning Microscope |
| SEM | Scanning Electron Microscope |
References
- Yu, Z.; Fischer, R. Light sensing and responses in fungi. Nat. Rev. Microbiol. 2019, 17, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Aguirre, J.; Herrera-Estrella, A.; Corrochano, L.M. The complexity of fungal vision. Microbiol. Spectr. 2016, 4, FUNK-0020-2016. [Google Scholar] [CrossRef] [PubMed]
- Fuller, K.K.; Dunlap, J.C.; Loros, J.J. Fungal Light Sensing at the Bench and Beyond. Adv. Genet. 2016, 96, 1–51. [Google Scholar] [PubMed]
- Tisch, D.; Schmoll, M. Light regulation of metabolic pathways in fungi. Appl. Microbiol. Biotechnol. 2010, 85, 1259–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayram, O.; Feussner, K.; Dumkow, M.; Herrfurth, C.; Feussner, I.; Braus, G.H. Changes of global gene expression and secondary metabolite accumulation during light-dependent Aspergillus nidulans development. Fungal Genet. Biol. 2016, 87, 30–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawlik, A.; Mazur, A.; Wielbo, J.; Koper, P.; Żebracki, K.; Kubik-Komar, A.; Janusz, G. RNA sequencing reveals differential gene expression of Cerrena unicolor in response to variable lighting conditions. Int. J. Mol. Sci. 2019, 20, 290. [Google Scholar] [CrossRef] [Green Version]
- Pazouki, M.; Panda, T. Understanding the morphology of fungi. Bioprocess. Eng. 2000, 22, 127–143. [Google Scholar] [CrossRef]
- Enebak, S.A.; Blanchette, R.A. Canker formation and decay in sugar maple and paper birch infected by Cerrena unicolor. Can. J. For. Res. 1989, 19, 225–231. [Google Scholar] [CrossRef]
- Roody, W.C. Mushrooms of West Virginia and the Central Appalachians; University Press of Kentucky: Lexington, KY, USA, 2003. [Google Scholar]
- Ko, K.S.; Jung, H.S. Phylogenetic re-evaluation of Trametes consors based on mitochondrial small subunit ribosomal DNA sequences. FEMS Microbiol. Lett. 1999, 170, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Westhuizen, G.C.A.v.d. The cultural characters, structure of the fruit body, and type of interfertility of Cerrena unicolor (Bull. ex Fr.) Murr. Can. J. Bot. 1963, 41, 1487–1499. [Google Scholar] [CrossRef]
- Janusz, G.; Rogalski, J.; Szczodrak, J. Increased production of laccase by Cerrena unicolor in submerged liquid cultures. World J. Microb. Biotechnol. 2007, 23, 1459–1464. [Google Scholar] [CrossRef]
- Hibi, M.; Hatahira, S.; Nakatani, M.; Yokozeki, K.; Shimizu, S.; Ogawa, J. Extracellular oxidases of Cerrena sp. complementarily functioning in artificial dye decolorization including laccase, manganese peroxidase, and novel versatile peroxidases. Biocatal Agric. Biotechnol. 2012, 1, 220–225. [Google Scholar] [CrossRef] [Green Version]
- Kachlishvili, E.; Metreveli, E.; Elisashvili, V. Modulation of Cerrena unicolor laccase and manganese peroxidase production. Springer Plus 2014, 3, 463. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhang, J.; Zhang, X.; Geng, A. Purification and characterization of a novel manganese peroxidase from white-rot fungus Cerrena unicolor BBP6 and its application in dye decolorization and denim bleaching. Proc. Biochem. 2017, 66, 222–229. [Google Scholar] [CrossRef]
- Belova, O.V.; Lisov, A.V.; Vinokurova, N.G.; Kostenevich, A.A.; Sapunova, L.I.; Lobanok, A.G.; Leontievsky, A.A. Xylanase and cellulase of fungus Cerrena unicolor VKM F-3196: Production, properties, and applications for the saccharification of plant material. Appl. Biochem. Microbiol. 2014, 50, 148–153. [Google Scholar] [CrossRef]
- Mizerska-Dudka, M.; Jaszek, M.; Blachowicz, A.; Rejczak, T.P.; Matuszewska, A.; Osinska-Jaroszuk, M.; Stefaniuk, D.; Janusz, G.; Sulej, J.; Kandefer-Szerszen, M. Fungus Cerrena unicolor as an effective source of new antiviral, immunomodulatory, and anticancer compounds. Int. J. Biol. Macromol. 2015, 79, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, A.; Ruminowicz-Stefaniuk, M.; Frąc, M.; Mazur, A.; Wielbo, J.; Janusz, G. The wood decay fungus Cerrena unicolor adjusts its metabolism to grow on various types of wood and light conditions. PLoS ONE 2019, 14, e0211744. [Google Scholar] [CrossRef]
- Janusz, G.; Sulej, J.; Jaszek, M.; Osinska-Jaroszuk, M. Effect of different wavelengths of light on laccase, cellobiose dehydrogenase, and proteases produced by Cerrena unicolor, Pycnoporus sanguineus and Phlebia lindtneri. Acta Biochim. Pol. 2016, 63, 223–228. [Google Scholar] [CrossRef]
- Pawlik, A.; Jaszek, M.; Sulej, J.; Janusz, G. Light-regulated synthesis of extra- and intracellular enzymes related to wood degradation by the white rot fungus Cerrena unicolor during solid-state fermentation on ash sawdust-based medium. Acta Biochim. Pol. 2019, 66, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ruchin, A. The effects of illumination on the early develompent of tailed and tailless amphibians. Banat’s J. Biotechnol. 2017, 8, 113–118. [Google Scholar] [CrossRef]
- Tabata, M.; Abe, Y. Cerrena unicolor isolated from the mycangia of a horntail, Tremex Iongicollis, in Kochi Prefecture, Japan. Mycosci 1995, 36, 447–450. [Google Scholar] [CrossRef]
- Nobles, M.K. Studies in forest pathology; identification of cultures of wood-rotting fungi. Can. J. Res. 1948, 26, 281–431. [Google Scholar] [CrossRef] [PubMed]
- Osma, J.F.; Moilanen, U.; Toca-Herrera, J.L.; Rodríguez-Couto, S. Morphology and laccase production of white-rot fungi grown on wheat bran flakes under semi-solid-state fermentation conditions. FEMS Microbiol. Lett. 2011, 318, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Corrochano, L.M.; Galland, P. Photomorphogenesis and gravitropism in fungi. In Growth, Differentiation and Sexuality, 3rd ed.; Wendland, J., Ed.; Springer International Publishing: Cham, Switzerland, 2016; Volume 1, pp. 235–266. [Google Scholar]
- Lauter, F.R.; Marchfelder, U.; Russo, V.E.; Yamashiro, C.T.; Yatzkan, E.; Yarden, O. Photoregulation of cot-1, a kinase-encoding gene involved in hyphal growth in Neurospora crassa. Fungal Genet. Biol. 1998, 23, 300–310. [Google Scholar] [CrossRef] [Green Version]
- Ambra, R.; Grimaldi, B.; Zamboni, S.; Filetici, P.; Macino, G.; Ballario, P. Photomorphogenesis in the hypogeous fungus Tuber borchii: Isolation and characterization of Tbwc-1, the homologue of the blue-light photoreceptor of Neurospora crassa. Fungal Genet. Biol. 2004, 41, 688–697. [Google Scholar] [CrossRef]
- Casas-Flores, S.; Rios-Momberg, M.; Bibbins, M.; Ponce-Noyola, P.; Herrera-Estrella, A. BLR-1 and BLR-2, key regulatory elements of photoconidiation and mycelial growth in Trichoderma atroviride. Microbiology 2004, 150, 3561–3569. [Google Scholar] [CrossRef] [Green Version]
- Friedl, M.A.; Schmoll, M.; Kubicek, C.P.; Druzhinina, I.S. Photostimulation of Hypocrea atroviridis growth occurs due to a cross-talk of carbon metabolism, blue light receptors and response to oxidative stress. Microbiology 2008, 154, 1229–1241. [Google Scholar] [CrossRef] [Green Version]
- Barrera, C.R. Formation and germination of fungal arthroconidia. Crit. Rev. Microbiol. 1985, 12, 271–292. [Google Scholar] [CrossRef]
- Lin, X.; Alspaugh, J.A.; Liu, H.; Harris, S. Fungal morphogenesis. Cold Spring Harb. Perspect. Med. 2014, 5, a019679. [Google Scholar] [CrossRef] [Green Version]
- Lauter, F.-R.; Yamashiro, C.T.; Yanofsky, C. Light stimulation of conidiation in Neurospora crassa: Studies with the wild-type strain and mutants wc-1, wc-2 and acon-2. J. Photochem. Photobiol. B 1997, 37, 203–211. [Google Scholar] [CrossRef]
- Sánchez-Murillo, R.I.; de la Torre-Martínez, M.; Aguirre-Linares, J.; Herrera-Estrella, A. Light-regulated asexual reproduction in Paecilomyces fumosoroseus. Microbiology 2004, 150, 311–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellanos, F.; Schmoll, M.; Martínez, P.; Tisch, D.; Kubicek, C.P.; Herrera-Estrella, A.; Esquivel-Naranjo, E.U. Crucial factors of the light perception machinery and their impact on growth and cellulase gene transcription in Trichoderma reesei. Fungal Genet. Biol. 2010, 47, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Buck, J.W. Effect of light on in vivo urediniospore germination, lesion development and sporulation of Puccinia hemerocallidis on daylily and Puccinia pelargoniizonalis on geranium. Mycologia 2011, 103, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Hoff, B.; Kamerewerd, J.; Sigl, C.; Mitterbauer, R.; Zadra, I.; Kürnsteiner, H.; Kück, U. Two components of a velvet-like complex control hyphal morphogenesis, conidiophore development, and penicillin biosynthesis in Penicillium chrysogenum. Eukaryot Cell 2010, 9, 1236–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlile, M.J. The photobiology of fungi. Ann. Rev. Plant. Physiol. 1965, 16, 175–202. [Google Scholar] [CrossRef]
- Krizsán, K.; Almási, É.; Merényi, Z.; Sahu, N.; Virágh, M.; Kószó, T.; Mondo, S.; Kiss, B.; Bálint, B.; Kües, U.; et al. Transcriptomic atlas of mushroom development reveals conserved genes behind complex multicellularity in fungi. Proc. Natl. Acad. Sci. USA 2019, 116, 7409–7418. [Google Scholar] [CrossRef] [Green Version]
- Janusz, G.; Mazur, A.; Chęcińska, A.; Małek, W.; Rogalski, J.; Ohga, S. Cloning and characterization of a lacease gene from biotechnologically important basidiomycete Cerrena unicolor. J. Fac. Agric. Kyushu Univ. 2012, 57, 41–49. [Google Scholar]
- Lindeberg, G.; Holm, G. Occurrence of tyrosinase and laccase in fruit bodies and mycelia of some hymenomycetes. Physiol. Plantarum 1952, 5, 100–114. [Google Scholar] [CrossRef]



| 1.5 % agar LH Synthetic Medium | Lighting Conditions | ||||
| White | Darkness | Red | Blue | Green | |
| Mycelium dimensions (mm × mm) | 56 × 55 ± 1.6 | 63 × 63 ± 1.2 | 60 × 61 ± 0.6 | 43 × 43 ± 1.7 | 58 × 60 ± 0.8 |
| Hyphae type and size (width, μm) | Skeletal hyphae (1.62–2.35); hyphae with single septa (2.03–3.33); septated hyphae producing arthrospores (1.86–3.3) | Skeletal hyphae (1.7–2.6); hyphae with single septa (2.76–3.98), branched hyphae (2.7–3.68) (rare); septated hyphae producing arthrospores (1.7–2.92) | Skeletal hyphae (1.78–2.6); hyphae with single septa (1.95–3.9); septated hyphae producing arthrospores (2.03–3.49) | Skeletal hyphae (1.7–2.35); hyphae with single septa (2.27–3.57); septated hyphae producing arthrospores (1.62–3.00, dominance) | Skeletal hyphae (1.9–2.7); hyphae with single septa (3.0–3.9); septated hyphae producing arthrospores (2.43–3.25) |
| Spore shape | Cylindrical (dominance); broadly ellipsoid | Cylindrical (dominance); broadly ellipsoid | Cylindrical (dominance); broadly ellipsoid | Cylindrical (dominance); broadly ellipsoid | Cylindrical (dominance); broadly ellipsoid |
| Spore size [μm × μm] | 3.0–7.0 × 1.7–3.0 | 3.0–11 × 2.0–3.5 | 3.5–10 × 2.0–3.5 | 3.5–13 × 1.5–3.5 | 2.5–8.5 × 2.0–3.5 |
| 1.5 % Agar Ash Sawdust Medium | Lighting Conditions | ||||
| White | Darkness | Red | Blue | Green | |
| Hyphae type and size (width, μm) | Skeletal hyphae (1.54–2.03); hyphae with single septa (1.94–2.63); septated hyphae producing arthrospores (1.54–2.51, rare) | Skeletal hyphae (1.7–2.51); hyphae with single septa (2.53–3.8); septated hyphae producing arthrospores (2.11–2.76, rare); branched hyphae (2.35–3.5, rare) | Skeletal hyphae (1.46–2.11); septated hyphae producing arthrospores (1.54–2.35, rare); hyphae with single septa (1.96–3.25, rare) | Skeletal hyphae (1.54–2.43); septated hyphae producing arthrospores (1.86–3.0, rare) | Skeletal hyphae (1.46–2.27, dominance); hyphae with single septa (1.95–3.65); septated hyphae producing arthrospores (1.82–2.61) |
| Spore shape | Cylindrical (dominance); broadly ellipsoid | Cylindrical (dominance); broadly ellipsoid | Cylindrical (dominance); broadly ellipsoid | Cylindrical (dominance); broadly ellipsoid | Cylindrical (dominance); broadly ellipsoid |
| Spore size (μm × μm) | 2.5–6.0 × 2.0–3.0 | 2.0–14 × 2.0–2.5 | 4.0–13 × 2.0–3.0 | 3.5–10 × 2.0–3.0 | 3.0–7.5 × 2.0–3.0 |
| Ash Sawdust Medium | Lighting Conditions | ||||
| White | Darkness | Red | Blue | Green | |
| Hyphae type and size (width, μm) | Skeletal hyphae (1.54–2.27); hyphae with single septa (1.7–2.63); septated hyphae producing arthrospores (1.54–3.0) | Skeletal hyphae (1.46–2.6); septated hyphae producing arthrospores (1.62–2.27); hyphae with single septa (1.91–2.7, rare) | Skeletal hyphae (1.48–2.27) (dominance); hyphae with single septa (1.7–2.63); septated hyphae producing arthrospores (1.66–2.68) | Skeletal hyphae (1.62–2.54, dominance); hyphae with single septa (1.7–3.3) | Skeletal hyphae (1.54–2.03, dominance); hyphae with single septa (1.7–3.12); septated hyphae producing arthrospores (1.62–2.6, rare) |
| Spore shape | Cylindrical (dominance); broadly ellipsoid | Cylindrical (dominance); broadly ellipsoid | Cylindrical (dominance); broadly ellipsoid | Cylindrical (dominance); broadly ellipsoid | Cylindrical (dominance); broadly ellipsoid |
| Spore size (μm × μm) | 3.0–6.0 × 1.7–2.5 | 3.5–10 × 2.0–3.0 | 3.0–8.5 × 2.0–3.0 | 3.5–8.5 × 2.0–3.0 | 3.5–7.5 × 2.0–3.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlik, A.; Jaszek, M.; Stefaniuk, D.; Świderska-Burek, U.; Mazur, A.; Wielbo, J.; Koper, P.; Żebracki, K.; Janusz, G. Combined Effect of Light and Nutrients on the Micromorphology of the White rot Fungus Cerrena unicolor. Int. J. Mol. Sci. 2020, 21, 1678. https://doi.org/10.3390/ijms21051678
Pawlik A, Jaszek M, Stefaniuk D, Świderska-Burek U, Mazur A, Wielbo J, Koper P, Żebracki K, Janusz G. Combined Effect of Light and Nutrients on the Micromorphology of the White rot Fungus Cerrena unicolor. International Journal of Molecular Sciences. 2020; 21(5):1678. https://doi.org/10.3390/ijms21051678
Chicago/Turabian StylePawlik, Anna, Magdalena Jaszek, Dawid Stefaniuk, Urszula Świderska-Burek, Andrzej Mazur, Jerzy Wielbo, Piotr Koper, Kamil Żebracki, and Grzegorz Janusz. 2020. "Combined Effect of Light and Nutrients on the Micromorphology of the White rot Fungus Cerrena unicolor" International Journal of Molecular Sciences 21, no. 5: 1678. https://doi.org/10.3390/ijms21051678
APA StylePawlik, A., Jaszek, M., Stefaniuk, D., Świderska-Burek, U., Mazur, A., Wielbo, J., Koper, P., Żebracki, K., & Janusz, G. (2020). Combined Effect of Light and Nutrients on the Micromorphology of the White rot Fungus Cerrena unicolor. International Journal of Molecular Sciences, 21(5), 1678. https://doi.org/10.3390/ijms21051678








