Butyrate Decreases ICAM-1 Expression in Human Oral Squamous Cell Carcinoma Cells
Abstract
:1. Introduction
2. Results
2.1. Cell Viability Upon SCFA Stimulation at Varying Concentrations
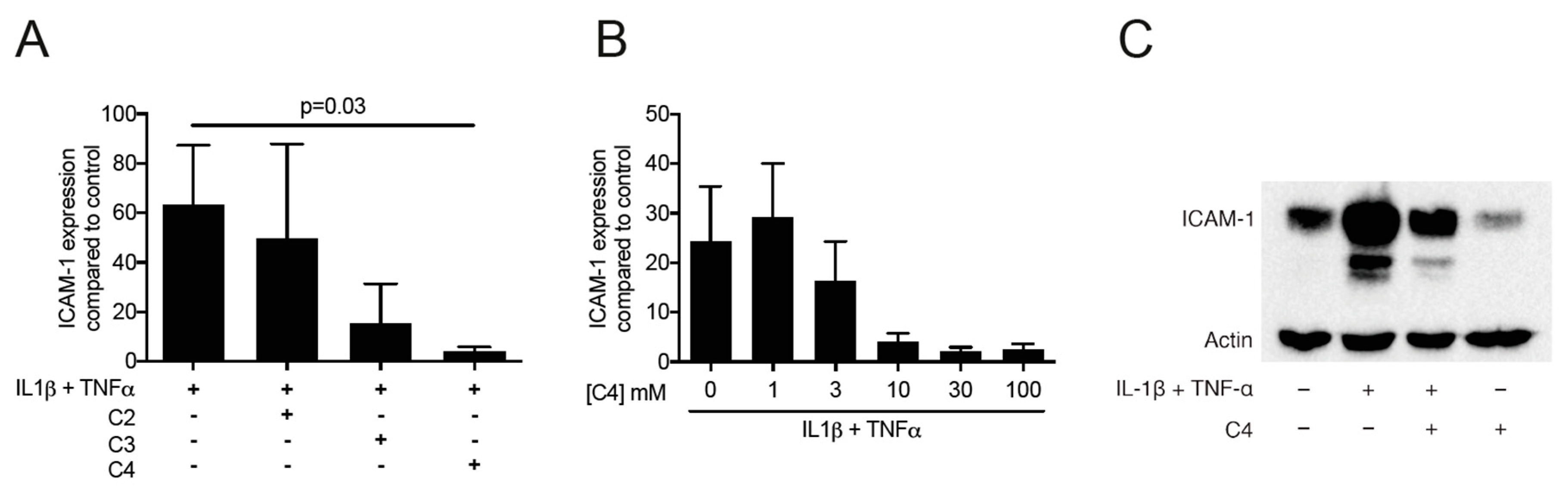
2.2. Butyrate but Not Acetate and Propionate Decrease the Expression of ICAM-1 in HSC-2 Cells
2.3. Activation of FFAR2 Can Mimic the Activity of Butyrate on ICAM-1 in HSC-2 Cells
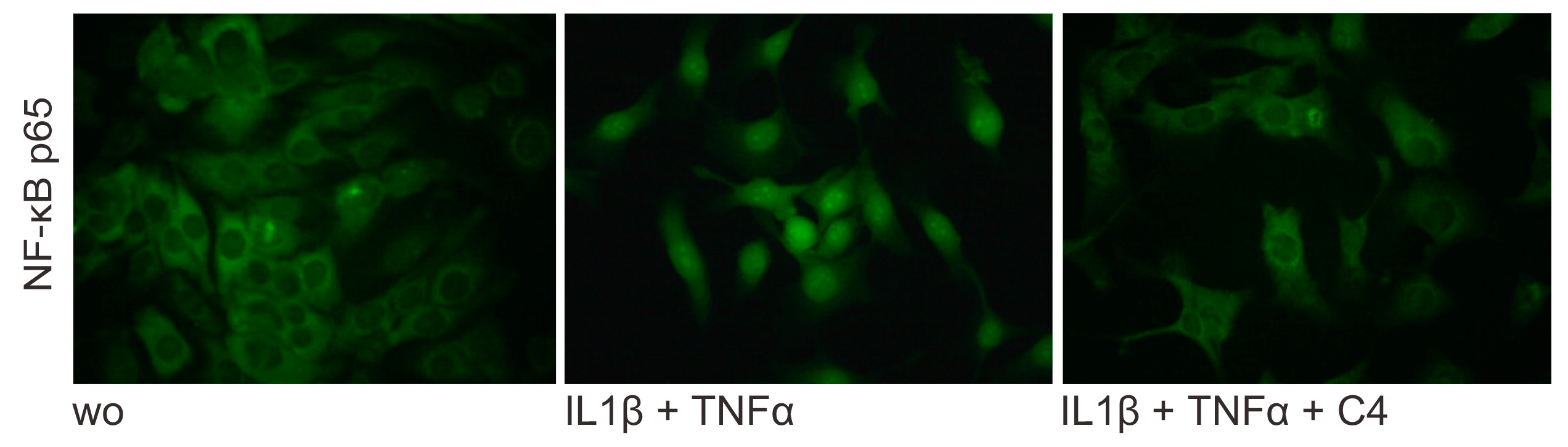
2.4. Butyrate Inhibits the Nuclear Translocation of p65 on HSC-2 cells
2.5. Butyrate Cannot Reverse the Acute Expression Levels of ICAM-1 in HSC-2 Cells
3. Discussion
4. Material and Methods
4.1. Cell Culture
4.2. Viability Assay
4.3. Cell Stimulation
4.4. qRT-PCR Analysis
4.5. Western Blot
4.6. Immunofluorescence
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mombelli, A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontol 2000 2018, 76, 85–96. [Google Scholar] [CrossRef]
- Moughal, N.A.; Adonogianaki, E.; Thornhill, M.H.; Kinane, D.F. Endothelial cell leukocyte adhesion molecule-1 (ELAM-1) and intercellular adhesion molecule-1 (ICAM-1) expression in gingival tissue during health and experimentally-induced gingivitis. J. Periodontal Res. 1992, 27, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, S.; Satoh, M.; Takiwaki, M.; Nomura, F. Current Status of Proteomic Technologies for Discovering and Identifying Gingival Crevicular Fluid Biomarkers for Periodontal Disease. Int. J. Mol. Sci 2018, 20, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanks, J.E.; Moll, T.; Eytner, R.; Vestweber, D. Stimulation of P-selectin glycoprotein ligand-1 on mouse neutrophils activates beta 2-integrin mediated cell attachment to ICAM-1. Eur J. Immunol 1998, 28, 433–443. [Google Scholar] [CrossRef]
- Rothlein, R.; Czajkowski, M.; O’Neill, M.M.; Marlin, S.D.; Mainolfi, E.; Merluzzi, V.J. Induction of intercellular adhesion molecule 1 on primary and continuous cell lines by pro-inflammatory cytokines. Regulation by pharmacologic agents and neutralizing antibodies. J. Immunol. 1988, 141, 1665–1669. [Google Scholar]
- Crawford, J.M.; Hopp, B. Junctional epithelium expresses the intercellular adhesion molecule ICAM-1. J. Periodontal Res. 1990, 25, 254–256. [Google Scholar] [CrossRef]
- Irie, K.; Tomofuji, T.; Ekuni, D.; Morita, M.; Shimazaki, Y.; Darveau, R.P. Impact of Oral Commensal Bacteria on Degradation of Periodontal Connective Tissue in Mice. J. Periodontol. 2015, 86, 899–905. [Google Scholar] [CrossRef]
- Crawford, J.M. Distribution of ICAM-1, LFA-3 and HLA-DR in healthy and diseased gingival tissues. J. Periodontal Res. 1992, 27, 291–298. [Google Scholar] [CrossRef]
- Yang, S.F.; Chen, M.K.; Hsieh, Y.S.; Chung, T.T.; Hsieh, Y.H.; Lin, C.W.; Su, J.L.; Tsai, M.H.; Tang, C.H. Prostaglandin E2/EP1 signaling pathway enhances intercellular adhesion molecule 1 (ICAM-1) expression and cell motility in oral cancer cells. J. Biol. Chem. 2010, 285, 29808–29816. [Google Scholar] [CrossRef] [Green Version]
- Chuang, J.Y.; Huang, Y.L.; Yen, W.L.; Chiang, I.P.; Tsai, M.H.; Tang, C.H. Syk/JNK/AP-1 signaling pathway mediates interleukin-6-promoted cell migration in oral squamous cell carcinoma. Int. J. Mol. Sci. 2014, 15, 545–559. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.T.; Haake, S.K.; Kim, J.W.; Park, N.H. Differential expression of interleukin-8 and intercellular adhesion molecule-1 by human gingival epithelial cells in response to Actinobacillus actinomycetemcomitans or Porphyromonas gingivalis infection. Oral. Microbiol. Immunol. 1998, 13, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Takigawa, M.; Takashiba, S.; Nagai, A.; Miyamoto, M.; Kurihara, H.; Murayama, Y. Role of cytokine in the induction of adhesion molecules on cultured human gingival fibroblasts. J. Periodontol. 1994, 65, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Oddera, S.; Silvestri, M.; Lantero, S.; Sacco, O.; Rossi, G.A. Downregulation of the expression of intercellular adhesion molecule (ICAM)-1 on bronchial epithelial cells by fenoterol, a beta2-adrenoceptor agonist. J. Asthma 1998, 35, 401–408. [Google Scholar] [CrossRef]
- Usui-Ouchi, A.; Ouchi, Y.; Ebihara, N. The peroxisome proliferator-activated receptor pan-agonist bezafibrate suppresses microvascular inflammatory responses of retinal endothelial cells and vascular endothelial growth factor production in retinal pigmented epithelial cells. Int. Immunopharmacol. 2017, 52, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int 2012, 95, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Singer, R.E.; Buckner, B.A. Butyrate and propionate: Important components of toxic dental plaque extracts. Infect. Immun. 1981, 32, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Meng, H.; Gao, X.; Xu, L.; Feng, X. Effect of non-surgical periodontal treatment on short chain fatty acid levels in gingival crevicular fluid of patients with generalized aggressive periodontitis. J. Periodontal Res. 2014, 49, 574–583. [Google Scholar] [CrossRef]
- Niederman, R.; Buyle-Bodin, Y.; Lu, B.Y.; Robinson, P.; Naleway, C. Short-chain carboxylic acid concentration in human gingival crevicular fluid. J. Dent. Res. 1997, 76, 575–579. [Google Scholar] [CrossRef]
- Cueno, M.E.; Ochiai, K. Gingival Periodontal Disease (PD) Level-Butyric Acid Affects the Systemic Blood and Brain Organ: Insights Into the Systemic Inflammation of Periodontal Disease. Front. Immunol. 2018, 9, 1158. [Google Scholar] [CrossRef]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2019. [Google Scholar] [CrossRef]
- Davie, J.R. Inhibition of histone deacetylase activity by butyrate. J. Nutr 2003, 133, 2485S–2493S. [Google Scholar] [CrossRef] [PubMed]
- Jeng, J.H.; Chan, C.P.; Ho, Y.S.; Lan, W.H.; Hsieh, C.C.; Chang, M.C. Effects of butyrate and propionate on the adhesion, growth, cell cycle kinetics, and protein synthesis of cultured human gingival fibroblasts. J. Periodontol. 1999, 70, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, S.; Sugano, N.; Nishihara, R.; Koshi, R.; Murai, M.; Yoshinuma, N.; Ochiai, K.; Ito, K. The effect of butyric acid on adhesion molecule expression by human gingival epithelial cells. J. Periodontal Res. 2008, 43, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Kurita-Ochiai, T.; Seto, S.; Suzuki, N.; Yamamoto, M.; Otsuka, K.; Abe, K.; Ochiai, K. Butyric acid induces apoptosis in inflamed fibroblasts. J. Dent. Res. 2008, 87, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Shirasugi, M.; Nishioka, K.; Yamamoto, T.; Nakaya, T.; Kanamura, N. Normal human gingival fibroblasts undergo cytostasis and apoptosis after long-term exposure to butyric acid. Biochem. Biophys. Res. Commun. 2017, 482, 1122–1128. [Google Scholar] [CrossRef]
- Kurita-Ochiai, T.; Fukushima, K.; Ochiai, K. Butyric acid-induced apoptosis of murine thymocytes, splenic T cells, and human Jurkat T cells. Infect. Immun. 1997, 65, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, T.M.; Westendorf, J.J. Histone deacetylase inhibitors promote osteoblast maturation. J. Bone Miner. Res. 2005, 20, 2254–2263. [Google Scholar] [CrossRef]
- Niederman, R.; Zhang, J.; Kashket, S. Short-chain carboxylic-acid-stimulated, PMN-mediated gingival inflammation. Crit. Rev. Oral. Biol. Med. 1997, 8, 269–290. [Google Scholar] [CrossRef]
- Tsuda, H.; Ochiai, K.; Suzuki, N.; Otsuka, K. Butyrate, a bacterial metabolite, induces apoptosis and autophagic cell death in gingival epithelial cells. J. Periodontal Res. 2010, 45, 626–634. [Google Scholar] [CrossRef]
- Evans, M.; Murofushi, T.; Tsuda, H.; Mikami, Y.; Zhao, N.; Ochiai, K.; Kurita-Ochiai, T.; Yamamoto, M.; Otsuka, K.; Suzuki, N. Combined effects of starvation and butyrate on autophagy-dependent gingival epithelial cell death. J. Periodontal Res. 2017, 52, 522–531. [Google Scholar] [CrossRef]
- Correa, R.O.; Vieira, A.; Sernaglia, E.M.; Lancellotti, M.; Vieira, A.T.; Avila-Campos, M.J.; Rodrigues, H.G.; Vinolo, M.A.R. Bacterial short-chain fatty acid metabolites modulate the inflammatory response against infectious bacteria. Cell Microbiol. 2017, 19, e12720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Wang, Y.; Meng, H.; Yu, J.; Lu, H.; Li, W.; Lu, R.; Zhao, Y.; Li, Q.; Su, L. Butyrate rather than LPS subverts gingival epithelial homeostasis by downregulation of intercellular junctions and triggering pyroptosis. J. Clin. Periodontol. 2019, 46, 894–907. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, A.; Morera, R.; Ciccocioppo, R.; Cazzola, P.; Gotti, S.; Tinozzi, F.P.; Tinozzi, S.; Corazza, G.R. Oral butyrate for mildly to moderately active Crohn’s disease. Aliment. Pharmacol. Ther. 2005, 22, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Butzner, J.D.; Parmar, R.; Bell, C.J.; Dalal, V. Butyrate enema therapy stimulates mucosal repair in experimental colitis in the rat. Gut 1996, 38, 568–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, S.; Omata, Y.; Hofmann, J.; Bottcher, M.; Iljazovic, A.; Sarter, K.; Albrecht, O.; Schulz, O.; Krishnacoumar, B.; Kronke, G.; et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 2018, 9, 55. [Google Scholar] [CrossRef] [Green Version]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.G.W.; Pires, E.; et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity 2019, 50, 432–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, J.; Shu, D.; Zheng, M.; Wang, J.; Luo, C.; Wang, Y.; Guo, F.; Zou, X.; Lv, X.; Li, Y.; et al. Microbial metabolite butyrate facilitates M2 macrophage polarization and function. Sci. Rep. 2016, 6, 24838. [Google Scholar] [CrossRef]
- Lee, C.; Kim, B.G.; Kim, J.H.; Chun, J.; Im, J.P.; Kim, J.S. Sodium butyrate inhibits the NF-kappa B signaling pathway and histone deacetylation, and attenuates experimental colitis in an IL-10 independent manner. Int. Immunopharmacol. 2017, 51, 47–56. [Google Scholar] [CrossRef]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-induced Inflammatory Bowel Disease Mice Model. EBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Chen, H.Z.; Xu, Z.D.; Wang, F.; Fang, H.; Bellanfante, O.; Chen, X.L. Sodium butyrate inhibits the production of HMGB1 and attenuates severe burn plus delayed resuscitation-induced intestine injury via the p38 signaling pathway. Burns 2018. [Google Scholar] [CrossRef]
- Pirozzi, C.; Francisco, V.; Guida, F.D.; Gomez, R.; Lago, F.; Pino, J.; Meli, R.; Gualillo, O. Butyrate Modulates Inflammation in Chondrocytes via GPR43 Receptor. Cell Physiol. Biochem. 2018, 51, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Guo, H.L.; Deng, X.; Zhu, T.T.; Xiong, J.F.; Xu, Y.H.; Xu, Y. Short-Chain Fatty Acids Inhibit Oxidative Stress and Inflammation in Mesangial Cells Induced by High Glucose and Lipopolysaccharide. Exp. Clin. Endocrinol. Diabetes 2017, 125, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Russo, I.; Luciani, A.; De Cicco, P.; Troncone, E.; Ciacci, C. Butyrate attenuates lipopolysaccharide-induced inflammation in intestinal cells and Crohn’s mucosa through modulation of antioxidant defense machinery. PLoS ONE 2012, 7, e32841. [Google Scholar] [CrossRef] [Green Version]
- Zapolska-Downar, D.; Siennicka, A.; Kaczmarczyk, M.; Kołodziej, B.; Naruszewicz, M. Butyrate inhibits cytokine-induced VCAM-1 and ICAM-1 expression in cultured endothelial cells: The role of NF-kappaB and PPARalpha. J. Nutr. Biochem. 2004, 15, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, S.; Sugano, N.; Ochiai, K.; Arai, N.; Ota, N.; Ito, K. Effects of sodium bicarbonate on butyric acid-induced epithelial cell damage in vitro. J. Oral Sci 2008, 50, 413–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, T.; Towatari, M.; Kosugi, H.; Saito, H. Up-regulation of costimulatory/adhesion molecules by histone deacetylase inhibitors in acute myeloid leukemia cells. Blood 2000, 96, 3847–3856. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.J.; Zaloga, G.P.; Hoggatt, A.M.; Labarrere, C.; Faulk, W.P. Short-chain fatty acids modulate gene expression for vascular endothelial cell adhesion molecules. Nutrition 2005, 21, 740–748. [Google Scholar] [CrossRef]
- Ogawa, H.; Rafiee, P.; Fisher, P.J.; Johnson, N.A.; Otterson, M.F.; Binion, D.G. Butyrate modulates gene and protein expression in human intestinal endothelial cells. Biochem. Biophys. Res. Commun 2003, 309, 512–519. [Google Scholar] [CrossRef]
- Vallabhapurapu, S.; Karin, M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 2009, 27, 693–733. [Google Scholar] [CrossRef]
- Magrin, G.L.; Di Summa, F.; Strauss, F.J.; Panahipour, L.; Mildner, M.; Benfatti, C.A.M.; Gruber, R. 10 mM of Butyrate Caused a 4-Fold Increase of β-arrestins-2; Department of Oral Biology, School of Dentistry, Medical University of Vienna: Vienna, Austria, 2020; Unpublished work. [Google Scholar]
- Gao, H.; Sun, Y.; Wu, Y.; Luan, B.; Wang, Y.; Qu, B.; Pei, G. Identification of beta-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-kappaB pathways. Mol. Cell 2004, 14, 303–317. [Google Scholar] [CrossRef]
- Lee, S.U.; In, H.J.; Kwon, M.S.; Park, B.O.; Jo, M.; Kim, M.O.; Cho, S.; Lee, S.; Lee, H.J.; Kwak, Y.S.; et al. beta-Arrestin 2 mediates G protein-coupled receptor 43 signals to nuclear factor-kappaB. Biol. Pharm. Bull. 2013, 36, 1754–1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magrin, G.L.; Di Summa, F.; Strauss, F.J.; Panahipour, L.; Mildner, M.; Benfatti, C.A.M.; Gruber, R. Three Hours Pre-exposure of HSC-2 Cells with Butyrate Could not Reduce the IL1β and TNFα-induced Increase of ICAM-1, Suggesting that It Requires the 24 h Exposure to Exert the Effects of Butyrate on ICAM-1 Expression; Department of Oral Biology, School of Dentistry, Medical University of Vienna: Vienna, Austria, 2020; Unpublished work. [Google Scholar]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J., Jr.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef] [PubMed]
- Ledebur, H.C.; Parks, T.P. Transcriptional regulation of the intercellular adhesion molecule-1 gene by inflammatory cytokines in human endothelial cells. Essential roles of a variant NF-kappa B site and p65 homodimers. J. Biol. Chem. 1995, 270, 933–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, W.; Jia, Y.; Liu, X.; Zhang, H.; Li, T.; Huang, W.; Chen, X.; Wang, F.; Sun, W.; Wu, H. Sodium butyrate activates NRF2 to ameliorate diabetic nephropathy possibly via inhibition of HDAC. J. Endocrinol. 2017, 232, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Su, W.; Wan, T.; Yu, J.; Zhu, W.; Tang, F.; Liu, G.; Olsen, N.; Liang, D.; Zheng, S.G. Sodium butyrate regulates Th17/Treg cell balance to ameliorate uveitis via the Nrf2/HO-1 pathway. Biochem. Pharmacol. 2017, 142, 111–119. [Google Scholar] [CrossRef]
- Sun, B.; Jia, Y.; Yang, S.; Zhao, N.; Hu, Y.; Hong, J.; Gao, S.; Zhao, R. Sodium butyrate protects against high-fat diet-induced oxidative stress in rat liver by promoting expression of nuclear factor E2-related factor 2. Br. J. Nutr. 2019, 122, 400–410. [Google Scholar] [CrossRef]
- Liu, J.; Chang, G.; Huang, J.; Wang, Y.; Ma, N.; Roy, A.C.; Shen, X. Sodium Butyrate Inhibits the Inflammation of Lipopolysaccharide-Induced Acute Lung Injury in Mice by Regulating the Toll-Like Receptor 4/Nuclear Factor kappaB Signaling Pathway. J. Agric. Food Chem. 2019, 67, 1674–1682. [Google Scholar] [CrossRef]
- Seo, Y.; Park, J.; Choi, W.; Ju Son, D.; Sung Kim, Y.; Kim, M.K.; Yoon, B.E.; Pyee, J.; Tae Hong, J.; Go, Y.M.; et al. Antiatherogenic Effect of Resveratrol Attributed to Decreased Expression of ICAM-1 (Intercellular Adhesion Molecule-1). Arter. Thromb. Vasc Biol. 2019, 39, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Youn, G.S.; Kwon, D.J.; Ju, S.M.; Choi, S.Y.; Park, J. Curcumin ameliorates TNF-alpha-induced ICAM-1 expression and subsequent THP-1 adhesiveness via the induction of heme oxygenase-1 in the HaCaT cells. BMB Rep. 2013, 46, 410–415. [Google Scholar] [CrossRef] [Green Version]
- Seo, W.Y.; Ju, S.M.; Song, H.Y.; Goh, A.R.; Jun, J.G.; Kang, Y.H.; Choi, S.Y.; Park, J. Celastrol suppresses IFN-gamma-induced ICAM-1 expression and subsequent monocyte adhesiveness via the induction of heme oxygenase-1 in the HaCaT cells. Biochem. Biophys. Res. Commun. 2010, 398, 140–145. [Google Scholar] [CrossRef]
- Mole, N.; Kennel-de March, A.; Martin, G.; Miller, N.; Bene, M.C.; Faure, G.C. High levels of soluble intercellular adhesion molecule-1 (ICAM-1) in crevicular fluid of periodontitis patients with plaque. J. Clin. Periodontol. 1998, 25, 754–758. [Google Scholar] [CrossRef]
- Xu, H.; Gonzalo, J.A.; St Pierre, Y.; Williams, I.R.; Kupper, T.S.; Cotran, R.S.; Springer, T.A.; Gutierrez-Ramos, J.C. Leukocytosis and resistance to septic shock in intercellular adhesion molecule 1-deficient mice. J. Exp. Med. 1994, 180, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, J.; Piao, C.; Shao, J.; Du, J. ICAM-1 suppresses tumor metastasis by inhibiting macrophage M2 polarization through blockade of efferocytosis. Cell Death Dis. 2015, 6, e1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, W.; Yao, L.; Li, L.; Zhang, J.; Place, A.T.; Minshall, R.D.; Liu, G. ICAM-1 regulates macrophage polarization by suppressing MCP-1 expression via miR-124 upregulation. Oncotarget 2017, 8, 111882–111901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [Green Version]
- Woelber, J.P.; Bremer, K.; Vach, K.; Konig, D.; Hellwig, E.; Ratka-Kruger, P.; Al-Ahmad, A.; Tennert, C. An oral health optimized diet can reduce gingival and periodontal inflammation in humans—a randomized controlled pilot study. BMC Oral Health 2016, 17, 28. [Google Scholar] [CrossRef] [Green Version]
- Woelber, J.P.; Gartner, M.; Breuninger, L.; Anderson, A.; Konig, D.; Hellwig, E.; Al-Ahmad, A.; Vach, K.; Dotsch, A.; Ratka-Kruger, P.; et al. The influence of an anti-inflammatory diet on gingivitis. A randomized controlled trial. J. Clin. Periodontol. 2019. [Google Scholar] [CrossRef]
- Gruber, R. Osteoimmunology: Inflammatory osteolysis and regeneration of the alveolar bone. J. Clin. Periodontol. 2019, 46 Suppl 21, 52–69. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Ahmed, K.; Gille, A.; Lu, S.; Grone, H.J.; Tunaru, S.; Offermanns, S. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat. Med. 2015, 21, 173–177. [Google Scholar] [CrossRef]
- Cantley, M.D.; Zannettino, A.C.W.; Bartold, P.M.; Fairlie, D.P.; Haynes, D.R. Histone deacetylases (HDAC) in physiological and pathological bone remodelling. Bone 2017, 95, 162–174. [Google Scholar] [CrossRef] [Green Version]
- Huynh, N.C.; Everts, V.; Ampornaramveth, R.S. Histone deacetylases and their roles in mineralized tissue regeneration. Bone Rep. 2017, 7, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Pourgonabadi, S.; Muller, H.D.; Mendes, J.R.; Gruber, R. Saliva initiates the formation of pro-inflammatory macrophages in vitro. Arch. Oral Biol 2017, 73, 295–301. [Google Scholar] [CrossRef] [PubMed]




| Cell Type | HSC-2 Cell Line | Gingival Fibroblasts | ||||
|---|---|---|---|---|---|---|
| Concentration | Acetate | Propionate | Butyrate | Acetate | Propionate | Butyrate |
| 100 mM | 39.2 ± 5 | 46.4 ± 5.1 | 53.5 ± 5 | 12.5 ± 1.2 | 11.9 ± 1.5 | 10.1 ± 0.9 |
| 30 mM | 45.8 ± 5.9 | 56.6 ± 5.8 | 95.8 ± 6 | 69.8 ± 4.5 | 74.5 ± 2.1 | 94 ± 0.5 |
| 10 mM | 104.7 ± 6.1 | 113 ± 6.4 | 122.7 ± 6.5 | 96.3 ± 1.2 | 102.7 ± 1.2 | 122.3 ± 3.1 |
| 1 mM | 125 ± 7.0 | 136 ± 5.6 | 139 ± 7.7 | 125.3 ± 6.7 | 130.4 ± 0.5 | 135.5 ± 6.5 |
| Primer | Sequence Forward | Sequence Reverse |
|---|---|---|
| hICAM-1 | cct tcc tca ccg tgt act gg | agc gta ggg taa ggt tct tgc |
| hARRB2 | caa ctc cac caa gac cgt caa ga | ttc gag ttg agc cac agg aca ctt |
| hGAPDH | aag cca cat cgc tca gac ac | gcc caa tac gac caa atc c |
| hActin | cca acc gcg aga aga tga | cca gag gcg tac agg gat ag |
| mICAM-1 | gtg atg ctc agg tat cca tcc a | cac agt tct caa agc aca gcg |
| mGAPDH | aac ttt ggc att gtg gaa gg | gga tgc agg gat gat gtt ct |
| mActin | cta agg cca acc gtg aaa ag | acc aga ggc ata cag gga ca |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magrin, G.L.; Di Summa, F.; Strauss, F.-J.; Panahipour, L.; Mildner, M.; Magalhães Benfatti, C.A.; Gruber, R. Butyrate Decreases ICAM-1 Expression in Human Oral Squamous Cell Carcinoma Cells. Int. J. Mol. Sci. 2020, 21, 1679. https://doi.org/10.3390/ijms21051679
Magrin GL, Di Summa F, Strauss F-J, Panahipour L, Mildner M, Magalhães Benfatti CA, Gruber R. Butyrate Decreases ICAM-1 Expression in Human Oral Squamous Cell Carcinoma Cells. International Journal of Molecular Sciences. 2020; 21(5):1679. https://doi.org/10.3390/ijms21051679
Chicago/Turabian StyleMagrin, Gabriel Leonardo, Francesca Di Summa, Franz-Josef Strauss, Layla Panahipour, Michael Mildner, Cesar Augusto Magalhães Benfatti, and Reinhard Gruber. 2020. "Butyrate Decreases ICAM-1 Expression in Human Oral Squamous Cell Carcinoma Cells" International Journal of Molecular Sciences 21, no. 5: 1679. https://doi.org/10.3390/ijms21051679
APA StyleMagrin, G. L., Di Summa, F., Strauss, F.-J., Panahipour, L., Mildner, M., Magalhães Benfatti, C. A., & Gruber, R. (2020). Butyrate Decreases ICAM-1 Expression in Human Oral Squamous Cell Carcinoma Cells. International Journal of Molecular Sciences, 21(5), 1679. https://doi.org/10.3390/ijms21051679









