Functional Ingredients From Brassicaceae Species: Overview and Perspectives
Abstract
1. Brassicaceae Family: A Rich Mine of Bioactive Phytochemicals
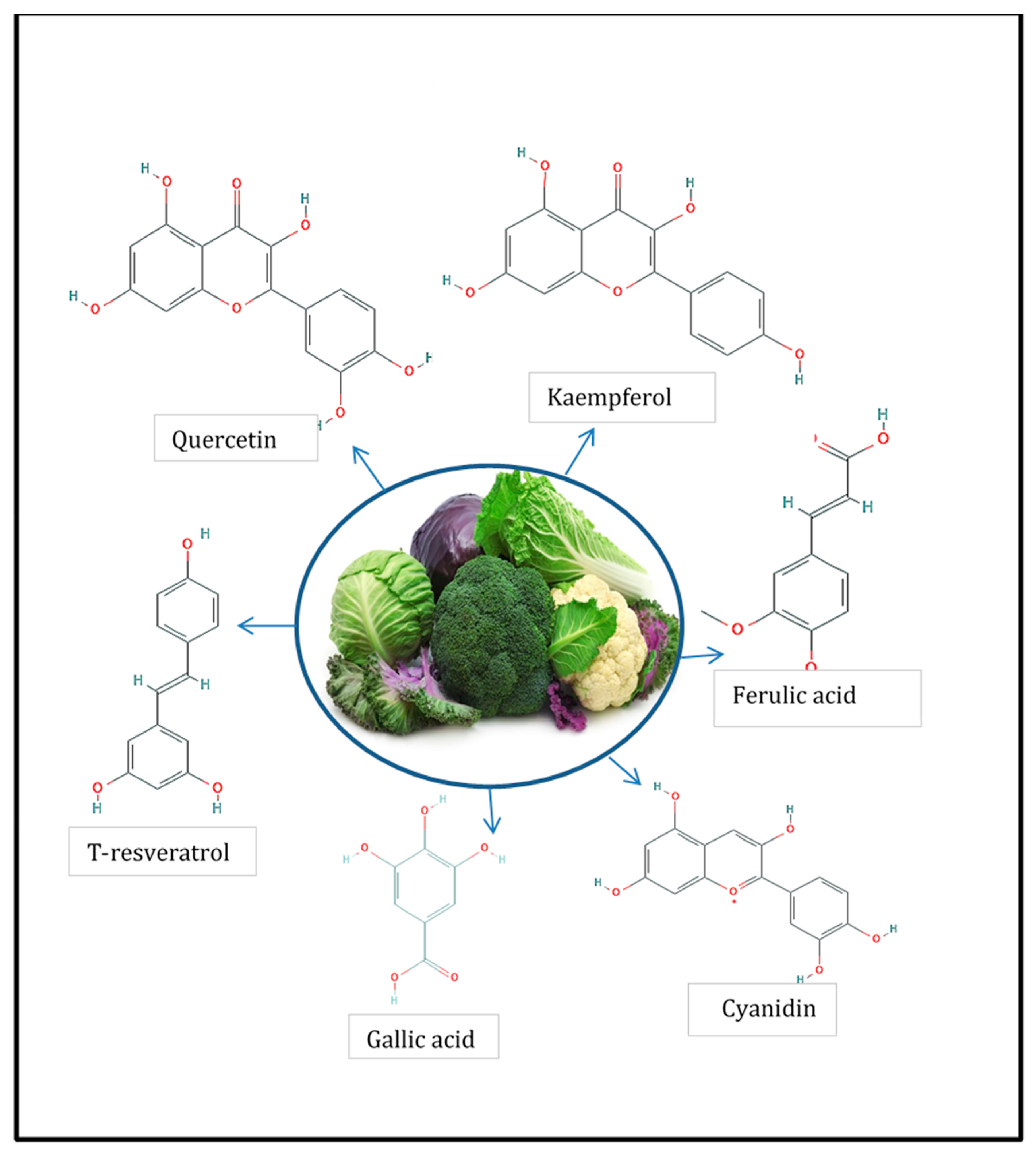
1.1. Phenolic Compounds
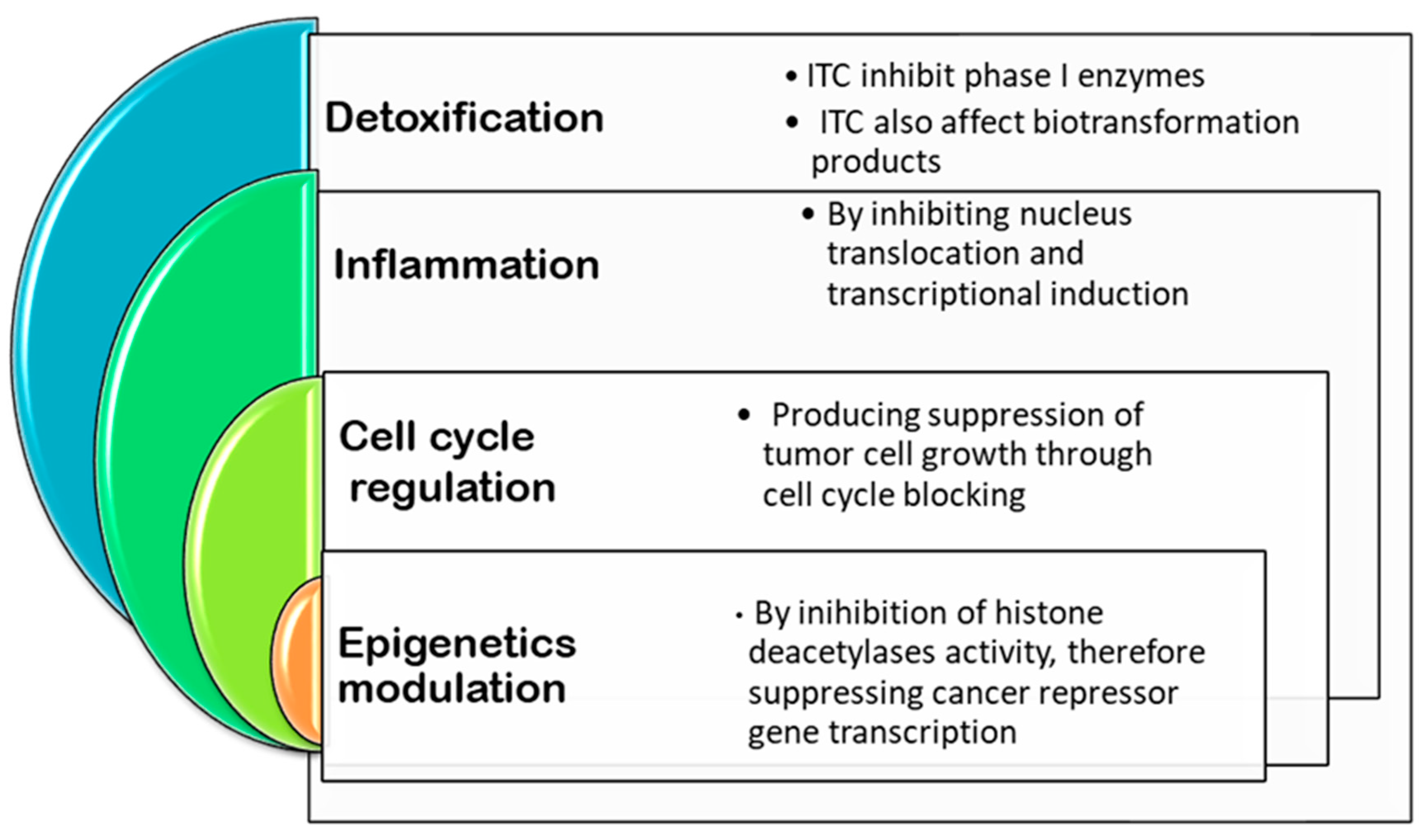
1.2. Organosulfur Compounds
1.3. Carotenoids
1.4. Other Terpenes Present in Brassicaceae Vegetables
1.5. Phytoalexins
1.6. Alkaloids
2. “Functional” Foods Based on Brassicas: Concepts and Relevance for Development of New Products
2.1. Origin of the “Functional Food” Concept
- Functional foods with an added (or enhanced) ingredient that is associated with a health benefit. Example: milk chocolate enriched with kale [77].
2.2. Functional Foods Based on Brassica Vegetables
3. Functionality: What Has Been Demonstrated and What Remains to Be Study
4. Food Products and Ingredients Enriched in Bioactives from Brassicaceae
5. Food Products and Ingredients from Brassica spp.—Certain Commercialization Aspects
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GSL | Glucosinolates |
| ITC | Isothiocyanates |
| CV | Cruciferous vegetables |
| SFN | Sulforaphane |
| GRA | Glucoraphanin |
References
- Jiménez-Morales, P.; Sánchez-León, G.; Vargas-Rincón, C. La Producción de Metabolitos Secundarios en la Familia Brassicaceae. Rev. Fac. Ciencias Básicas 2014, 9, 282. [Google Scholar]
- Branca, F.; Argento, S.; Alessandro, T. Assessing genetic reserves in Sicily (Italy): The Brassica wild relatives case study. In Agrobiodiversity Conservation: Securing the Diversity of Crop Wild Relatives and Landraces; Maxted, N., Ehsan Dulloo, M., Ford-Lloyd, B.V., Frese, L., Iriondo, J.M., Pinheiro de Carvalho, M.A.A., Eds.; CABI: Wallingford, Oxfordshire, UK, 2012; pp. 52–58. [Google Scholar]
- Pinheiro de Carvalho, M.Â. Agrobiodiversity Conservation: Securing the Diversity of Crop Wild Relatives and Landraces; CABI: Wallingford, Oxfordshire, UK, 2012. [Google Scholar]
- Branca, F.; Chiarenza, L.; Ragusa, L.; Argento, S. Morphological Characterization of the ECPGR Wild Brassica Species Collection. Acta horticulturae. Acta Hortic. 2013, 1005, 157–164. [Google Scholar] [CrossRef]
- Argento, S.; Melilli, M.G.; Branca, F. Enhancing Greenhouse Tomato-Crop Productivity by Using Brassica macrocarpa Guss. Leaves for Controlling Root-Knot Nematodes. Agronomy 2019, 9, 820. [Google Scholar] [CrossRef]
- Branca, F.; Lucia, R.; Alessandro, T.; Lo Scalzo, R.; Picchi, V.; Argento, S. The Glucosinolates and Variation of Antioxidant Compounds in Seeds and Sprouts of Broccoli (Brassica oleracea L. var. italica) and Rocket (Eruca sativa L.) in Relation to Temperature and Germinative Stage. Acta Hortic. 2013, 1005, 271–278. [Google Scholar] [CrossRef]
- Galletti, S.; Bagatta, M.; Branca, F.; Argento, S.; De Nicola, G.R.; Cianchetta, S.; Iori, R.; Ninfali, P. Isatis canescens is a rich source of glucobrassicin and other health-promoting compounds. J. Sci. Food Agric. 2015, 95, 158–164. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic Compounds in Brassica Vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef]
- Cheynier, V. Phenolic compounds: From plants to foods. Phytochem. Rev. 2012, 11, 153–177. [Google Scholar] [CrossRef]
- Todaro, A.; Cavallaro, R.; Argento, S.; Branca, F.; Spagna, G. Study and Characterization of Polyphenol Oxidase from Eggplant (Solanum melongena L.). J. Agric. Food Chem. 2011, 59, 11244–11248. [Google Scholar] [CrossRef]
- Li, Z.; Lee, H.W.; Liang, X.; Liang, D.; Wang, Q.; Huang, D.; Ong, C.N. Profiling of Phenolic Compounds and Antioxidant Activity of 12 Cruciferous Vegetables. Molecules 2018, 23, 1139. [Google Scholar] [CrossRef]
- Fusari, C.; Beretta, H.; Locatelli, D.; Nazareno, M.; Camargo, A. Seasonal isothiocyanates variation and market availability of Brassicaceae species consumed in Mendoza. Rev. Fac. Cienc. Agrar. 2019, 51, 403–408. [Google Scholar]
- Baenas, N.; Gómez-Jodar, I.; Moreno, D.A.; García-Viguera, C.; Periago, P.M. Broccoli and radish sprouts are safe and rich in bioactive phytochemicals. Postharvest Biol. Technol. 2017, 127, 60–67. [Google Scholar] [CrossRef]
- Loedolff, B.; Brooks, J.; Stander, M.; Peters, S.; Kossmann, J. High light bio-fortification stimulates de novo synthesis of resveratrol in Diplotaxis tenuifolia (wild rocket) micro-greens. Funct. Foods Health Dis. 2017, 7, 859–872. [Google Scholar] [CrossRef]
- Matera, R.; Gabbanini, S.; De Nicola, G.R.; Iori, R.; Petrillo, G.; Valgimigli, L. Identification and analysis of isothiocyanates and new acylated anthocyanins in the juice of Raphanus sativus cv. Sango sprouts. Food Chem. 2012, 133, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Moreno, D.A.; Pérez-Balibrea, S.; Ferreres, F.; Gil-Izquierdo, Á.; García-Viguera, C. Acylated anthocyanins in broccoli sprouts. Food Chem. 2010, 123, 358–363. [Google Scholar] [CrossRef]
- Lo Scalzo, R.; Genna, A.; Branca, F.; Chedin, M.; Chassaigne, H. Anthocyanin composition of cauliflower (Brassica oleracea L. var. botrytis) and cabbage (B. oleracea L. var. capitata) and its stability in relation to thermal treatments. Food Chem. 2008, 107, 136–144. [Google Scholar] [CrossRef]
- Otsuki, T.; Matsufuji, H.; Takeda, M.; Toyoda, M.; Goda, Y. Acylated anthocyanins from red radish (Raphanus sativus L.). Phytochemistry 2002, 60, 79–87. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J. Red cabbage anthocyanins: Profile, isolation, identification, and antioxidant activity. Food Res. Int. 2013, 51, 303–309. [Google Scholar] [CrossRef]
- Anjum, N.A.; Gill, S.S.; Ahmad, I.; Pacheco, M.; Duarte, A.C.; Umar, S.; Khan, N.A.; Pereira, M.E. The Plant Family Brassicaceae: An Introduction. In The Plant Family Brassicaceae: Contribution Towards Phytoremediation; Anjum, N.A., Ahmad, I., Pereira, M.E., Duarte, A.C., Umar, S., Khan, N.A., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 1–33. [Google Scholar]
- Bell, L.; Yahya, H.N.; Oloyede, O.O.; Methven, L.; Wagstaff, C. Changes in rocket salad phytochemicals within the commercial supply chain: Glucosinolates, isothiocyanates, amino acids and bacterial load increase significantly after processing. Food Chem. 2017, 221, 521–534. [Google Scholar] [CrossRef]
- Traka, M.; Mithen, R.J.P.R. Glucosinolates, isothiocyanates and human health. Phytochem. Rev. 2009, 8, 269–282. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Cartea, M.E.; Velasco, P.J.P.R. Glucosinolates in Brassica foods: Bioavailability in food and significance for human health. Phytochem. Rev. 2008, 7, 213–229. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef]
- Baenas, N.; Piegholdt, S.; Schloesser, A.; Moreno, D.A.; García-Viguera, C.; Rimbach, G.; Wagner, A.E. Metabolic Activity of Radish Sprouts Derived Isothiocyanates in Drosophila melanogaster. Int. J. Mol. Sci. 2016, 17, 251. [Google Scholar] [CrossRef] [PubMed]
- Blažević, I.; Mastelić, J. Glucosinolate degradation products and other bound and free volatiles in the leaves and roots of radish (Raphanus sativus L.). Food Chem. 2009, 113, 96–102. [Google Scholar] [CrossRef]
- Yi, G.; Lim, S.; Chae, W.B.; Park, J.E.; Park, H.R.; Lee, E.J.; Huh, J.H. Root Glucosinolate Profiles for Screening of Radish (Raphanus sativus L.) Genetic Resources. J. Agric. Food Chem. 2016, 64, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Fechner, J.; Kaufmann, M.; Herz, C.; Eisenschmidt, D.; Lamy, E.; Kroh, L.W.; Hanschen, F.S. The major glucosinolate hydrolysis product in rocket (Eruca sativa L.), sativin, is 1,3-thiazepane-2-thione: Elucidation of structure, bioactivity, and stability compared to other rocket isothiocyanates. Food Chem. 2018, 261, 57–65. [Google Scholar] [CrossRef]
- Franco, P.; Spinozzi, S.; Pagnotta, E.; Lazzeri, L.; Ugolini, L.; Camborata, C.; Roda, A. Development of a liquid chromatography–electrospray ionization–tandem mass spectrometry method for the simultaneous analysis of intact glucosinolates and isothiocyanates in Brassicaceae seeds and functional foods. J. Chromatogr. A 2016, 1428, 154–161. [Google Scholar] [CrossRef]
- Kuang, P.; Song, D.; Yuan, Q.; Yi, R.; Lv, X.; Liang, H. Separation and purification of sulforaphene from radish seeds using macroporous resin and preparative high-performance liquid chromatography. Food Chem. 2013, 136, 342–347. [Google Scholar] [CrossRef]
- Kim, J.-W.; Kim, M.-B.; Lim, S.-B. Formation and Stabilization of Raphasatin and Sulforaphene from Radish Roots by Endogenous Enzymolysis. Prev. Nutr. Food Sci. 2015, 20, 119–125. [Google Scholar] [CrossRef]
- Yu, B.; Lydiate, D.J.; Young, L.W.; Schäfer, U.A.; Hannoufa, A.J.T.R. Enhancing the carotenoid content of Brassica napus seeds by downregulating lycopene epsilon cyclase. Transgenic Res. 2008, 17, 573–585. [Google Scholar] [CrossRef]
- Frede, K.; Schreiner, M.; Baldermann, S. Light quality-induced changes of carotenoid composition in pak choi Brassica rapa ssp. chinensis. J. Photochem. Photobiol. B Biol. 2019, 193, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.; Olsson, K.; Engqvist, G.; Ekvall, J.; Olsson, M.; Nyman, M.; Åkesson, B. Variation in the content of glucosinolates, hydroxycinnamic acids, carotenoids, total antioxidant capacity and low-molecular-weight carbohydrates in Brassica vegetables. J. Sci. Food Agric. 2006, 86, 528–538. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, C.; Wang, Y.; Yao, X.; Wang, F.; Wu, J.; King, G.J.; Liu, K. Disruption of a Carotenoid Cleavage Dioxygenase 4 gene converts flower colour from white to yellow in Brassica species. New Phytol. 2015, 206, 1513–1526. [Google Scholar] [CrossRef] [PubMed]
- Guzman, I.; Yousef, G.G.; Brown, A.F. Simultaneous Extraction and Quantitation of Carotenoids, Chlorophylls, and Tocopherols in Brassica Vegetables. J. Agric. Food Chem. 2012, 60, 7238–7244. [Google Scholar] [CrossRef] [PubMed]
- Park, W.T.; Kim, J.K.; Park, S.; Lee, S.-W.; Li, X.; Kim, Y.B.; Uddin, M.R.; Park, N.I.; Kim, S.-J.; Park, S.U. Metabolic Profiling of Glucosinolates, Anthocyanins, Carotenoids, and Other Secondary Metabolites in Kohlrabi (Brassica oleracea var. gongylodes). J. Agric. Food Chem. 2012, 60, 8111–8116. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.-H.; Kim, N.-H.; Seo, M.-S.; Jin, M.; Park, S.U.; Arasu, M.V.; Kim, S.-J.; Al-Dhabi, N.A. Molecular characterization of glucosinolates and carotenoid biosynthetic genes in Chinese cabbage (Brassica rapa L. ssp. pekinensis). Saudi J. Biol. Sci. 2018, 25, 71–82. [Google Scholar] [CrossRef]
- Walsh, R.P.; Bartlett, H.; Eperjesi, F. Variation in Carotenoid Content of Kale and Other Vegetables: A Review of Pre- and Post-harvest Effects. J. Agric. Food Chem. 2015, 63, 9677–9682. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Akhtar, H.; Zaheer, K.; Ali, R. Dietary Sources of Lutein and Zeaxanthin Carotenoids and Their Role in Eye Health. Nutrients 2013, 5, 1169–1185. [Google Scholar] [CrossRef]
- Mageney, V.; Baldermann, S.; Albach, D.C. Intraspecific Variation in Carotenoids of Brassica oleracea var. sabellica. J. Agric. Food Chem. 2016, 64, 3251–3257. [Google Scholar] [CrossRef]
- Fernández-León, M.F.; Fernández-León, A.M.; Lozano, M.; Ayuso, M.C.; González-Gómez, D. Identification, quantification and comparison of the principal bioactive compounds and external quality parameters of two broccoli cultivars. J. Funct. Foods 2012, 4, 465–473. [Google Scholar] [CrossRef]
- Kaulmann, A.; André, C.M.; Schneider, Y.-J.; Hoffmann, L.; Bohn, T. Carotenoid and polyphenol bioaccessibility and cellular uptake from plum and cabbage varieties. Food Chem. 2016, 197, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Podsędek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT—Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Kurilich, A.C.; Tsau, G.J.; Brown, A.; Howard, L.; Klein, B.P.; Jeffery, E.H.; Kushad, M.; Wallig, M.A.; Juvik, J.A. Carotene, Tocopherol, and Ascorbate Contents in Subspecies of Brassica oleracea. J. Agric. Food Chem. 1999, 47, 1576–1581. [Google Scholar] [CrossRef] [PubMed]
- Dan, L.; Yi, Y.; Yongxin, L.; Chengjun, S. Analysis of Tocopherols and Tocotrienols in Pharmaceuticals and Foods: A Critical Review. Curr. Pharm. Anal. 2015, 11, 66–78. [Google Scholar] [CrossRef]
- Yu, W.; Simmons-Menchaca, M.; Gapor, A.; Sanders, B.G.; Kline, K. Induction of apoptosis in human breast cancer cells by tocopherols and tocotrienols. Nutr. Cancer 1999, 33, 26–32. [Google Scholar] [CrossRef]
- Mo, H.; Elson, C.E. Apoptosis and Cell-Cycle Arrest in Human and Murine Tumor Cells Are Initiated by Isoprenoids. J. Nutr. 1999, 129, 804–813. [Google Scholar] [CrossRef]
- Lampi, A.-M.; Kamal-Eldin, A.; Piironen, V. Tocopherols and tocotrienols from oil and cereal grains. Funct. Foods Biochem. Process. Asp. 2002, 1–38. [Google Scholar]
- Ling, W.H.; Jones, P.J.H. Dietary phytosterols: A review of metabolism, benefits and side effects. Life Sci. 1995, 57, 195–206. [Google Scholar] [CrossRef]
- Gül, M.; Amar, S. Sterols and the phytosterol content in oilseed rape (Brassica napus L.). J. Cell & Mol. Biol. 2006, 5, 71–79. [Google Scholar]
- Sharma, A.; Rai, P.K.; Prasad, S. GC–MS detection and determination of major volatile compounds in Brassica juncea L. leaves and seeds. Microchem. J. 2018, 138, 488–493. [Google Scholar] [CrossRef]
- Ahuja, I.; Kissen, R.; Bones, A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012, 17, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.P.; Sattely, E.S. Biosynthesis of cabbage phytoalexins from indole glucosinolate. Proc. Natl. Acad. Sci. USA 2017, 114, 1910–1915. [Google Scholar] [CrossRef] [PubMed]
- Pedras, M.S.C.; Zheng, Q.-A.; Gadagi, R.S.; Rimmer, S.R. Phytoalexins and polar metabolites from the oilseeds canola and rapeseed: Differential metabolic responses to the biotroph Albugo candida and to abiotic stress. Phytochemistry 2008, 69, 894–910. [Google Scholar] [CrossRef] [PubMed]
- Pedras, M.S.C.; Adio, A.M.; Suchy, M.; Okinyo, D.P.O.; Zheng, Q.-A.; Jha, M.; Sarwar, M.G. Detection, characterization and identification of crucifer phytoalexins using high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry. J. Chromatogr. A 2006, 1133, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Pedras, M.S.C.; Ahiahonu, P.W.K. Metabolism and detoxification of phytoalexins and analogs by phytopathogenic fungi. Phytochemistry 2005, 66, 391–411. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Montaut, S.; Zaharia, I.L.; Gai, Y.; Ward, D.E. Transformation of the host-selective toxin destruxin B by wild crucifers: Probing a detoxification pathway. Phytochemistry 2003, 64, 957–963. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Sorensen, J.L.; Okanga, F.I.; Zaharia, I.L. Wasalexins A and B, new phytoalexins from wasabi: Isolation, synthesis, and antifungal activity. Bioorganic Med. Chem. Lett. 1999, 9, 3015–3020. [Google Scholar] [CrossRef]
- Monde, K.; Takasugi, M.; Shirata, A. Three sulphur-containing stress metabolites from Japanese radish. Phytochemistry 1995, 39, 581–586. [Google Scholar] [CrossRef]
- Nee, M. Plant Alkaloids: A Guide to Their Discovery and Distribution; Raffauf, R.F., Ed.; Brittonia: New York, NY, USA, 1998; Volume 50, p. 55. [Google Scholar]
- Guriya, R.; Moon, A.; Talreja, K. Phytochemical profiling and characterization of bioactive compounds from Brassica oleracea. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 825–831. [Google Scholar]
- Khalid, A.; Mohammed, A.D.; Al-Maliki, M. Effect of phenolic and alkaloid compounds extracted from Brassica oleracea var. capitata seed on glucose level in blood of alloxan- induced diabetes rabbits. World J. Exp. Biosci. 2014, 2, 24–29. [Google Scholar]
- Yannai, S. Dictionary of Food; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Shah, M.A.; Sarker, M.M.R.; Gousuddin, M. Antidiabetic Potential of Brassica oleracea var. italica in Type 2 Diabetic Sprague Dawley (sd) Rats. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 462–469. [Google Scholar]
- Chauhan, E.S.T.; Tiwari, A.; Singh, A. Phytochemical screening of red cabbage (Brassica oleracea) powder and juice—A comparative study. J. Med. Plants Stud. 2016, 4, 196–199. [Google Scholar]
- Brock, A.; Herzfeld, T.; Paschke, R.; Koch, M.; Dräger, B. Brassicaceae contain nortropane alkaloids. Phytochemistry 2006, 67, 2050–2057. [Google Scholar] [CrossRef] [PubMed]
- Villaño, D.; Gironés-Vilapana, A.; García-Viguera, C.; Moreno, D.A. Development of Functional Foods. In Innovation Strategies in the Food Industry; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2016; Chapter 10; pp. 191–210. [Google Scholar] [CrossRef]
- Serafini, M.; Stanzione, A.; Foddai, S. Functional foods: Traditional use and European legislation. Int. J. Food Sci. Nutr. 2012, 63, 7–9. [Google Scholar] [CrossRef]
- Bagchi, D. Nutraceutical and Functional Food Regulations in the United States and Around the World. In Nutraceutical and Functional Food Regulations in the United States and Around the World; Bagchi, D., Ed.; Academic Press: San Diego, CA, USA, 2008; pp. ix–xii. [Google Scholar] [CrossRef]
- Powers, J.-P.; Farrell, M.; McMullin, C.; Retik, L.; White, J. Regulation of dietary supplements and functional foods in Canada. In Nutraceutical and Functional Food Regulations in the United States and around the World (Third Edition); Bagchi, D., Ed.; Academic Press: San Diego, CA, USA, 2019; Chapter 17; pp. 235–252. [Google Scholar] [CrossRef]
- Baenas, N.; Abellán, Á.; Rivera, S.; Moreno, D.A.; García-Viguera, C.; Domínguez-Perles, R. Foods and Suplements. In Polyphenols: Properties, Recovery and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 327–362. [Google Scholar]
- Bigliardi, B.; Galati, F. Innovation trends in the food industry: The case of functional foods. Trends Food Sci. Technol. 2013, 31, 118–129. [Google Scholar] [CrossRef]
- Poli, A.; Barbagallo, C.M.; Cicero, A.F.G.; Corsini, A.; Manzato, E.; Trimarco, B.; Bernini, F.; Visioli, F.; Bianchi, A.; Canzone, G.; et al. Nutraceuticals and functional foods for the control of plasma cholesterol levels. An intersociety position paper. Pharmacol. Res. 2018, 134, 51–60. [Google Scholar] [CrossRef]
- Fuentes-Alventosa, J.M. Caracterización de Componentes Bioactivos del Espágarrago Verde: Obtención de Ingredientes Funcionales a Partir de Los Subproductos Generados Durante su Transformación Industrial; University of Cordoba: Cordoba, Spain, 2010. [Google Scholar]
- Carvalho, J.C.S.; Romoff, P.; Lannes, S.C.d.S. Improvement of nutritional and physicochemical proprieties of milk chocolates enriched with kale (Brassica olereacea var. acephala) and grape (Vitis vinífera). Food Sci. Technol. 2018, 38, 551–560. [Google Scholar] [CrossRef]
- Ouraji, M.; Alimi, M.; Motamedzadegan, A.; Shokoohi, S. Faba bean protein in reduced fat/cholesterol mayonnaise: Extraction and physico-chemical modification process. J. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Zanoni, F.; Primiterra, M.; Angeli, N.; Zoccatelli, G. Microencapsulation by spray-drying of polyphenols extracted from red chicory and red cabbage: Effects on stability and color properties. Food Chem. 2020, 307, 125535. [Google Scholar] [CrossRef]
- Singh, N.; Aditika; Rani, S.; Chaurasia, O.P. Vegetable Microgreens Farming in High-Altitude Region of Trans-Himalayas to Maintain Nutritional Diet of Indian Troops. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019. [Google Scholar] [CrossRef]
- Granado-Lorencio, F.; Herrero-Barbudo, C.; Acién-Fernández, G.; Molina-Grima, E.; Fernández-Sevilla, J.M.; Pérez-Sacristán, B.; Blanco-Navarro, I. In vitro bioaccesibility of lutein and zeaxanthin from the microalgae Scenedesmus almeriensis. Food Chem. 2009, 114, 747–752. [Google Scholar] [CrossRef]
- Baenas, N.; Fusari, C.; Moreno, D.A.; Valero, D.; García-Viguera, C. Biostimulation of bioactive compounds in radish sprouts (Raphanus sativus ‘Rambo’) by priming seeds and spray treatments with elicitors. ISHS Acta Hortic. 2017, 659–663. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Ser, S.L.; Cumming, J.R.; Ku, K.-M. Comparative Phytonutrient Analysis of Broccoli By-Products: The Potentials for Broccoli By-Product Utilization. Molecules 2018, 23, 900. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Marhuenda, J.; García-Viguera, C.; Zafrilla, P.; Moreno, D.A. Influence of Cooking Methods on Glucosinolates and Isothiocyanates Content in Novel Cruciferous Foods. Foods 2019, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Villaño, D.; García-Viguera, C.; Moreno, D.A. Optimizing elicitation and seed priming to enrich broccoli and radish sprouts in glucosinolates. Food Chem. 2016, 204, 314–319. [Google Scholar] [CrossRef]
- Zang, Y.X.; Ge, J.L.; Huang, L.H.; Gao, F.; Lv, X.S.; Zheng, W.-W.; Hong, S.-B.; Zhu, Z.-J. Leaf and root glucosinolate profiles of Chinese cabbage (Brassica rapa ssp. pekinensis) as a systemic response to methyl jasmonate and salicylic acid elicitation. J. Zhejiang Univ. Sci. B 2015, 16, 696–708. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Drabińska, N.; Rosell, C.M.; Fadda, C.; Anders, A.; Jeliński, T.; Ostaszyk, A. Broccoli leaf powder as an attractive by-product ingredient: Effect on batter behaviour, technological properties and sensory quality of gluten-free mini sponge cake. Food Sci. Tehnol. 2019, 54, 1121–1129. [Google Scholar] [CrossRef]
- Liang, J.L.; Yeow, C.C.; Teo, K.C.; Gnanaraj, C.; Chang, Y.P. Valorizing cabbage (Brassica oleracea L. var. capitata) and capsicum (Capsicum annuum L.) wastes: In vitro health-promoting activities. J. Food Sci. Technol. 2019, 56, 4696–4704. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Cardoso, S.M.; Wessel, D.F.; Coimbra, M.A. Microwave assisted dehydration of broccoli by-products and simultaneous extraction of bioactive compounds. Food Chem. 2018, 246, 386–393. [Google Scholar] [CrossRef]
- Yang, Y. Scientific Substantiation of Functional Food Health Claims in China. J. Nutr. 2008, 138, 1199S–1205S. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Valverde, J.; Kehoe, K.; Reilly, K.; Rai, D.K.; Barry-Ryan, C. Development of a Novel Functional Soup Rich in Bioactive Sulforaphane Using Broccoli (Brassica oleracea L. ssp. italica) Florets and Byproducts. Food Bioprocess Technol. 2014, 7, 1310–1321. [Google Scholar] [CrossRef]
- Masci, A.; Mattioli, R.; Costantino, P.; Baima, S.; Morelli, G.; Punzi, P.; Giordano, C.; Pinto, A.; Donini, L.M.; d’Erme, M.; et al. Neuroprotective Effect of Brassica oleracea Sprouts Crude Juice in a Cellular Model of Alzheimer’s Disease. Oxid. Med. Cell Longev. 2015, 2015, 781938. [Google Scholar] [CrossRef] [PubMed]
- Saban Guler, M.; Sanlier, N. The Benefits of Brassica Vegetables on Human Health. J. Hum. Heal. Res. 2012, 1, 104. [Google Scholar]
- Brandi, G.; Amagliani, G.; Schiavano, G.F.; De Santi, M.; Sisti, M. Activity of Brassica oleracea Leaf Juice on Foodborne Pathogenic Bacteria. J. Food Prot. 2006, 69, 2274–2279. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.K.; Mithen, R.; Johnson, I.T. Effects of Brassica vegetable juice on the induction of apoptosis and aberrant crypt foci in rat colonic mucosal crypts in vivo. Carcinogenesis 2003, 24, 491–495. [Google Scholar] [CrossRef][Green Version]
- McNaughton, S.A.; Marks, G.C. Development of a food composition database for the estimation of dietary intakes of glucosinolates, the biologically active constituents of cruciferous vegetables. Br. J. Nutr. 2003, 90, 687–697. [Google Scholar] [CrossRef]
- Herron, J.D.; Nurrenbern, S.C. Chemical Education Research: Improving Chemistry Learning. J. Chem. Educ. 1999, 76, 1353. [Google Scholar] [CrossRef]
- Van Poppel, G.; Verhagen, D.T.; Verhagen, H.; Goldbohm, R.A. Brassica vegetables and cancer prevention: Epidemiology and mechanisms. Adv. Exp. Med. Biol. 2000, 472, 159–168. [Google Scholar]
- Mitsiogianni, M.; Koutsidis, G.; Mavroudis, N.; Trafalis, D.T.; Botaitis, S.; Franco, R.; Zoumpourlis, V.; Amery, T.; Galanis, A.; Pappa, A.; et al. The Role of Isothiocyanates as Cancer Chemo-Preventive, Chemo-Therapeutic and Anti-Melanoma Agents. Antioxidants 2019, 8, 106. [Google Scholar] [CrossRef]
- Hu, J.; Hu, Y.; Hu, Y.; Zheng, S. Intake of cruciferous vegetables is associated with reduced risk of ovarian cancer: A meta-analysis. Asia Pac. J. Clin. Nutr. 2015, 24, 101–109. [Google Scholar]
- Tse, G.; Eslick, G.D. Cruciferous Vegetables and Risk of Colorectal Neoplasms: A Systematic Review and Meta-Analysis. Nutr. Cancer 2014, 66, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Mao, Q.; Wang, X.; Zhou, F.; Luo, J.; Wang, C.; Lin, Y.; Zheng, X.; Xie, L. Cruciferous Vegetables Consumption and Risk of Renal Cell Carcinoma: A Meta-Analysis. Nutr. Cancer 2013, 65, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Y.; Luo, Y.; Lu, M.D.; Xu, X.W.; Lin, H.D.; Zheng, Z.Q. Cruciferous vegetable consumption and the risk of pancreatic cancer: A meta-analysis. World J. Surg. Oncol. 2015, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Abbaoui, B.; Lucas, C.R.; Riedl, K.M.; Clinton, S.K.; Mortazavi, A. Cruciferous Vegetables, Isothiocyanates, and Bladder Cancer Prevention. Mol. Nutr. Food Res. 2018, 62, e1800079. [Google Scholar] [CrossRef]
- Jia, X.; Zhong, L.; Song, Y.; Hu, Y.; Wang, G.; Sun, S. Consumption of citrus and cruciferous vegetables with incident type 2 diabetes mellitus based on a meta-analysis of prospective study. Prim. Care Diabetes 2016, 10, 272–280. [Google Scholar] [CrossRef]
- Zhang, Z.; Bergan, R.; Shannon, J.; Slatore, C.G.; Bobe, G.; Takata, Y. The Role of Cruciferous Vegetables and Isothiocyanates for Lung Cancer Prevention: Current Status, Challenges, and Future Research Directions. Mol. Nutr. Food Res. 2018, 62, 1700936. [Google Scholar] [CrossRef]
- Zhang, N.-Q.; Ho, S.C.; Mo, X.-F.; Lin, F.-Y.; Huang, W.-Q.; Luo, H.; Huang, J.; Zhang, C.-X. Glucosinolate and isothiocyanate intakes are inversely associated with breast cancer risk: A case–control study in China. Br. J. Nutr. 2018, 119, 957–964. [Google Scholar] [CrossRef]
- Wu, Q.J.; Xie, L.; Zheng, W.; Vogtmann, E.; Li, H.L.; Yang, G.; Ji, B.T.; Gao, Y.T.; Shu, X.O.; Xiang, Y.B. Cruciferous vegetables consumption and the risk of female lung cancer: A prospective study and a meta-analysis. Ann. Oncol. 2013, 24, 1918–1924. [Google Scholar] [CrossRef]
- Johnson, I.T. Cruciferous Vegetables and Risk of Cancers of the Gastrointestinal Tract. Mol. Nutr. Food Res. 2018, 62, 1701000. [Google Scholar] [CrossRef]
- Gianfredi, V.; Vannini, S.; Moretti, M.; Villarini, M.; Bragazzi, N.L.; Izzotti, A.; Nucci, D. Sulforaphane and Epigallocatechin Gallate Restore Estrogen Receptor Expression by Modulating Epigenetic Events in the Breast Cancer Cell Line MDA-MB-231: A Systematic Review and Meta-Analysis. Lifestyle Genom. 2017, 10, 126–135. [Google Scholar] [CrossRef]
- Lam, T.K.; Gallicchio, L.; Lindsley, K.; Shiels, M.; Hammond, E.; Tao, X.G.; Chen, L.; Robinson, K.A.; Caulfield, L.E.; Herman, J.G.; et al. Cruciferous vegetable consumption and lung cancer risk: A systematic review. Cancer Epidemiol. Biomark. Prev. 2009, 18, 184–195. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC); World Health Organization. All Cancers Data Sheet; International Agency for Research on Cancer: Lyon, France, 2018; Volume 876. [Google Scholar]
- Murillo, G.; Mehta, R.G. Cruciferous vegetables and cancer prevention. Nutr. Cancer 2014, 41, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Gründemann, C.; Huber, R. Chemoprevention with isothiocyanates—From bench to bedside. Cancer Lett. 2018, 414, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Kelleher, M.O.; Eggleston, I.M. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur. J. Nutr. 2008, 47, 73–88. [Google Scholar] [CrossRef]
- Cox, D.N.; Poelman, A.A.M. Towards greater vegetable consumption: Change the product or change the person? Case studies of two vegetable commodities. Food Res. Int. 2015, 69, 348–356. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Martínez-Ballesta, M.C.; Carvajal, M.; García-Viguera, C.; Moreno, D.A. Broccoli-Derived By-Products—A Promising Source of Bioactive Ingredients. J. Food Sci. 2010, 75, C383–C392. [Google Scholar] [CrossRef]
- Drabińska, N.; Ciska, E.; Szmatowicz, B.; Krupa-Kozak, U. Broccoli by-products improve the nutraceutical potential of gluten-free mini sponge cakes. Food Chem. 2018, 267, 170–177. [Google Scholar] [CrossRef]
- Klopsch, R.; Baldermann, S.; Voss, A.; Rohn, S.; Schreiner, M.; Neugart, S. Narrow-Banded UVB Affects the Stability of Secondary Plant Metabolites in Kale (Brassica oleracea var. sabellica) and Pea (Pisum sativum) Leaves Being Added to Lentil Flour Fortified Bread: A Novel Approach for Producing Functional Foods. Foods 2019, 8, 427. [Google Scholar] [CrossRef]
- Heo, Y.; Kim, M.-J.; Lee, J.-W.; Moon, B. Muffins enriched with dietary fiber from kimchi by-product: Baking properties, physical–chemical properties, and consumer acceptance. Food Sci. Nutr. 2019, 7, 1778–1785. [Google Scholar] [CrossRef]
- Ye, J.-H.; Huang, L.-Y.; Terefe, N.S.; Augustin, M.A. Fermentation-based biotransformation of glucosinolates, phenolics and sugars in retorted broccoli puree by lactic acid bacteria. Food Chem. 2019, 286, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.X.; Wang, J.H.; McAuley, C.; Augustin, M.A.; Terefe, N.S. Fermentation for enhancing the bioconversion of glucoraphanin into sulforaphane and improve the functional attributes of broccoli puree. J. Funct. Foods 2019, 61, 103461. [Google Scholar] [CrossRef]
- Lafarga, T.; Acién-Fernández, F.G.; Castellari, M.; Villaró, S.; Bobo, G.; Aguiló-Aguayo, I. Effect of microalgae incorporation on the physicochemical, nutritional, and sensorial properties of an innovative broccoli soup. LWT 2019, 111, 167–174. [Google Scholar] [CrossRef]
- Ashfaq, F.; Butt, M.S.; Bilal, A.; Tehseen, S.; Suleria, H.A.R. Effect of cabbage or its aqueous extract incorporated croquettes on chemical composition and storage stability in relation to antioxidant potential and sensory profile. J. Food Process. Preserv. 2020, 44, e14291. [Google Scholar] [CrossRef]
- Song, G.-H.; Park, E.-S.; Lee, S.-M.; Park, D.-B.; Park, K.-Y. Beneficial Outcomes of Kimchi Prepared with Amtak Baechu Cabbage and Salting in Brine Solution: Anticancer Effects in Pancreatic and Hepatic Cancer Cells. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, T.; Gallagher, E.; Bademunt, A.; Bobo, G.; Echeverria, G.; Viñas, I.; Aguiló-Aguayo, I. Physiochemical and nutritional characteristics, bioaccessibility and sensory acceptance of baked crackers containing broccoli co-products. Int. J. Food Sci. Technol. 2019, 54, 634–640. [Google Scholar] [CrossRef]
- Shi, M.; Ying, D.-Y.; Hlaing, M.M.; Ye, J.-H.; Sanguansri, L.; Augustin, M.A. Development of broccoli by-products as carriers for delivering EGCG. Food Chem. 2019, 301, 125301. [Google Scholar] [CrossRef]
- Prokopov, T.; Goranova, Z.; Baeva, M.; Slavov, A.; Galanakis, C.M. Effects of powder from white cabbage outer leaves on sponge cake quality. Int. Agrophys. 2015, 29, 493–500. [Google Scholar] [CrossRef]
- García-Saldaña, J.S.; Campas-Baypoli, O.N.; López-Cervantes, J.; Sánchez-Machado, D.I.; Cantú-Soto, E.U.; Rodríguez-Ramírez, R. Microencapsulation of sulforaphane from broccoli seed extracts by gelatin/gum arabic and gelatin/pectin complexes. Food Chem. 2016, 201, 94–100. [Google Scholar] [CrossRef]
- Sánchez, F.M.; García, F.; Calvo, P.; Bernalte, M.J.; González-Gómez, D. Optimization of broccoli microencapsulation process by complex coacervation using response surface methodology. Innov. Food Sci. Emerg. Technol. 2016, 34, 243–249. [Google Scholar] [CrossRef]
- Soler-Rivas, C.; Marín, F.R.; Santoyo, S.; García-Risco, M.R.; Señoráns, F.J.; Reglero, G. Testing and Enhancing the in Vitro Bioaccessibility and Bioavailability of Rosmarinus officinalis Extracts with a High Level of Antioxidant Abietanes. J. Agric. Food Chem. 2010, 58, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Riar, H.; Khatkar, S.; Khatkar, A.; Arora, N.; Mann, S.; Panghal, A.; Kumar, S. The conceptual understanding of nutrikinetics: A futuristic approach for designing health foods. Nutr. Food Sci. 2019. [Google Scholar] [CrossRef]
- Klug, T.V.; Martínez-Hernández, G.B.; Collado, E.; Artés, F.; Artés-Hernández, F. Effect of Microwave and High-Pressure Processing on Quality of an Innovative Broccoli Hummus. Food Bioprocess Technol. 2018, 11, 1464–1477. [Google Scholar] [CrossRef]
- Valenzuela, B.A.; Valenzuela, R.; Sanhueza, J.; Morales, I.G. Alimentos funcionales, nutraceúticos y foshu: ¿Vamos hacia un nuevo concepto de alimentación? Revista chilena de nutrición. Rev. Chil. Nutr. 2014, 41, 198–204. [Google Scholar] [CrossRef][Green Version]
- Cai, Y.X.; Augustin, M.A.; Jegasothy, H.; Wang, J.H.; Terefe, N.S. Mild heat combined with lactic acid fermentation: A novel approach for enhancing sulforaphane yield in broccoli puree. Food Funct. 2020, 11, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Mazzucotelli, C.A.; González-Aguilar, G.A.; Villegas-Ochoa, M.A.; Domínguez-Avila, A.J.; Ansorena, M.R.; Di Scala, K.C. Chemical characterization and functional properties of selected leafy vegetables for innovative mixed salads. J. Food Biochem. 2018, 42, e12461. [Google Scholar] [CrossRef]
- Barakat, H.; Reim, V.; Rohn, S. Stability of saponins from chickpea, soy and faba beans in vegetarian, broccoli-based bars subjected to different cooking techniques. Food Res. Int. 2015, 76, 142–149. [Google Scholar] [CrossRef]
- Atwell, L.L.; Hsu, A.; Wong, C.P.; Stevens, J.F.; Bella, D.; Yu, T.-W.; Pereira, C.B.; Löhr, C.V.; Christensen, J.M.; Dashwood, R.H.; et al. Absorption and chemopreventive targets of sulforaphane in humans following consumption of broccoli sprouts or a myrosinase-treated broccoli sprout extract. Mol. Nutr. Food Res. 2015, 59, 424–433. [Google Scholar] [CrossRef]

| Functional Property Addressed in the Meta-Analysis/Health Claim | Bioactive Compounds/Vegetables/Ingredients to Which the Bioactivity Is Attributed | Reference |
|---|---|---|
| Chemoprevention of melanoma | Isothiocyanates | [99] |
| May reduce ovarian cancer | Cruciferous vegetables | [100] |
| Protect against colon cancer | Cruciferous vegetables | [101] |
| May decrease risk of renal cancer | Cruciferous vegetables | [102] |
| Might be inversely associated with pancreatic cancer | Cruciferous vegetables | [103] |
| Chemoprevention activities against bladder cancer | Isothiocyanates | [104] |
| Inversely associated with type 2 diabetes | Cruciferous vegetables | [105] |
| Inversely associated with lung cancer | Cruciferous vegetables | [106] |
| Inversely associated with breast cancer | Glucosinolates (GSL) and isothiocyanates (ITC) | [107] |
| Decreased risk of renal cell carcinoma | Cruciferous vegetables | [102] |
| May reduce risk of developing lung cancer in females | Cruciferous vegetables | [108] |
| Decreased risk of developing colorectal and gastric cancer | Cruciferous vegetables | [109] |
| Chemoprevention of breast cancer | Sulforaphane (SFN)/Epicathechin Gallate | [110] |
| Weakly and inversely associated with lung cancer | Cruciferous vegetables | [111] |
| Functional Food | Nutrients and Bioactive Compounds | Effects of Processing on Bioactive Compounds | References |
|---|---|---|---|
| Juice from Broccoli sprouts (Brassica oleracea L. var. botrytis subvar. cymosa) | SFN and Glucoraphanin (GRA) | Less amount of SFN present than expected from GRA dosage Lost of GLS/ITC during processing | [88] |
| Lentil flour fortified bread with addition of kale (Brassica oleracea var. sabellica) and pea leaves | Carotenoids, Chlorophylls Flavonoid glycosides Hydroxycinnamic acid derivatives | Less quantities of carotenoids and chlorophylls. Formation of derivatives (pheophytins) Losses of flavonoid glycosides and hydroxycinnamic acid derivatives | [119] |
| Muffins enriched with dietary fiber from kimchi byproducts | Dietary fiber | Enhanced of antioxidant capacity by adding kimchi fiber Decrease of color, height, and volume of the muffins Increased of hardness due to the weakening of gluten | [120] |
| Milk chocolate enriched with kale (Brassica oleracea var. acephala) and grapes | Phenolic compounds Dietary fiber Minerals | Some phenolic compounds transferred from kale or grapes to milk chocolate. However, the antiradical activity was not increased Enhanced total amount of fiber and minerals due to the addition of kale powder | [77] |
| Broccoli puree inoculated with lactic acid | Phenolic compounds GLS | Phytochemical total content was enhanced due to the fermentation of the lactic bacteria. The total content of sugar was lower than original broccoli puree | [121] |
| Broccoli puree inoculated with lactic acid | GRA SFN-nitrile | Improved stability of SFN Improved antioxidant capacity Preferential formation of SFN-nitrile (less potential as inducer of phase II detoxification enzymes than SFN) instead of SFN. | [122] |
| Broccoli soup with microalgae addition | Phenolic compounds | Improved antioxidant capacity due to the incorporation of the microalgae rich in bioaccessible phenolic compounds Preferential formation of SFN-nitrile instead of SFN. | [123] |
| Croquets with addition of red and green cabbage aqueous extract | Phenolic compounds | Improved antioxidant activity, better in croquets with green cabbage than croquets with red cabbage Organoleptic analysis indicates acceptability for consumers | [124] |
| “Kimchi” Prepared with Amtap Baechu Cabbage salted in Brine Solution | Gluconasturtin β-carotene, Pyropheophorbide A | Increase of the anticancer effect of “kimchi” | [125] |
| Baked crackers with addition of broccoli byproducts | Phenolic compounds Dietary fiber GLS | Improved antioxidant capacity Organoleptic properties unaffected by elaboration | [126] |
| Puree and juice made with Broccoli by-products (powder) | Epigallocatechin gallate | Improved antioxidant anticancer and anti-inflammatory activity by increased Epigallocatechin-gallate in puree Juices is not an optimal carrier of Epigallocatechin-gallate | [127] |
| Sponge cake with substitution of white flour (10% and 20%) by White Cabbage byproduct powder | Total dietary fiber | Increase of dietary fiber Decrease of total quantity of fat and carbohydrates Slight but acceptable decrease of organoleptic properties | [128] |
| Microencapsulation of polyphenols extracted from red chicory and red cabbage (nutraceutical) | Phenolic compounds | Stabilization of pH-dependent light-absorption properties of polyphenols Improvement of the thermal stability of polyphenols, mostly from red cabbage | [79] |
| Microencapsulated SFN from broccoli seed extracts (nutraceutical) | SFN | Powdered complex from Arabic gum and gelatin for encapsulating SFN from broccoli seeds. | [129] |
| Microencapsulated of Broccoli ingredient (nutraceutical) | Phenolic compounds Chlorophylls Carotenoids | Powdered complex obtained by coacervation for the stability of chlorophylls content Odor masking effects to improved acceptability for consumer | [130] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez, D.; Abellán-Victorio, A.; Beretta, V.; Camargo, A.; Moreno, D.A. Functional Ingredients From Brassicaceae Species: Overview and Perspectives. Int. J. Mol. Sci. 2020, 21, 1998. https://doi.org/10.3390/ijms21061998
Ramirez D, Abellán-Victorio A, Beretta V, Camargo A, Moreno DA. Functional Ingredients From Brassicaceae Species: Overview and Perspectives. International Journal of Molecular Sciences. 2020; 21(6):1998. https://doi.org/10.3390/ijms21061998
Chicago/Turabian StyleRamirez, Daniela, Angel Abellán-Victorio, Vanesa Beretta, Alejandra Camargo, and Diego A. Moreno. 2020. "Functional Ingredients From Brassicaceae Species: Overview and Perspectives" International Journal of Molecular Sciences 21, no. 6: 1998. https://doi.org/10.3390/ijms21061998
APA StyleRamirez, D., Abellán-Victorio, A., Beretta, V., Camargo, A., & Moreno, D. A. (2020). Functional Ingredients From Brassicaceae Species: Overview and Perspectives. International Journal of Molecular Sciences, 21(6), 1998. https://doi.org/10.3390/ijms21061998