Pic-Producing Escherichia coli Induces High Production of Proinflammatory Mediators by the Host Leading to Death by Sepsis
Abstract
:1. Introduction
2. Results
2.1. Pic-Producing E. coli (F5) Induces Lethal Sepsis in Mice
2.2. Pic is Essential for the Permanence of the Bacteria in the Bloodstream
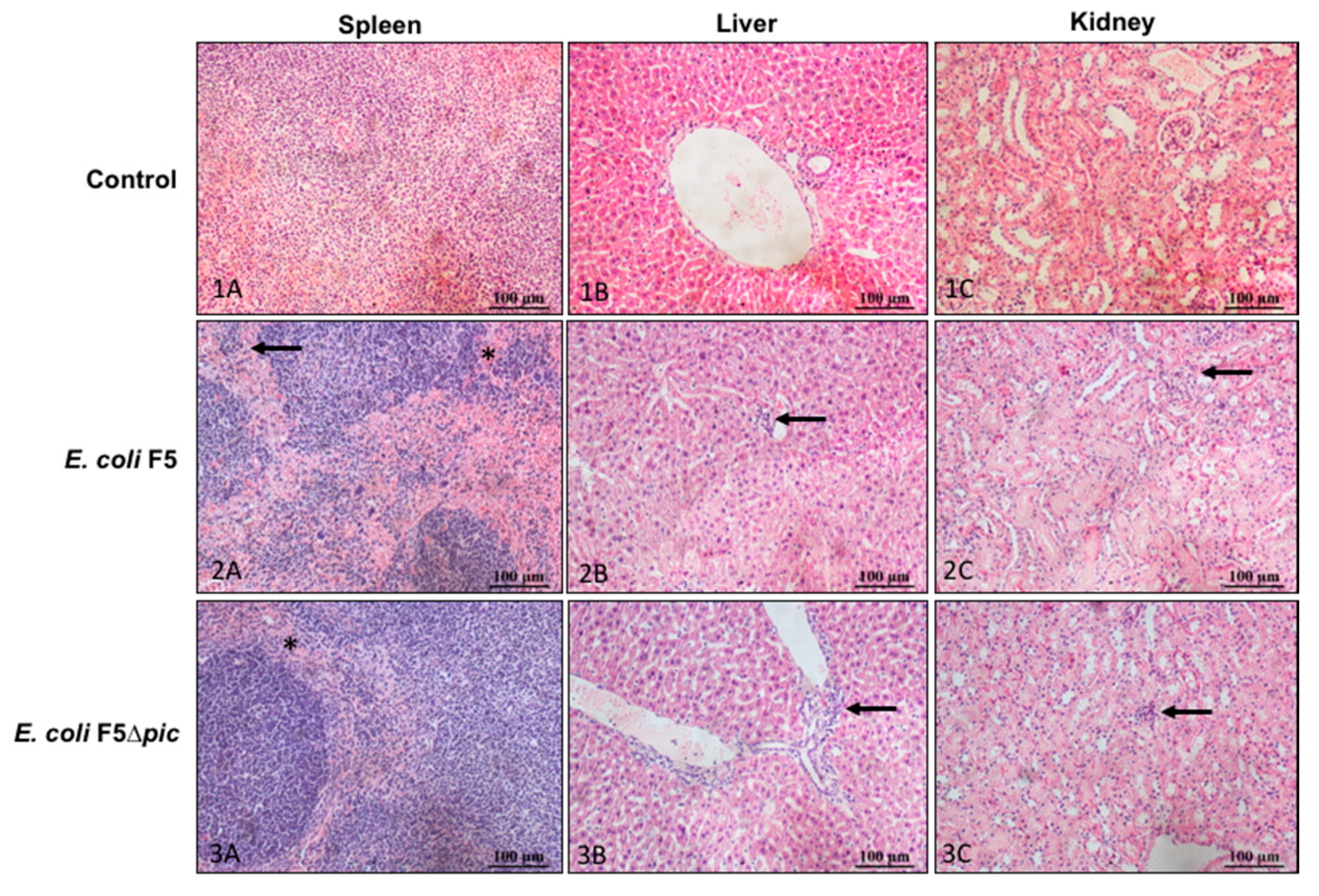
2.3. Infection Induces Histological Changes in the Organs of Animals
2.4. Pic-Producing E. coli Promotes Decrease of leukocytes in the Blood, but Does Not Induce Alterations in the Leukocytes Total Number in Bone Marrow Cells, Spleen, and Peritoneal Cavity
2.5. E. coli F5 Compromises the Expression of Costimulatory Molecules and Affects the Expression of iNOS by Leukocytes in the Peritoneal Cavity
2.6. The Expression of Costimulatory Molecules in CD4 T Lymphocytes in the Spleen is Compromised
2.7. Pic-Producing E. coli Promotes Exacerbated Increase of Inflammatory Mediators in Serum and Peritoneum
3. Discussion
4. Material and Methods
4.1. Bacterial Samples
4.2. Animals
4.3. Bacteria Intraperitoneal Inoculation
4.4. Experimental Design
4.5. Blood Acquisition
4.6. Peritoneal Cell Harvesting
4.7. Colony Forming Units (CFU) Determination
4.8. Histopathological Evaluation
4.9. Hydrogen Peroxide Production
4.10. Counting of Spleen and Bone Marrow Cells
4.11. Immunophenotyping of Spleen and Peritoneum Cells
4.12. Determination of Nitric Oxide (NO) Production
4.13. Quantification of Cytokines
4.14. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Appendix A

References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Janeway, J.R.C. Innate immune recognition: Mechanisms and pathways. Immunol Rev. 2000, 173, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Lupu, F.; Keshari, R.S.; Lambris, J.D.; Coggeshall, K.M. Crosstalk between the coagulation and complement systems in sepsis. Thromb Res. 2014, 133, 28–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Poll, T.; Van de Veerdonk, F.L.; Scicluna, B.P.; Netea, M.G. The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 2017, 17, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Minasyan, H. Sepsis and septic shock: Pathogenesis and treatment perspectives. J. Crit. Care. 2017, 40, 229–242. [Google Scholar] [CrossRef]
- Rulim, A.L.L.; Rulim, M.A.B.; Rolim Neto, M.L.; Pita, P.; Batista, I.L. Sepsis Epidemiology: Settings and Contexts. Int. Arch. Med. 2017, 10, 155. [Google Scholar] [CrossRef]
- Machado, F.R.; Mazza, B.F. Improving mortality in sepsis: Analysis of clinical trials. Shock 2010, 4, 54–58. [Google Scholar] [CrossRef] [Green Version]
- Mayr, F.B.; Yende, S.; Angus, S. Epidemiology of severe sepsis. Virulence 2014, 5, 4–11. [Google Scholar] [CrossRef] [Green Version]
- Miquelin, P.R.S.; Reis, G.R. Comparação entre as taxas de morbimortalidade de pacientes com septicemia em todos os estados da federação e o Distrito Federal. Amazon. Sci. Health. 2016, 4, 420–424. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- World Health Statistics 2016: Monitoring health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland; Available online: https://www.who.int/gho/publications/world_health_statistics/2016/en/ (accessed on 11 June 2019).
- Croxen, M.A.; Law, R.J.; Scholz, R.; Keeney, K.M.; Wlodarska, M.; Finlay, B.B. Recent advances in understanding enteric pathogenic Escherichia coli. Clin. Microbiol Rev. 2013, 26, 822–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laupland, K.B.; Gregson, D.B.; Church, D.L.; Ross, T.; Pitout, J.D. Incidence, risk factors and outcomes of Escherichia coli bloodstream infections in a large Canadian region. Clin. Microbiol. Infect. 2008, 14, 1041–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Kraker, M.E.A.; Jarlier, V.; Monen, J.C.M.; Heuer, O.E.; Van De Sande, N.; Grundmann, H. The changing epidemiology of bacteraemias in Europe: Trends from the European Antimicrobial Resistance Surveillance System. Clin. Microbiol. Infect. 2013, 19, 860–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.R.; Russo, T.A. Extraintestinal pathogenic Escherichia coli: The other bad E. coli. J. Lab. Clin. Med. 2002, 139, 155–162. [Google Scholar] [CrossRef]
- Miajlovic, H.; Aoga, M.M.; Collins, C.J.; Rogers, T.R.; Smith, S.G.J. Characterization of Escherichia coli bloodstream isolates associated with mortality. J. Med. Microbiol. 2016, 65, 71–79. [Google Scholar] [CrossRef]
- Herzog, K.; Engeler Dusel, J.; Hugentobler, M.; Beutin, L.; Sägesser, G.; Stephan, R.; Hachler, H.; Nuesch-Inderbinen, M. Diarrheagenic enteroaggregative Escherichia coli causing urinary tract infection and bacteremia leading to sepsis. Infection 2014, 42, 441–444. [Google Scholar] [CrossRef] [Green Version]
- Mora-Rillo, M.; Fernández-Romero, N.; Navarro-Sanfrancisco, C.; Díez-Sebastián, J.; Romero-Gómez, M.P.; Fernández, F.A.; Lopez, J.R.A.; Mingorance, J. Impact of virulence genes on sepsis severity and survival in Escherichia coli bacteremia. Virulence 2015, 6, 93–100. [Google Scholar] [CrossRef]
- Abreu, A.G.; Abe, C.M.; Nunes, K.O.; Moraes, C.T.; Chavez-Duenas, L.; Navarro-Garcia, F.; Barbosa, A.S.; Piazza, R.M.F.; Elias, W.P. The serine protease Pic as a virulence factor of atypical enteropathogenic Escherichia coli. Gut microbes. 2016, 7, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Henderson, I.R.; Czeczulin, J.; Eslava, C.; Noriega, F.; Nataro, J.P. Characterization of Pic, a secreted protease of Shigella flexneri and enteroagregative Escherichia coli. Infect. Immun. 1999, 67, 5587–5596. [Google Scholar] [CrossRef] [Green Version]
- Parham, N.J.; Srinivasan, U.; Desvaux, M.; Foxman, B.; Marrs, C.F.; Henderson, I.R. PicU, a second serine protease autotransporter of uropathogenic Escherichia coli. FEMS Microbiol Lett. 2004, 230, 73–83. [Google Scholar] [CrossRef]
- Rasko, D.A.; Webster, D.R.; Sahl, J.W.; Bashir, A.; Boisen, N.; Scheutz, F. Origins of the E. coli strain causing an outbreak of hemolytic-uremic syndrome in Germany. N. Engl. J. Med. 2011, 365, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Rajakumar, K.; Sasakawa, C.; Adler, B. Use of a novel approach, termed island probing, identifies the Shigella flexneri she pathogenicity island which encodes a homolog of the Immunoglobulin A protease-like family of proteins. Infect. Immun. 1997, 65, 4606–4614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhullar, K.; Zarepour, M.; Yu, H.; Yang, H.; Croxen, M.; Stahl, M.; Vallance, B.A. The Serine Protease Autotransporter Pic Modulates Citrobacter rodentium Pathogenesis and Its Innate Recognition by the Host. Infect. Immun. 2015, 83, 2636–2650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, I.R.; Navarro-Garcia, F.; Desvaux, M.; Fernandez, R.C.; Ala’Aldeen, D. Type V protein secretion pathway: The autotransporter story. Microbiol. Mol. Biol. Rev. 2004, 68, 692–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leyton, D.L.; Rossiter, A.E.; Henderson, I.R. From self sufficiency to dependence: Mechanisms and factors important for autotransporter biogenesis. Nat. Rev. Microbiol. 2012, 10, 213–225. [Google Scholar] [CrossRef]
- Ruiz-Perez, F.; Wahid, R.; Faherty, C.S.; Kolappaswamy, K.; Rodriguez, L.; Santiago, A.; Murphy, E.; Cross, A.; Sztein, M.B.; Nataro, J.P. Serine protease autotransporters from Shigella flexneri and pathogenic Escherichia coli target a broad range of leukocyte glycoproteins. Proc. Natl. Acad. Sci. USA 2011, 108, 12881–12886. [Google Scholar] [CrossRef] [Green Version]
- Dutta, P.R.; Cappello, R.; Navarro-Garcia, F.; Nataro, J.P. Functional comparison of serine protease autotransporters of enterobacteriaceae. Infect. Immun. 2002, 70, 7105–7113. [Google Scholar] [CrossRef] [Green Version]
- Munera, D.; Ritchie, J.M.; Hatzios, S.K.; Bronson, R.; Fang, G.; Schadt, E.E.; Davis, B.M.; Waldor, M.K. Autotransporters but not pAA are critical for rabbit colonization by Shiga toxin-producing Escherichia coli O104:H4. Nat. Commun. 2014, 5, 3080. [Google Scholar] [CrossRef] [Green Version]
- Abreu, A.G.; Fraga, T.R.; Martinez, A.P.G.; Kondo, M.Y.; Juliano, M.A.; Juliano, L.; Navarro-Garcia, F.; Isaac, L.; Barbosa, A.S.; Elias, W.P. The serine protease Pic from enteroaggregative Escherichia coli mediates immune evasion by the direct cleavage of complement proteins. J. Infect. Dis. 2015, 212, 106–115. [Google Scholar] [CrossRef]
- Durand, D.; Contreras, C.A.; Mosquito, S.; Ruíz, J.; Cleary, T.G.; Ochoa, T.J. pic gene of enteroaggregative Escherichia coli and its association with diarrhea in Peruvian children. Pathog. Dis. 2016, 74, ftw054. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.H.; Poulsen, A.; Rasmussen, S.H.S.; Struve, C.; Engberg, J.H.; Friis-Møller, A.; Boisen, N.; Jønsson, R.; Petersen, R.F.; Petersen, A.M.; et al. Genetic virulence profile of Enteroaggregative Escherichia coli strains isolated from Danish children with either acute or persistent diarrhea. Front. Cell Infect. Microbiol. 2017, 7, 230. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.B.; Abreu, A.G.; Nunes, K.O.; Gomes, T.A.T.; Piazza, R.M.F.; Elias, W.P. Distribution of serine protease autotransporters of Enterobacteriaceae in typical and atypical enteroaggregative Escherichia coli. Infect. Genet. Evol. 2017, 50, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, P.H.Q.S.; Lima, A.A.M.; Guedes, M.M.; Havt, A.; Bona, M.D.; Rey, L.C.; Soares, A.M.; Guerrant, R.L.; Weigl, B.H.; Lima, I.F.N. Molecular characterization of virulence and antimicrobial resistance profile of Shigella species isolated from children with moderate to severe diarrhea in northeastern Brazil. Diagn Microbiol. Infect. Dis. 2018, 90, 198–205. [Google Scholar] [CrossRef]
- Piva, I.C.; Pereira, A.L.; Ferraz, L.R.; Silva, R.S.N.; Vieira, A.C.; Blanco, J.E.; Blanco, M.; Blanco, J.; Giugliano, L.G. Virulence markers of enteroaggregative Escherichia coli isolated from children and adults with diarrhea in Brasília, Brazil. J. Clin. Microbiol. 2003, 41, 1827–1832. [Google Scholar] [CrossRef] [Green Version]
- Ayala-Lujan, J.L.; Vijayakumar, V.; Gong, M.; Smith, R.; Santiago, A.E.; Ruiz-Perez, F. Broad spectrum activity of a lectin-like bacterial serine protease family on human leukocytes. Plos ONE 2014, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Macmicking, J.D.; Nathan, C.; Horn, G.; Chartrain, N.; Fletcher, D.S.; Trumbauer, M.; Stevens, K.; Xie, Q.K.; Sokol, K.; Hutchinson, N. Altered Responses to Bacterial Infection and Endotoxic Shock in Mice Lacking Inducible Nitric Oxide Synthase. Cell 1995, 61, 641–650. [Google Scholar] [CrossRef] [Green Version]
- Benjamim, C.F.; Silva, J.S.; Fortes, Z.B.; Oliveira, M.A.; Ferreira, S.H.; Cunha, F.Q. Inhibition of leukocyte rolling by nitric oxide during sepsis leads to reduced migration of active microbicidal neutrophils. Infect. Immun. 2002, 70, 3602–3610. [Google Scholar] [CrossRef] [Green Version]
- Craciun, F.L.; Schuller, E.R.; Schuller, D.G. Early enhanced local neutrophil recruitment in peritonitis-induced sepsis improves bacterial clearance and survival. J. Immunol. 2010, 185, 6930–6938. [Google Scholar] [CrossRef] [Green Version]
- Navarini, A.A.; Lang, K.S.; Verschoor, A.; Recher, M.; Zinkernagel, A.S.; Nizet, V.; Odermatt, B.; Hengartner, H.; Zinkernagel, R.M. Innate immune-induced depletion of bone marrow neutrophils aggravates systemic bacterial infections. Proc. Natl. Acad. Sci. USA 2009, 106, 7107–7112. [Google Scholar] [CrossRef] [Green Version]
- Maciel, M.C.G.; Fialho, E.M.S.; Guerra, R.N.M.; Borges, V.M.; Kwasniewski, F.H.; Nascimento, F.R.F. Tityus serrulatus scorpion venom improves survival and lung inflammation in lethal sepsis induced by CLP in mice. Toxicon 2014, 89, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rios, C.E.P.; Abreu, A.G.; Braga Filho, J.A.F.; Nascimento, J.R.; Guerra, R.N.M.; Amaral, F.M.M.; Maciel, M.C.G.; Nascimento, F.R.F. Chenopodium ambrosioides L. improves phagocytic activity and decreases bacterial growth and the systemic inflammatory response in sepsis induced by cecal ligation and puncture. Front. Microbiol. 2017. [Google Scholar] [CrossRef] [Green Version]
- Beneyto, L.A.P.; Luis, O.R.; Luis, C.S.; Simón, O.C.; Rentero, D.B.; Bayarri, V.M. Prognostic value of interleukin 6 for death of patients with sepsis. Med. Clin. (Barc.) 2016, 147, 281–286. [Google Scholar] [CrossRef]
- Zhu, T.; Liao, X.; Feng, T.; Wu, Q.; Zhang, J.; Cao, X.; Li, H. Plasma Monocyte Chemoattractant Protein 1 as a Predictive Marker for Sepsis Prognosis: A Prospective Cohort Study. Tohoku J. Exp. Med. 2017, 241, 139–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sans, T.; Rull, A.; Luna, J.; Mackness, B.; Mackness, M.; Joven, J.; Ibañez, M.; Pariente, R.; Rodriguez, M.; Ortin, X.; et al. Monocyte chemoattractant protein-1 and paraoxonase-1 and 3 levels in patients with sepsis treated in an intensive care unit: A preliminary report. Clin. Chem. Lab. Med. 2012, 50, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.V.; Ferezin, C.Z.; Pereira, A.C.; Rodrigues, G.J.; Grando, M.D.; Bonaventura, D.; Bendhack, L.M. Augmented nitric oxide production and up-regulation of endothelial nitric oxide synthase during cecal ligation and perforation. Nitric Oxide 2012, 27, 59–66. [Google Scholar] [CrossRef]
- Ellis, C.G.; Bateman, R.M.; Sharpe, M.D.; Sibbald, W.J.; Gill, R. Effect of a maldistribution of microvascular blood flow on capillary O(2) extraction in sepsis. J. Physiol. Heart Circ. Physiol. 2002, 282, H156–H164. [Google Scholar] [CrossRef]
- Trzeciak, S.; Dellinger, R.P.; Parrillo, J.E.; Guglielmi, M.; Bajaj, J.; Abate, N.L.; Arnold, R.C.; Colilla, S.; Zanotti, S.; Hollenberg, S.M. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: Relationship to hemodynamics, oxygen transport, and survival. Ann. Emerg. Med. 2007, 49, 88–98. [Google Scholar] [CrossRef]
- Datsenko, K.A.; Wanner, B.L. One step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef] [Green Version]
- Chaveroche, M.K.; Ghigo, J.M.; s d’Enfert, C. A rapid method for efficient gene replacement in the filamentous fungus Aspergillus nidulans. Nucleic Acids Res. 2000, 28, E97. [Google Scholar] [CrossRef]
- Picard, B.; Garcia, J.S.; Gouriou, S.; Duriez, P.; Brahimi, N.; Bingen, E.; Elion, J.; Denamur, E. The link between phylogeny and virulence in Escherichia coli extraintestinal infection. Infect. Immun. 1999, 67, 546–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pick, E.; Mizel, D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J. Immunol. Methods. 1981, 46, 211–226. [Google Scholar] [CrossRef]
- Pick, E.; Keisari, Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J. Immunol. Methods 1980, 38, 161–170. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide: Biol. Chem. 2001, 5, 62–71. [Google Scholar] [CrossRef] [PubMed]








| Organ | Group | Hemorrhage | Infiltrated | Necrosis | Edema |
|---|---|---|---|---|---|
| Kidneys | Control | 0 | 0 | 0 | 0 |
| F5 | 1.0 ± 0.6 | 1.4 ± 0.5 | 0 | 1.6 ± 0.5 | |
| F5∆pic | 0.16 ± 0.3 | 1.33 ± 0.4 | 0 | 0.83 ± 0.7 | |
| Spleen | Control | 0 | 0 | 0 | 0 |
| F5 | 1.2 ± 1.2 | 2.25 ± 0.4 | 0 | 1.2 ± 1.0 | |
| F5∆pic | 0.8 ± 0.7 | 2.0 ± 0.6 | 0 | 1.8 ± 1.2 | |
| Liver | Control | 0 | 0 | 0 | 0 |
| F5 | 0 | 1.0 ± 0.0 | 0 | 0.2 ± 0.4 | |
| F5∆pic | 0 | 0.83 ± 0.7 | 0 | 0.66 ± 0.7 |
| Cells Counting | Control | E. coli F5 | E. coli F5∆pic |
|---|---|---|---|
| Spleen (× 106/mL) | 26.86 ± 6.40 | 38.68 ± 11.84 | 38.68 ± 9.50 |
| Bone marrow (× 106/mL) | 4.74 ± 1.74 | 4.48 ± 1.77 | 5.77 ± 1.02 |
| Peritoneum (× 106/mL) | 1.568 ± 0.26 | 0.99 ± 0.43 | 1.332 ± 0.68 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutra, I.L.; Araújo, L.G.; Assunção, R.G.; Lima, Y.A.; Nascimento, J.R.; Vale, A.A.M.; Alves, P.C.S.; Trovão, L.O.; Santos, A.C.M.; Silva, R.M.; et al. Pic-Producing Escherichia coli Induces High Production of Proinflammatory Mediators by the Host Leading to Death by Sepsis. Int. J. Mol. Sci. 2020, 21, 2068. https://doi.org/10.3390/ijms21062068
Dutra IL, Araújo LG, Assunção RG, Lima YA, Nascimento JR, Vale AAM, Alves PCS, Trovão LO, Santos ACM, Silva RM, et al. Pic-Producing Escherichia coli Induces High Production of Proinflammatory Mediators by the Host Leading to Death by Sepsis. International Journal of Molecular Sciences. 2020; 21(6):2068. https://doi.org/10.3390/ijms21062068
Chicago/Turabian StyleDutra, Itaynara L., Lorena G. Araújo, Raissa G. Assunção, Yago A. Lima, Johnny R. Nascimento, André A. M. Vale, Patrícia C. S. Alves, Liana O. Trovão, Ana Carolina M. Santos, Rosa M. Silva, and et al. 2020. "Pic-Producing Escherichia coli Induces High Production of Proinflammatory Mediators by the Host Leading to Death by Sepsis" International Journal of Molecular Sciences 21, no. 6: 2068. https://doi.org/10.3390/ijms21062068
APA StyleDutra, I. L., Araújo, L. G., Assunção, R. G., Lima, Y. A., Nascimento, J. R., Vale, A. A. M., Alves, P. C. S., Trovão, L. O., Santos, A. C. M., Silva, R. M., Silva, L. A., Maciel, M. C. G., de Sousa, E. M., Elias, W. P., Nascimento, F. R. F., & Abreu, A. G. (2020). Pic-Producing Escherichia coli Induces High Production of Proinflammatory Mediators by the Host Leading to Death by Sepsis. International Journal of Molecular Sciences, 21(6), 2068. https://doi.org/10.3390/ijms21062068






