Thymosin β4-Enhancing Therapeutic Efficacy of Human Adipose-Derived Stem Cells in Mouse Ischemic Hindlimb Model
Abstract
:1. Introduction
2. Results
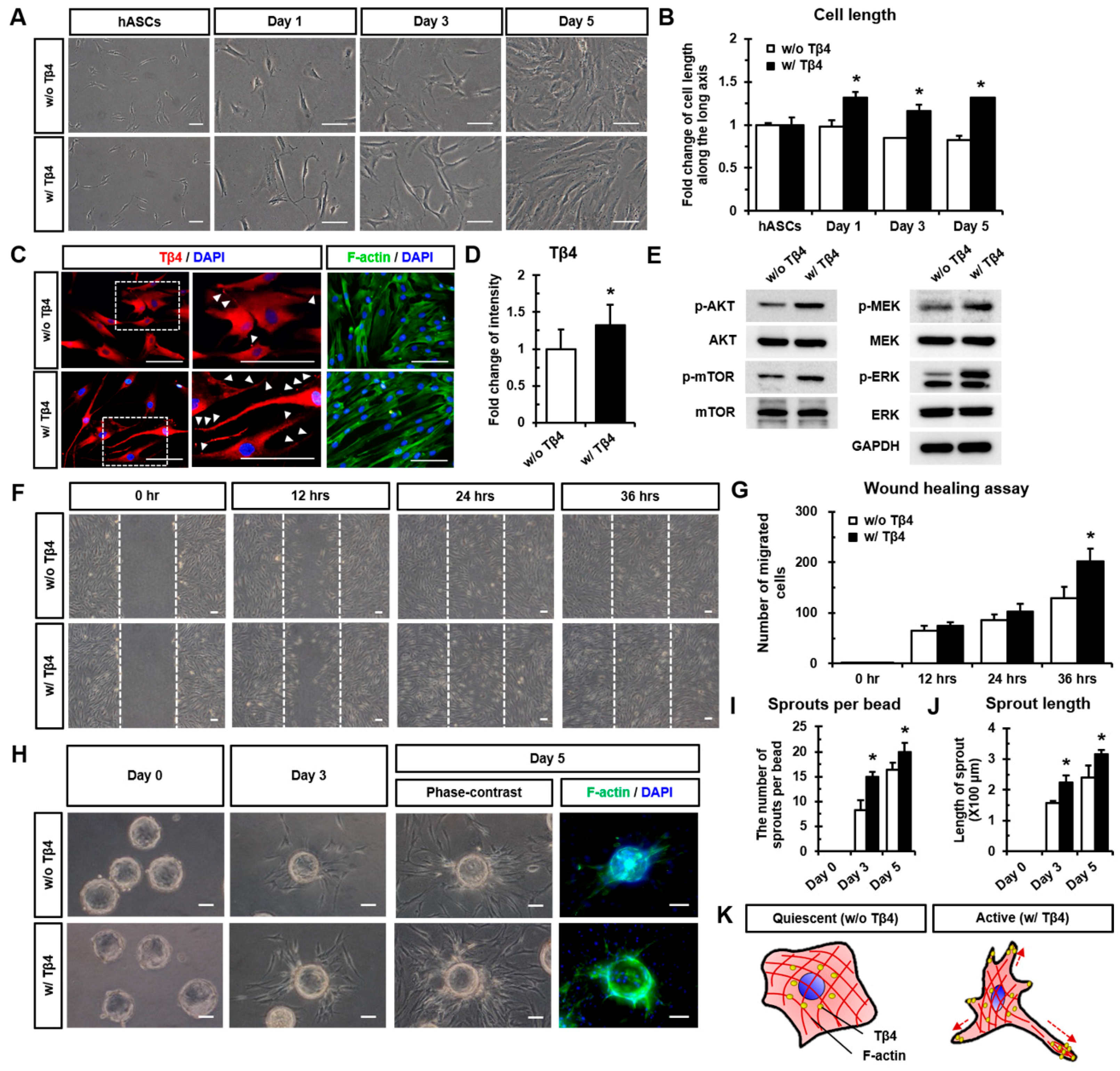
2.1. Treatment with Tβ4 Promotes Morphological Changes in hASCs
2.2. Tβ4 Induces the Active State of hASCs
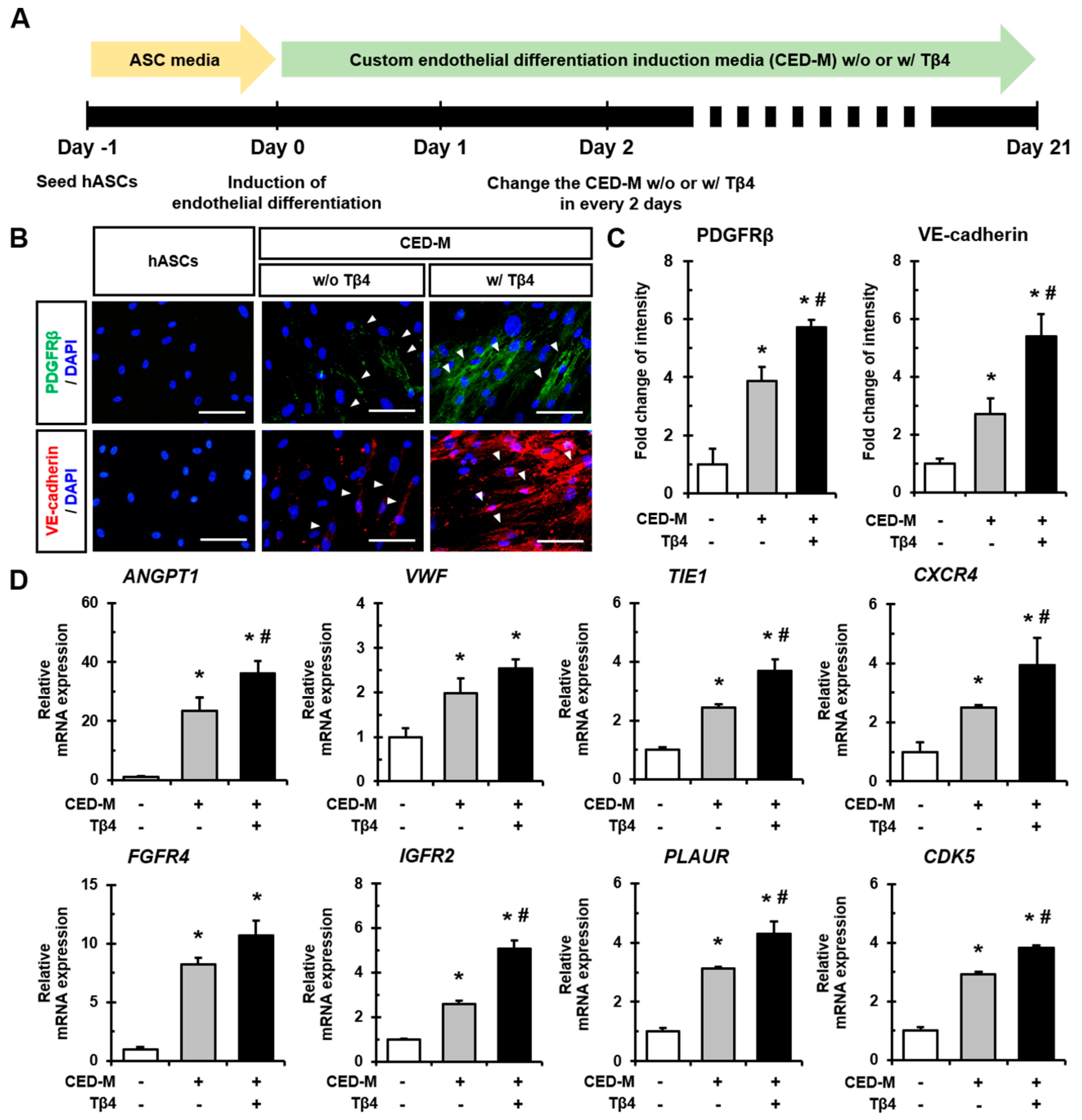
2.3. Additional Treatment with Tβ4 Enhances Endothelial Differentiation of hASCs
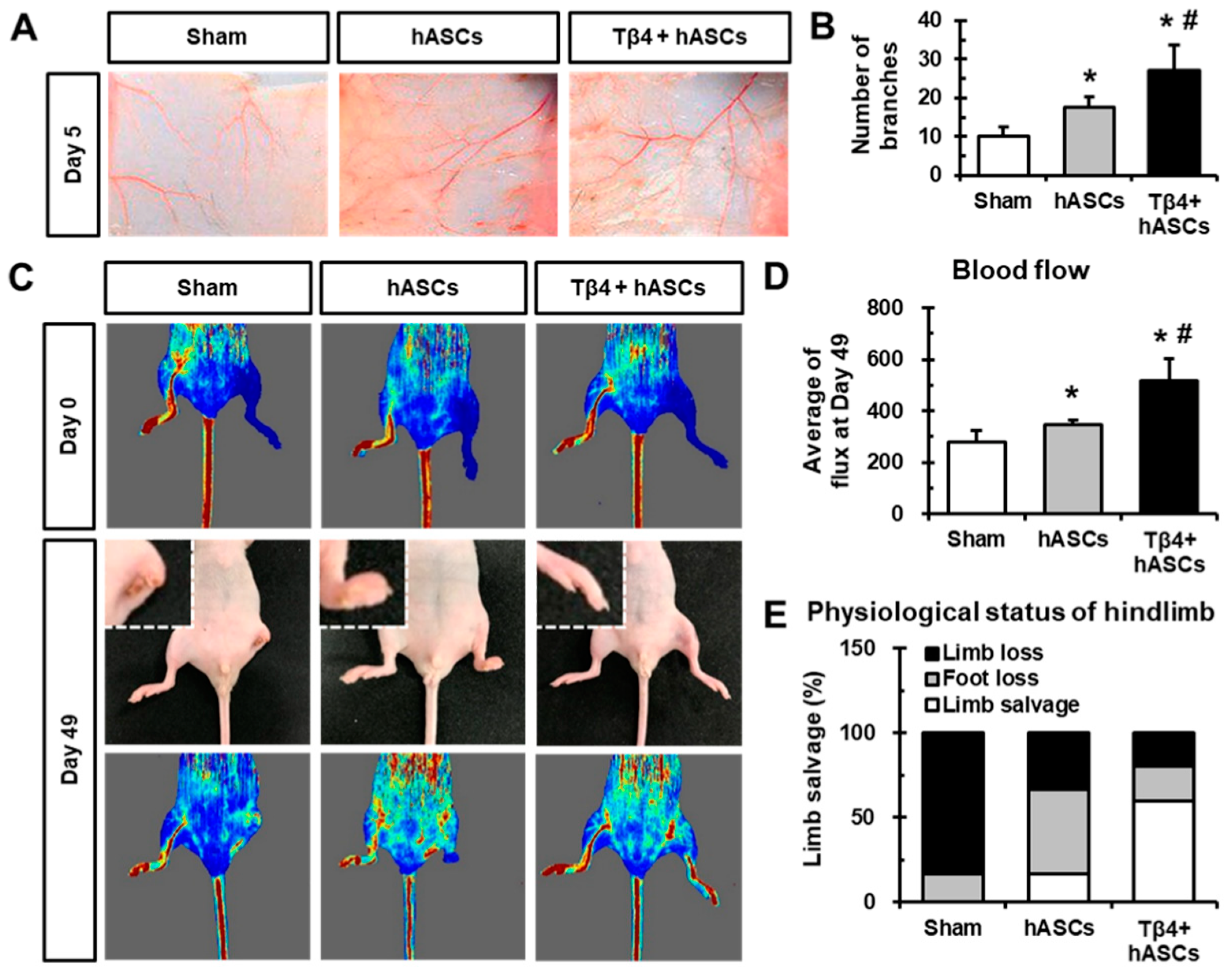
2.4. Transplantation of hASCs in Combination with Tβ4 Improves Blood Vessel Recruitment and Blood Flow After Ischemia
3. Discussion
4. Materials and Methods
4.1. Cell Source
4.2. Real-Time Polymerase Chain Reaction
4.3. Immunofluorescence Staining
4.4. Western Blot Analysis
4.5. Wound Healing Assay
4.6. Microbead Sprouting Assay
4.7. Induction of Endothelial Differentiation
4.8. Dorsal Window Chamber Assay
4.9. Mouse Hindlimb Ischemia Model
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhang, S.H.; Cho, S.W.; Lim, J.M.; Kang, J.M.; Lee, T.J.; Yang, H.S.; Song, Y.S.; Park, M.H.; Kim, H.S.; Yoo, K.J.; et al. Locally delivered growth factor enhances the angiogenic efficacy of adipose-derived stromal cells transplanted to ischemic limbs. Stem Cells 2009, 27, 1976–1986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweitzer, K.S.; Johnstone, B.H.; Garrison, J.; Rush, N.I.; Cooper, S.; Traktuev, D.O.; Feng, D.; Adamowicz, J.J.; Van Demark, M.; Fisher, A.J.; et al. Adipose stem cell treatment in mice attenuates lung and systemic injury induced by cigarette smoking. Am. J. Respir. Crit. Care Med. 2011, 183, 215–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Wang, F.; Mei, H.; Wang, S.; Cheng, L. Human Adipose Mesenchymal Stem Cells Show More Efficient Angiogenesis Promotion on Endothelial Colony-Forming Cells than Umbilical Cord and Endometrium. Stem Cells Int. 2018, 2018, 7537589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopatina, T.; Favaro, E.; Grange, C.; Cedrino, M.; Ranghino, A.; Occhipinti, S.; Fallo, S.; Buffolo, F.; Gaykalova, D.A.; Zanone, M.M.; et al. PDGF enhances the protective effect of adipose stem cell-derived extracellular vesicles in a model of acute hindlimb ischemia. Sci. Rep. 2018, 8, 17458. [Google Scholar] [CrossRef]
- Kim, J.; Jung, Y. Potential role of thymosin Beta 4 in liver fibrosis. Int. J. Mol. Sci. 2015, 16, 10624–10635. [Google Scholar] [CrossRef] [Green Version]
- Low, T.L.; Hu, S.K.; Goldstein, A.L. Complete amino acid sequence of bovine thymosin beta 4: A thymic hormone that induces terminal deoxynucleotidyl transferase activity in thymocyte populations. Proc. Natl. Acad. Sci. USA 1981, 78, 1162–1166. [Google Scholar] [CrossRef] [Green Version]
- Ho, J.H.; Chuang, C.H.; Ho, C.Y.; Shih, Y.R.; Lee, O.K.; Su, Y. Internalization is essential for the antiapoptotic effects of exogenous thymosin beta-4 on human corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2007, 48, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Huff, T.; Muller, C.S.; Otto, A.M.; Netzker, R.; Hannappel, E. beta-Thymosins, small acidic peptides with multiple functions. Int. J. Biochem. Cell Biol. 2001, 33, 205–220. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, F.; Xu, S.; Yu, L.; Fu, G. Thymosin beta4 activates integrin-linked kinase and decreases endothelial progenitor cells apoptosis under serum deprivation. J. Cell. Physiol. 2011, 226, 2798–2806. [Google Scholar] [CrossRef]
- Qiu, F.Y.; Song, X.X.; Zheng, H.; Zhao, Y.B.; Fu, G.S. Thymosin beta4 induces endothelial progenitor cell migration via PI3K/Akt/eNOS signal transduction pathway. J. Cardiovasc. Pharmacol. 2009, 53, 209–214. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, J.; Bi, X.; Gao, J.; Shen, Z.; Zhu, J.; Fu, G. Thymosin beta4 promotes endothelial progenitor cell angiogenesis via a vascular endothelial growth factordependent mechanism. Mol. Med. Rep. 2018, 18, 2314–2320. [Google Scholar] [PubMed] [Green Version]
- Grant, D.S.; Rose, W.; Yaen, C.; Goldstein, A.; Martinez, J.; Kleinman, H. Thymosin beta4 enhances endothelial cell differentiation and angiogenesis. Angiogenesis 1999, 3, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.J.; Song, S.; Seo, H.R.; Shin, J.H.; Choi, S.C.; Park, J.H.; Yu, C.W.; Hong, S.J.; Lim, D.S. Human endothelial colony forming cells from adult peripheral blood have enhanced sprouting angiogenic potential through up-regulating VEGFR2 signaling. Int. J. Cardiol. 2015, 197, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Choi, S.C.; Park, C.Y.; Park, J.H.; Choi, J.H.; Joo, H.J.; Hong, S.J.; Lim, D.S. Transplantation of Immortalized CD34+ and CD34- Adipose-Derived Stem Cells Improve Cardiac Function and Mitigate Systemic Pro-Inflammatory Responses. PLoS ONE 2016, 11, e0147853. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Villalobos, M.A.; Choron, R.L.; Chang, S.; Brown, S.A.; Carpenter, J.P.; Tulenko, T.N.; Zhang, P. Fibroblast growth factor and vascular endothelial growth factor play a critical role in endotheliogenesis from human adipose-derived stem cells. J. Vasc. Surg. 2017, 65, 1483–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, Y.; Chun, B.; Potthoff, S.A.; Kazi, N.; Brolin, T.J.; Orhan, D.; Yang, H.C.; Ma, L.J.; Kon, V.; Myohanen, T.; et al. Thymosin beta4 and its degradation product, Ac-SDKP, are novel reparative factors in renal fibrosis. Kidney Int. 2013, 84, 1166–1175. [Google Scholar] [CrossRef] [Green Version]
- Shah, R.; Reyes-Gordillo, K.; Rojkind, M. Thymosin beta4 inhibits PDGF-BB induced activation, proliferation, and migration of human hepatic stellate cells via its actin-binding domain. Expert Opin. Biol. Ther. 2018, 18 (Suppl. 1), 177–184. [Google Scholar] [CrossRef]
- Kupatt, C.; Bock-Marquette, I.; Boekstegers, P. Embryonic endothelial progenitor cell-mediated cardioprotection requires Thymosin beta4. Trends Cardiovasc. Med. 2008, 18, 205–210. [Google Scholar] [CrossRef]
- Gupta, S.; Li, L. The role of Thymosin beta4 in angiotensin II-induced cardiomyocytes growth. Expert Opin. Biol. Ther. 2018, 18 (Suppl. 1), 105–110. [Google Scholar] [CrossRef]
- Bock-Marquette, I.; Saxena, A.; White, M.D.; Dimaio, J.M.; Srivastava, D. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature 2004, 432, 466–472. [Google Scholar] [CrossRef]
- Morishita, R.; Aoki, M.; Kaneda, Y.; Ogihara, T. Gene therapy in vascular medicine: Recent advances and future perspectives. Pharmacol. Ther. 2001, 91, 105–114. [Google Scholar] [CrossRef]
- Evans, M.A.; Smart, N.; Dube, K.N.; Bollini, S.; Clark, J.E.; Evans, H.G.; Taams, L.S.; Richardson, R.; Levesque, M.; Martin, P.; et al. Thymosin beta4-sulfoxide attenuates inflammatory cell infiltration and promotes cardiac wound healing. Nat. Commun. 2013, 4, 2081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollard, T.D.; Borisy, G.G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2003, 112, 453–465. [Google Scholar] [CrossRef] [Green Version]
- De Lucas, B.; Perez, L.M.; Galvez, B.G. Importance and regulation of adult stem cell migration. J. Cell. Mol. Med. 2018, 22, 746–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, T.; Park, H.C.; Son, K.M.; Kwon, J.H.; Park, J.C.; Yang, H.C. Effects of thymosin beta4 on wound healing of rat palatal mucosa. Int. J. Mol. Med. 2014, 34, 816–821. [Google Scholar] [CrossRef] [Green Version]
- Philp, D.; Nguyen, M.; Scheremeta, B.; St-Surin, S.; Villa, A.M.; Orgel, A.; Kleinman, H.K.; Elkin, M. Thymosin beta4 increases hair growth by activation of hair follicle stem cells. FASEB J. 2004, 18, 385–387. [Google Scholar] [CrossRef] [Green Version]
- Hong, K.O.; Lee, J.I.; Hong, S.P.; Hong, S.D. Thymosin beta4 induces proliferation, invasion, and epithelial-to-mesenchymal transition of oral squamous cell carcinoma. Amino Acids 2016, 48, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.C.; Hu, C.H.; Tang, M.C.; Wang, W.S.; Chen, P.M.; Su, Y. Thymosin beta4 triggers an epithelial-mesenchymal transition in colorectal carcinoma by upregulating integrin-linked kinase. Oncogene 2007, 26, 2781–2790. [Google Scholar] [CrossRef] [Green Version]
- Piao, Z.; Hong, C.S.; Jung, M.R.; Choi, C.; Park, Y.K. Thymosin beta4 induces invasion and migration of human colorectal cancer cells through the ILK/AKT/beta-catenin signaling pathway. Biochem. Biophys. Res. Commun. 2014, 452, 858–864. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, P.; Duval, S.; Su, L.; Xiong, Q.; Zhang, J. Thymosin beta4 increases the potency of transplanted mesenchymal stem cells for myocardial repair. Circulation 2013, 128 (Suppl. 1), S32–S41. [Google Scholar] [CrossRef] [Green Version]
- Jeon, B.J.; Yang, Y.; Kyung Shim, S.; Yang, H.M.; Cho, D.; Ik Bang, S. Thymosin beta-4 promotes mesenchymal stem cell proliferation via an interleukin-8-dependent mechanism. Exp. Cell Res. 2013, 319, 2526–2534. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.H.; Ma, W.H.; Su, Y.; Tseng, K.C.; Kuo, T.K.; Lee, O.K. Thymosin beta-4 directs cell fate determination of human mesenchymal stem cells through biophysical effects. J. Orthop. Res. 2010, 28, 131–138. [Google Scholar] [PubMed]
- Grant, D.S.; Kinsella, J.L.; Kibbey, M.C.; LaFlamme, S.; Burbelo, P.D.; Goldstein, A.L.; Kleinman, H.K. Matrigel induces thymosin beta 4 gene in differentiating endothelial cells. J. Cell Sci. 1995, 108 (Suppl. 12), 3685–3694. [Google Scholar] [PubMed]
- Wang, L.; Chopp, M.; Szalad, A.; Lu, X.; Lu, M.; Zhang, T.; Zhang, Z.G. Angiopoietin-1/Tie2 signaling pathway contributes to the therapeutic effect of thymosin beta4 on diabetic peripheral neuropathy. Neurosci. Res. 2019, 147, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Smart, N.; Dube, K.N.; Riley, P.R. Identification of Thymosin beta4 as an effector of Hand1-mediated vascular development. Nat. Commun. 2010, 1, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulussen, M.; Landuyt, B.; Schoofs, L.; Luyten, W.; Arckens, L. Thymosin beta 4 mRNA and peptide expression in phagocytic cells of different mouse tissues. Peptides 2009, 30, 1822–1832. [Google Scholar] [CrossRef]
- Nemolato, S.; Van Eyken, P.; Cabras, T.; Cau, F.; Fanari, M.U.; Locci, A.; Fanni, D.; Gerosa, C.; Messana, I.; Castagnola, M.; et al. Expression pattern of thymosin beta 4 in the adult human liver. Eur. J. Histochem. 2011, 55, e25. [Google Scholar] [CrossRef] [Green Version]
- Smart, N.; Risebro, C.A.; Melville, A.A.; Moses, K.; Schwartz, R.J.; Chien, K.R.; Riley, P.R. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature 2007, 445, 177–182. [Google Scholar] [CrossRef]
- Rui, L.; Yu, N.; Hong, L.; Feng, H.; Chunyong, H.; Jian, M.; Zhe, Z.; Shengshou, H. Extending the time window of mammalian heart regeneration by thymosin beta 4. J. Cell. Mol. Med. 2014, 18, 2417–2424. [Google Scholar] [CrossRef]
- Bollini, S.; Riley, P.R.; Smart, N. Thymosin beta4: Multiple functions in protection, repair and regeneration of the mammalian heart. Expert Opin. Biol. Ther. 2015, 15 (Suppl. 1), S163–S174. [Google Scholar] [CrossRef]
- Shang, T.; Li, S.; Zhang, Y.; Lu, L.; Cui, L.; Guo, F.F. Hypoxia promotes differentiation of adipose-derived stem cells into endothelial cells through demethylation of ephrinB2. Stem Cell Res. Ther. 2019, 10, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Kim, H.; Cho, H.; Bae, Y.; Suh, K.; Jung, J. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell Physiol. Biochem. 2007, 20, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Joo, H.J.; Kim, M.; Choi, S.C.; Lee, J.I.; Hong, S.J.; Lim, D.S. Transplantation of Adipose-Derived Stem Cell Sheet Attenuates Adverse Cardiac Remodeling in Acute Myocardial Infarction. Tissue Eng. Part A 2017, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Lim, I.-R.; Park, C.-Y.; Joo, H.J.; Noh, J.-M.; Choi, S.-C.; Hong, S.J.; Lim, D.-S. Thymosin β4-Enhancing Therapeutic Efficacy of Human Adipose-Derived Stem Cells in Mouse Ischemic Hindlimb Model. Int. J. Mol. Sci. 2020, 21, 2166. https://doi.org/10.3390/ijms21062166
Kim J-H, Lim I-R, Park C-Y, Joo HJ, Noh J-M, Choi S-C, Hong SJ, Lim D-S. Thymosin β4-Enhancing Therapeutic Efficacy of Human Adipose-Derived Stem Cells in Mouse Ischemic Hindlimb Model. International Journal of Molecular Sciences. 2020; 21(6):2166. https://doi.org/10.3390/ijms21062166
Chicago/Turabian StyleKim, Jong-Ho, I-Rang Lim, Chi-Yeon Park, Hyung Joon Joo, Ji-Min Noh, Seung-Cheol Choi, Soon Jun Hong, and Do-Sun Lim. 2020. "Thymosin β4-Enhancing Therapeutic Efficacy of Human Adipose-Derived Stem Cells in Mouse Ischemic Hindlimb Model" International Journal of Molecular Sciences 21, no. 6: 2166. https://doi.org/10.3390/ijms21062166




