Protective Effects of Recombinant Human Soluble Thrombomodulin on Lipopolysaccharide-Induced Acute Kidney Injury
Abstract
1. Introduction
2. Results
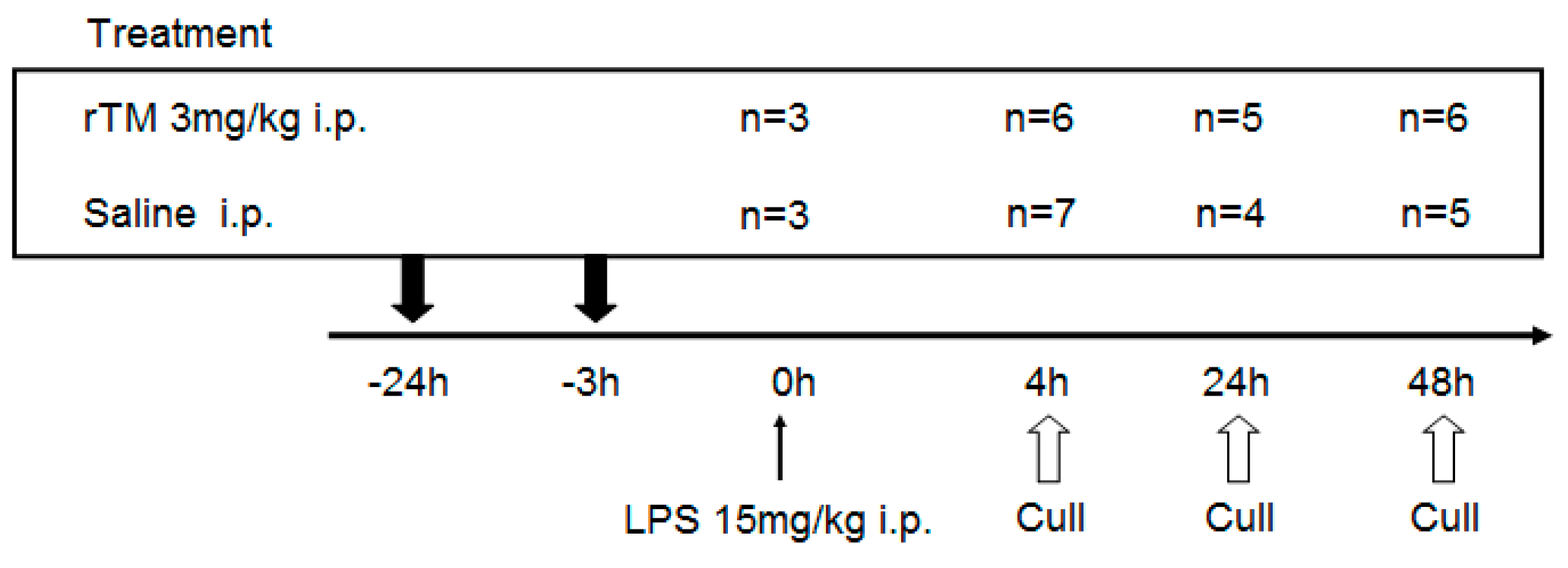
2.1. Experimental Schedule
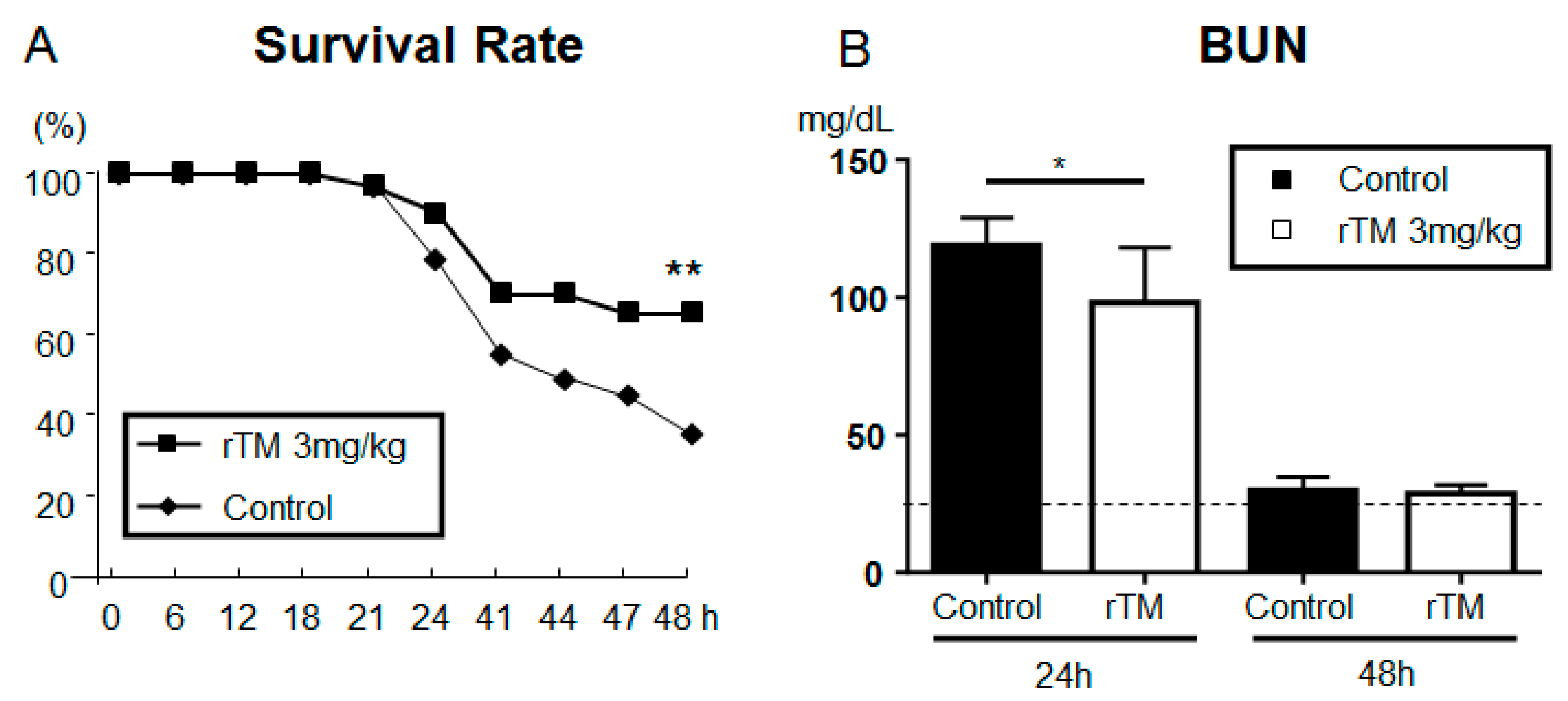
2.2. Recombinant Human Soluble TM after LPS-Induced AKI Model
2.3. Serum Biomarkers in AKI
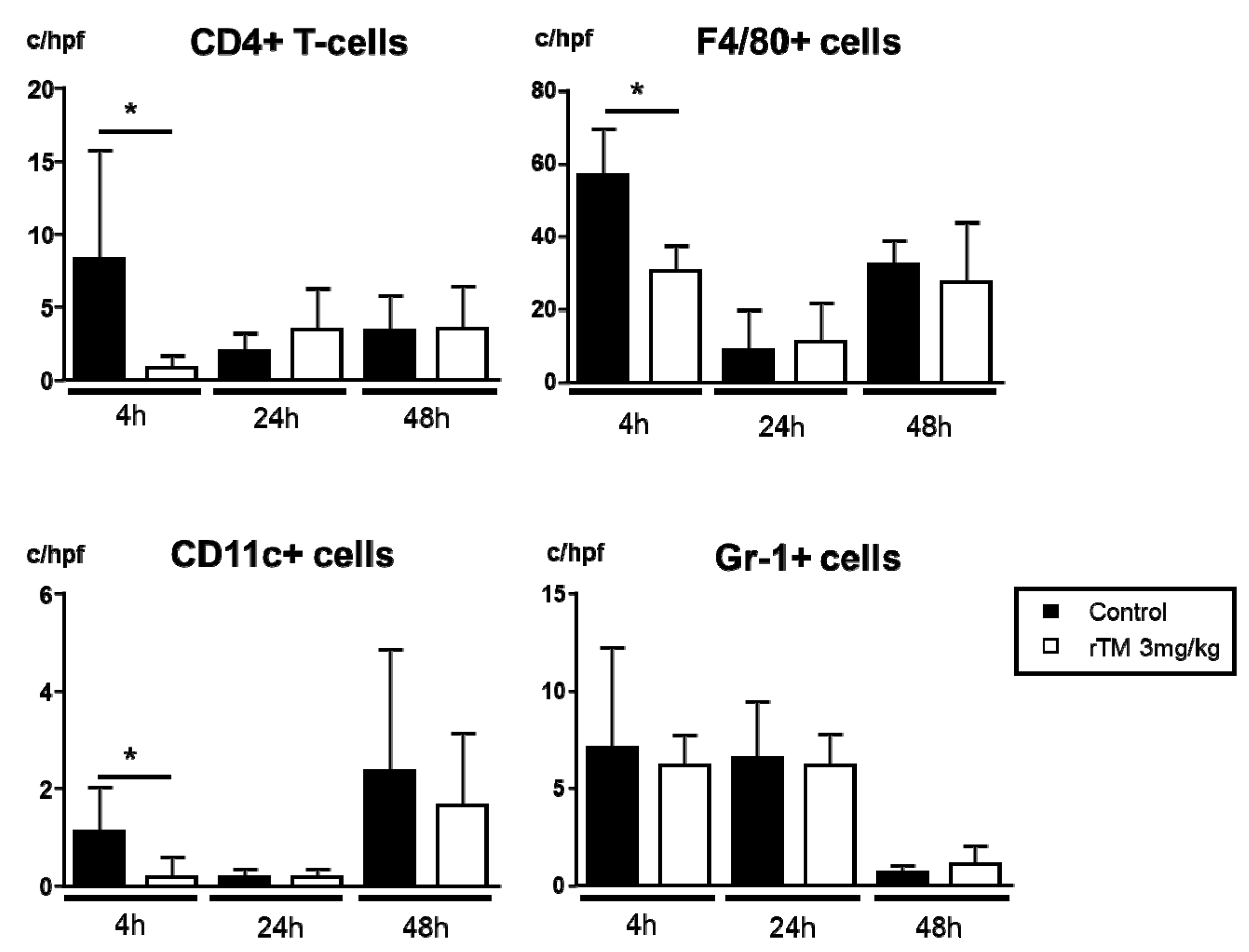
2.4. Infiltration of CD4+ T-Cells, CD11c+ Cells, Macrophages, and Neutrophils in the Kidney
2.5. Renal mRNA Expression in LPS-Induced AKI
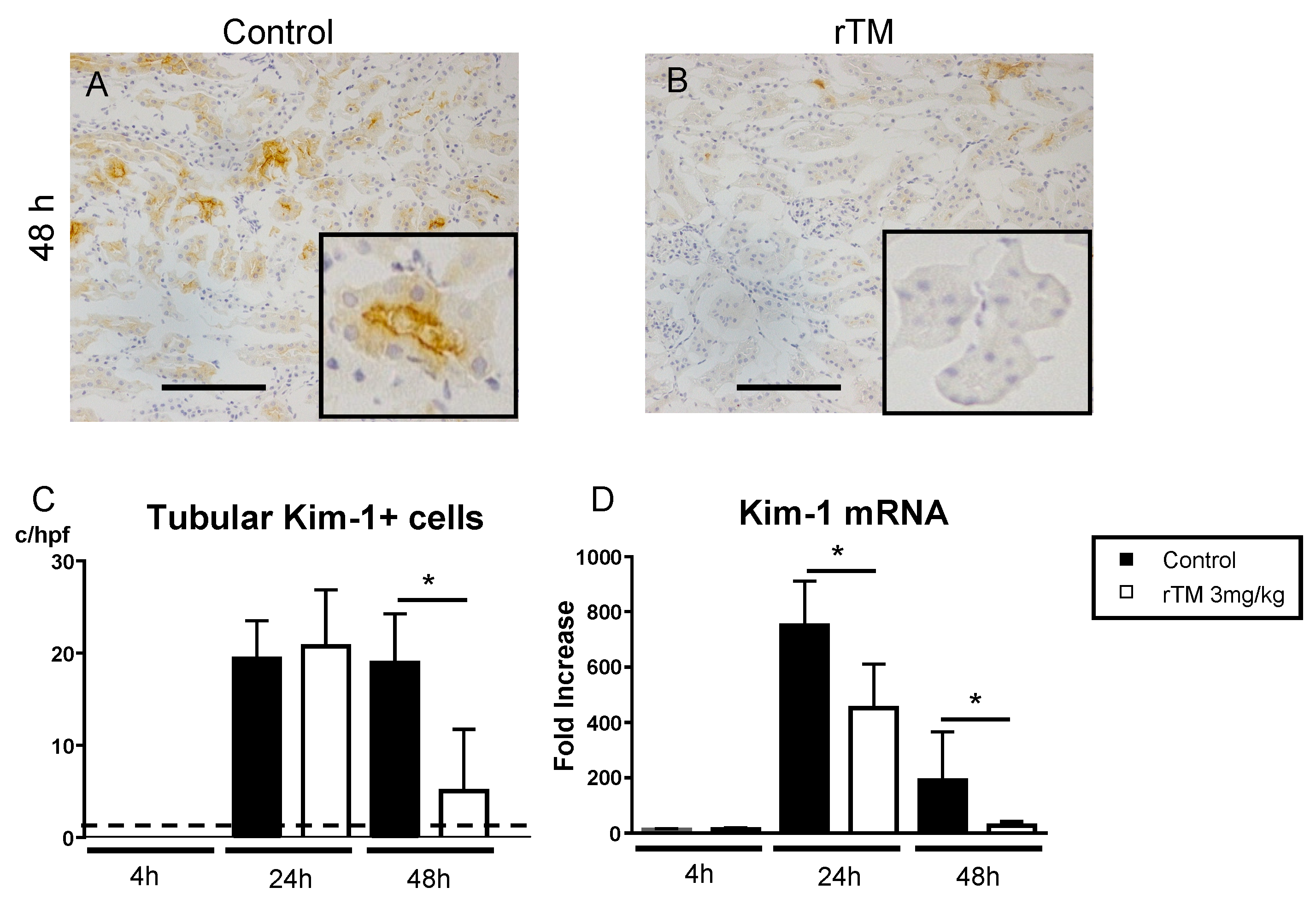
2.6. Renal Kidney Injury Molecule-1 Expression
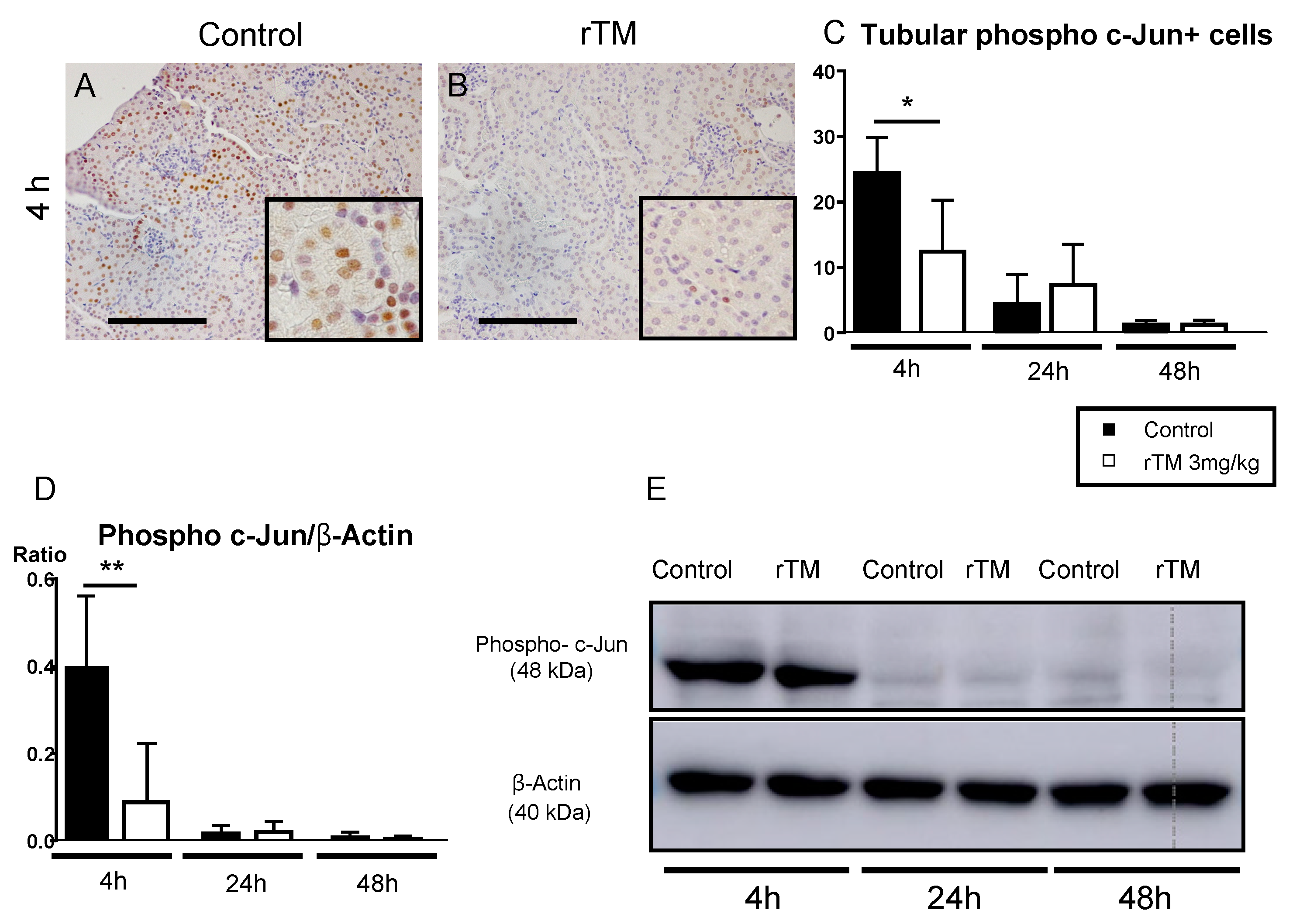
2.7. Renal and Splenic Phospho c-Jun+ Cells
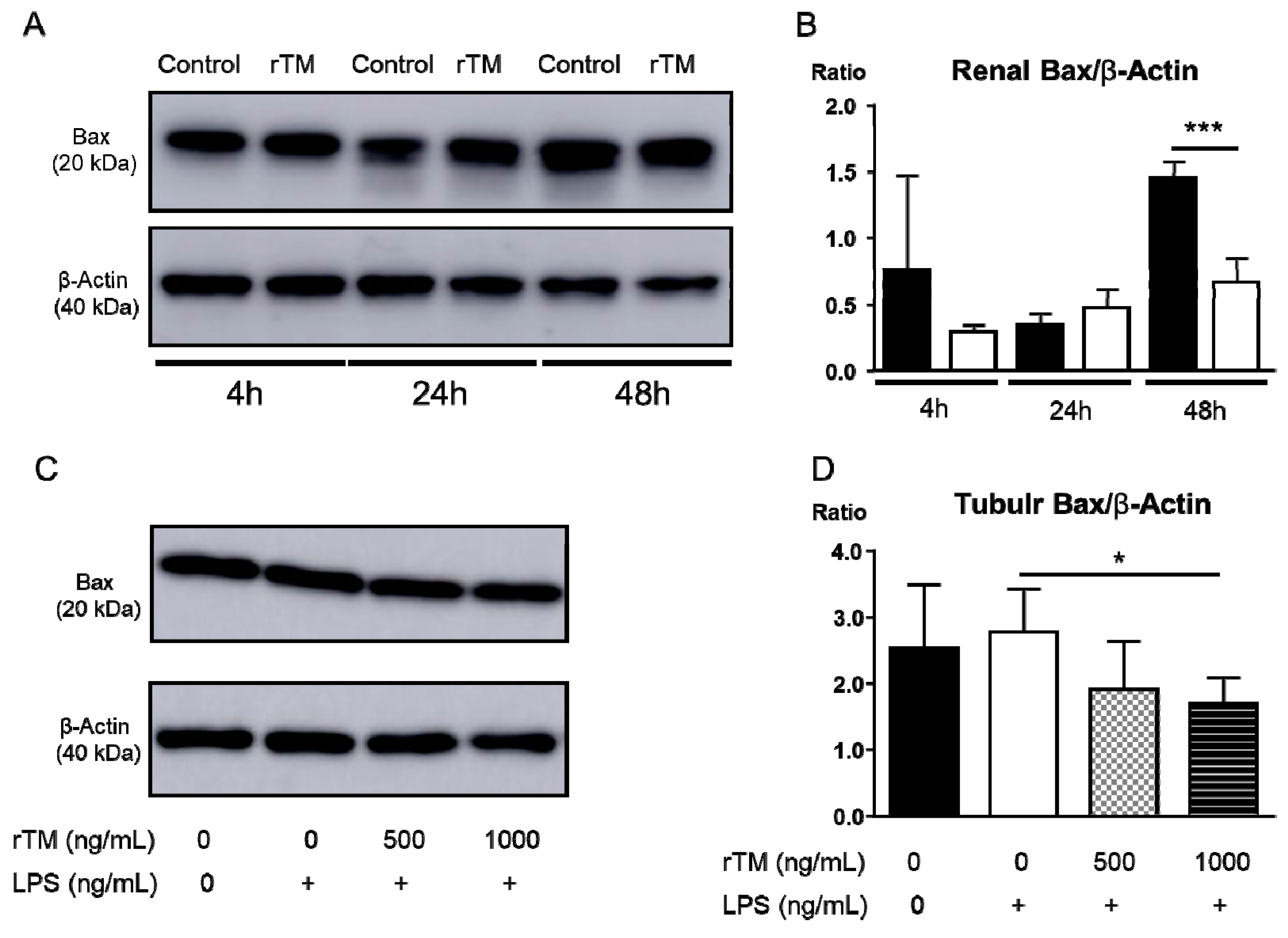
2.8. rTM Mediates Apoptosis in Kidney and Tubular Epithelial Cells
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animals
4.3. Murine Model of Endotoxin-Induced Acute Kidney Injury
4.4. Assessment of Renal Injury
4.5. Measurement of mRNA Expression in the Kidney by Real-Time PCR
4.6. Serum Cytokine Quantitation by Enzyme-Linked Immunosorbent Assay
4.7. Cell Culture
4.8. Western Blotting Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AKI | acute kidney injury |
| IL | interleukin |
| TNF | tumor necrosis factor |
| TM | thrombomodulin |
| rTM | recombinant human soluble TM |
| APC | anticoagulant protein C |
| LPS | lipopolysaccharide |
| IFN-γ | interferon-gamma |
| i.p. | intraperitoneally |
| BUN | blood urea nitrogen |
| CCL2/MCP-1 | chemokine ligand 2/monocyte chemoattractant protein-1 |
| Kim-1 | kidney injury molecule-1 |
| TEC | tubular epithelial cells |
| APCs | antigen-presenting cells |
| TLR4 | toll-like receptor4 |
| JNK | c-Jun N-terminal kinase |
| Hsp70 | heat shock protein 70 |
| ANOVA | analysis of variance |
References
- Devarajan, P. Emerging biomarkers of acute kidney injury. Contrib. Nephrol. 2007, 156, 203–212. [Google Scholar] [PubMed]
- Bone, R.C. The pathogenesis of sepsis. Ann. Intern. Med. 1991, 115, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Groeneveld, A.B.; Tran, D.D.; van der Meulen, J.; Nauta, J.J.; Thijs, L.G. Acute renal failure in the medical intensive care unit: Predisposing, complicating factors and outcome. Nephron 1991, 59, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, K.; Björk, P.; Bergenfeldt, M.; Hageman, R.; Thompson, R.C. Interleukin-1 receptor antagonist reduces mortality from endotoxin shock. Nature 1990, 348, 550–552. [Google Scholar] [CrossRef] [PubMed]
- Tracey, K.J.; Fong, Y.; Hesse, D.G.; Manogue, K.R.; Lee, A.T.; Kuo, G.C.; Lowry, S.F.; Cerami, A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 1987, 330, 662–664. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, A.; Kinsey, G.R.; Okusa, M.D. Immune mechanisms and novel pharmacological therapies of acute kidney injury. Curr. Drug Targets 2009, 10, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Day, Y.J.; Huang, L.; Ye, H.; Li, L.; Linden, J.; Okusa, M.D. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: The role of CD4+ T-cells and IFN-gamma. J. Immunol. 2006, 176, 3108–3114. [Google Scholar] [CrossRef]
- Day, Y.J.; Huang, L.; Ye, H.; Linden, J.; Okusa, M.D. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: Role of macrophages. Am. J. Physiol. Renal Physiol. 2005, 288, F722–F731. [Google Scholar] [CrossRef]
- Esmon, C.T. Thrombomodulin as a model of molecular mechanisms that modulate protease specificity and function at the vessel surface. FASEB J. 1995, 9, 946–955. [Google Scholar] [CrossRef]
- Weiler, H.; Isermann, B.H. Thrombomodulin. J. Thromb. Haemost. 2003, 1, 1515–1524. [Google Scholar] [CrossRef]
- Van de Wouwer, M.; Collen, D.; Conway, E.M. Thrombomodulin-protein C-EPCR system: Integrated to regulate coagulation and inflammation. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Conway, E.M.; Nowakowski, B.; Steiner-Mosonyi, M. Human neutrophils synthesize thrombomodulin that does not promote thrombin-dependent protein C activation. Blood 1992, 80, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Conway, E.M.; Nowakowski, B. Biologically active thrombomodulin is synthesized by adherent synovial fluid cells and is elevated in synovial fluid of patients with rheumatoid arthritis. Blood 1993, 81, 726–733. [Google Scholar] [CrossRef]
- McCachren, S.S.; Diggs, J.; Weinberg, J.B.; Dittman, W.A. Thrombomodulin expression by human blood monocytes and by human synovial tissue lining macrophages. Blood 1991, 78, 3128–3132. [Google Scholar] [CrossRef]
- Maillard, C.; Berruyer, M.; Serre, C.M.; Amiral, J.; Dechavanne, M.; Delmas, P.D. Thrombomodulin is synthesized by osteoblasts, stimulated by 1,25-(OH)2D3 and activates protein C at their cell membrane. Endocrinology 1993, 133, 668–674. [Google Scholar] [CrossRef]
- Grey, S.T.; Tsuchida, A.; Hau, H.; Orthner, C.L.; Salem, H.H.; Hancock, W.W. Selective inhibitory effects of the anticoagulant activated protein C on the responses of human mononuclear phagocytes to LPS, IFN-gamma, or phorbol ester. J. Immunol. 1994, 153, 3664–3672. [Google Scholar]
- Ito, T.; Maruyama, I. Thrombomodulin: Protectorate God of the vasculature in thrombosis and inflammation. J. Thromb. Haemost. 2011, 9, 168–173. [Google Scholar] [CrossRef]
- Ito, T.; Kawahara, K.; Okamoto, K.; Yamada, S.; Yasuda, M.; Imaizumi, H.; Nawa, Y.; Meng, X.; Shrestha, B.; Hashiguchi, T.; et al. Proteolytic cleavage of high mobility group box 1 protein by thrombin-thrombomodulin complexes. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1825–1830. [Google Scholar] [CrossRef]
- Esmon, C.T. The regulation of natural anticoagulant pathways. Science 1987, 235, 1348–1352. [Google Scholar] [CrossRef]
- Ghose, P.; Ali, A.Q.; Fang, R.; Forbes, D.; Ballard, B.; Ismail, N. The interaction between IL-18 and IL-18 receptor limits the magnitude of protective immunity and enhances pathogenic responses following infection with intracellular bacteria. J. Immunol. 2011, 187, 1333–1346. [Google Scholar] [CrossRef]
- Ichimura, T.; Bonventre, J.V.; Bailly, V.; Wei, H.; Hession, C.A.; Cate, R.L.; Sanicola, M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J. Biol. Chem. 1998, 273, 4135–4142. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, Y.; Kinoshita, K.; Hino, S.; Yano, T.; Niki, K.; Hirooka, Y.; Kishimoto, K.; Funauchi, M.; Matsumura, I. Signaling Rho-kinase mediates inflammation and apoptosis in T cells and renal tubules in cisplatin nephrotoxicity. Am. J. Physiol. Renal Physiol. 2015, 308, F899–F909. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, Y.; Nikolic-Paterson, D.J.; Snelgrove, S.L.; Akiba, H.; Yagita, H.; Holdsworth, S.R.; Kitching, A.R. Endogenous Tim-1 (Kim-1) Promotes T-cell Responses and Cell-Mediated Injury in Experimental Crescentic Glomerulonephritis. Kidney Int. 2012, 81, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, Y.; Kinoshita, K.; Yano, T.; Shiga, T.; Hino, S.; Niki, K.; Kishimoto, K.; Funauchi, M.; Matsumura, I. Estimation of Kidney Injury molecule-1 (Kim-1) in Patients with Lupus Nephritis Y. Lupus 2014, 23, 769–777. [Google Scholar] [CrossRef]
- Nozaki, Y.; Nikolic-Paterson, D.J.; Yagita, H.; Akiba, H.; Holdsworth, S.R.; Kitching, A.R. Tim-1 promotes cisplatin nephrotoxicity. Am. J. Physiol. Renal Physiol. 2011, 301, F1098–F1104. [Google Scholar] [CrossRef]
- Iba, T.; Yagi, Y.; Kidokoro, A.; Fukunaga, M.; Fukunaga, T. Increased plasma levels of soluble thrombomodulin in patients with sepsis and organ failure. Surg. Today 1995, 25, 585–590. [Google Scholar] [CrossRef]
- Vincent, J.L.; Francois, B.; Zabolotskikh, I.; Daga, M.K.; Lascarrou, J.B.; Kirov, M.Y.; Pettilä, V.; Wittebole, X.; Meziani, F.; Mercier, E.; et al. Effect of a Recombinant Human Soluble Thrombomodulin on Mortality in Patients with Sepsis-Associated Coagulopathy: The SCARLET Randomized Clinical Trial. JAMA 2019, 321, 1993–2002. [Google Scholar] [CrossRef]
- Ramesh, G.; Reeves, W.B. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J. Clin. Investig. 2002, 110, 835–842. [Google Scholar] [CrossRef]
- Ramesh, G.; Reeves, W.B. TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am. J. Physiol. Renal Physiol. 2003, 285, F610–F618. [Google Scholar] [CrossRef]
- Razzaque, M.S.; Koji, T.; Kumatori, A.; Taguchi, T. Cisplatin-induced apoptosis in human proximal tubular epithelial cells is associated with the activation of the Fas/Fas ligand system. Histochem. Cell Biol. 1999, 111, 359–365. [Google Scholar] [CrossRef]
- Rabb, H.; Daniels, F.; O’Donnell, M.; Haq, M.; Saba, S.R.; Keane, W.; Tang, W.W. Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am. J. Physiol. Renal Physiol. 2000, 279, F525–F531. [Google Scholar] [PubMed]
- Ysebaert, D.K.; De Greef, K.E.; Vercauteren, S.R.; Ghielli, M.; Verpooten, G.A.; Eyskens, E.J.; De Broe, M.E. Identification and kinetics of leukocytes after severe ischaemia/reperfusion renal injury. Nephrol. Dial. Transplant. 2000, 15, 1562–1574. [Google Scholar] [PubMed]
- Burne, M.J.; Daniels, F.; El Ghandour, A.; Mauiyyedi, S.; Colvin, R.B.; O’Donnell, M.P.; Rabb, H. Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J. Clin. Investig. 2001, 108, 1283–1290. [Google Scholar]
- Liu, M.; Chien, C.C.; Burne-Taney, M.; Molls, R.R.; Racusen, L.C.; Colvin, R.B.; Rabb, H. A pathophysiologic role for T lymphocytes in murine acute cisplatin nephrotoxicity. J. Am. Soc. Nephrol. 2006, 17, 765–774. [Google Scholar]
- Pober, J.S.; Cotran, R.S. Cytokines and endothelial cell biology. Physiol. Rev. 1990, 70, 427–451. [Google Scholar]
- Rabb, H.; O’Meara, Y.M.; Maderna, P.; Coleman, P.; Brady, H.R. Leukocytes, cell adhesion molecules and ischemic acute renal failure. Kidney Int. 1997, 5, 1463–1468. [Google Scholar]
- Van de Wouwer, M.; Conway, E.M. Novel functions of thrombomodulin in inflammation. Crit. Care Med. 2004, 32, S254–S261. [Google Scholar]
- Molitoris, B.A.; Sutton, T.A. Endothelial injury and dysfunction: Role in the extension phase of acute renal failure. Kidney Int. 2004, 66, 496–499. [Google Scholar]
- Sutton, T.A.; Fisher, C.J.; Molitoris, B.A. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 2002, 62, 1539–1549. [Google Scholar]
- Conway, E.M.; Van de Wouwer, M.; Pollefeyt, S.; Jurk, K.; Van Aken, H.; De Vriese, A.; Weitz, J.I.; Weiler, H.; Hellings, P.W.; Schaeffer, P.; et al. The lectin-like domain of thrombomodulin confers protection from neutrophil-mediated tissue damage by suppressing adhesion molecule expression via nuclear factor kappaB and mitogen-activated protein kinase pathways. J. Exp. Med. 2002, 196, 565–577. [Google Scholar]
- Ma, C.Y.; Shi, G.Y.; Shi, C.S.; Kao, Y.C.; Lin, S.W.; Wu, H.L. Monocytic thrombomodulin triggers LPS- and gram-negative bacteria-induced inflammatory response. J. Immunol. 2012, 188, 6328–6337. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, Y.; Hino, S.; Ri, J.; Sakai, K.; Nagare, Y.; Kawanishi, M.; Niki, K.; Funauchi, M.; Matsumura, I. Lipopolysaccharide-Induced Acute Kidney Injury Is Dependent on an IL-18 Receptor Signaling Pathway. Int. J. Mol. Sci. 2017, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, P.N.; Dyanov, H.M.; Park, P.; Wang, J.; Newell, K.A.; Quigg, R.J. Acute renal failure in endotoxemia is caused by TNF acting directly on TNF receptor-1 in kidney. J. Immunol. 2002, 168, 5817–5823. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Wang, Y.; Minto, A.W.; Quigg, R.J.; Cunningham, P.N. Acute renal failure in endotoxemia is dependent on caspase activation. J. Am. Soc. Nephrol. 2004, 15, 3093–3102. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.; Nimnual, A.; Zong, W.X.; Kennedy, N.J.; Flavell, R.A.; Thompson, C.B.; Bar-Sagi, D.; Davis, R.J. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH(2)-terminal kinase. Mol. Cell Biol. 2002, 22, 4929–4942. [Google Scholar] [CrossRef]
- Davis, R.J. Signal transduction by the JNK group of MAP kinases. Cell 2000, 103, 239–252. [Google Scholar] [CrossRef]
- Terada, Y.; Inoshita, S.; Kuwana, H.; Kobayashi, T.; Okado, T.; Ichijo, H.; Sasaki, S. Important role of apoptosis signal-regulating kinase 1 in ischemic acute kidney injury. Biochem. Biophys. Res. Commun. 2007, 364, 1043–1049. [Google Scholar] [CrossRef]
- Ma, Q.; Devarajan, P. Induction of proapoptotic Daxx following ischemic acute kidney injury. Kidney Int. 2008, 74, 310–318. [Google Scholar] [CrossRef]
- Tsuruta, F.; Sunayama, J.; Mori, Y.; Hattori, S.; Shimizu, S.; Tsujimoto, Y.; Yoshioka, K.; Masuyama, N.; Gotoh, Y. JNK promotes Bax translocation to mitochondria through phosphorylation of 14-3-3 proteins. EMBO J. 2004, 23, 1889–1899. [Google Scholar] [CrossRef]
- Murphy, K.M.; Streips, U.N.; Lock, R.B. Bax Membrane Insertion During Fas(CD95)-induced Apoptosis Precedes Cytochrome C Release and Is Inhibited by Bcl-2. Oncogene 1999, 18, 5991–5999. [Google Scholar] [CrossRef]
- Ichimura, T.; Asseldonk, E.J.P.V.; Humphreys, B.D.; Gunaratnam, L.; Duffield, J.S.; Bonventre, J.V. Kidney Injury molecule-1 is a Phosphatidylserine Receptor That Confers a Phagocytic Phenotype on Epithelial Cells. J. Clin. Investig. 2008, 118, 1657–1668. [Google Scholar] [CrossRef] [PubMed]

| Gene Expressions | 4 h | 24 h | 48 h |
|---|---|---|---|
| Control versus rTM | Control versus rTM | Control versus rTM | |
| Cytokines | |||
| IL-10 | 36.5 ± 3.9 versus 35.8 ± 7.3 | 84.0 ± 19.1 versus 91.4 ± 17.8 | 31.1 ± 9.6 versus 54.6 ± 9.4 * |
| IL-18 | 2.4 ± 0.2 versus 2.9 ± 0.5 | 2.9 ± 0.4 versus 4.4 ± 0.5 | 1.3 ± 0.5 versus 1.2 ± 0.3 |
| IFN-γ | 8.6 ± 1.5 versus 11.4 ± 4.4 | 2.1 ± 0.5 versus 1.5 ± 0.2 * | 0.5 ± 0.1 versus 0.8 ± 0.2 |
| TNF | 12.7 ± 1.6 versus 8.7 ± 1.8 * | 10.8 ± 4.7 versus 4.3 ± 0.6 * | 2.8 ± 0.3 versus 3.3 ± 0.3 |
| Chemokines | |||
| CCL2/MCP-1 | 245.9 ± 27.9 versus 187.2 ± 30.1 * | 38.1 ± 4.9 versus 25.8 ± 2.4 * | 17.0 ± 3.1 versus 24.4 ± 3.6 |
| TLR4 | 1.9 ± 0.3 versus 2.1 ± 0.3 | 1.1 ± 0.2 versus 1.9 ± 0.6 | 1.3 ± 0.1 versus 1.7 ± 0.3 |
| Th-cell subset transcription factors | |||
| GATA3 | 0.6 ± 0.2 versus 1.0 ± 0.4 | 0.3 ± 0.1 versus 0.3 ± 0.1 | 0.5 ± 0.1 versus 0.6 ± 0.5 |
| T-bet | 6.5 ± 3.4 versus 0.8 ± 0.6 * | 0.3 ± 0.1 versus 0.3 ± 0.1 | 0.3 ± 0.2 versus 0.2 ± 0.1 |
| Gene Expression | Forward Primer | Reverse Primer |
|---|---|---|
| 18SrRNA | GTAACCCGTTGAACCCCATTC | GCCTCACTAAACCATCCAATCG |
| TNF | CGATCACCCCGAAGTTCAGTA | GGTGCCTATGTCTCAGCCTCTT |
| CCL2/MCP-1 | AAAAACCTGGATCGGAACCAA | CGGGTCAACTTCACATTCAAAG |
| GATA3 | AGGGACATCCTGCGCGAACTGT | CATCTTCCGGTTTCGGGTCTGG |
| T-bet | CCTGGACCCAACTGTCAACT | AACTGTGTTCCCGAGGTGTC |
| Gene Expression | Gene Database Number |
|---|---|
| 18SrRNA | NM_026744.3 |
| IFN-γ | NM_008337.3 |
| IL-10 | NM_010548.1 |
| IL-18 | NM_008360.1 |
| Kim-1 | NM_134248.1 |
| TLR4 | Mm00445273_m1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nozaki, Y.; Ri, J.; Sakai, K.; Niki, K.; Funauchi, M.; Matsumura, I. Protective Effects of Recombinant Human Soluble Thrombomodulin on Lipopolysaccharide-Induced Acute Kidney Injury. Int. J. Mol. Sci. 2020, 21, 2519. https://doi.org/10.3390/ijms21072519
Nozaki Y, Ri J, Sakai K, Niki K, Funauchi M, Matsumura I. Protective Effects of Recombinant Human Soluble Thrombomodulin on Lipopolysaccharide-Induced Acute Kidney Injury. International Journal of Molecular Sciences. 2020; 21(7):2519. https://doi.org/10.3390/ijms21072519
Chicago/Turabian StyleNozaki, Yuji, Jinhai Ri, Kenji Sakai, Kaoru Niki, Masanori Funauchi, and Itaru Matsumura. 2020. "Protective Effects of Recombinant Human Soluble Thrombomodulin on Lipopolysaccharide-Induced Acute Kidney Injury" International Journal of Molecular Sciences 21, no. 7: 2519. https://doi.org/10.3390/ijms21072519
APA StyleNozaki, Y., Ri, J., Sakai, K., Niki, K., Funauchi, M., & Matsumura, I. (2020). Protective Effects of Recombinant Human Soluble Thrombomodulin on Lipopolysaccharide-Induced Acute Kidney Injury. International Journal of Molecular Sciences, 21(7), 2519. https://doi.org/10.3390/ijms21072519





