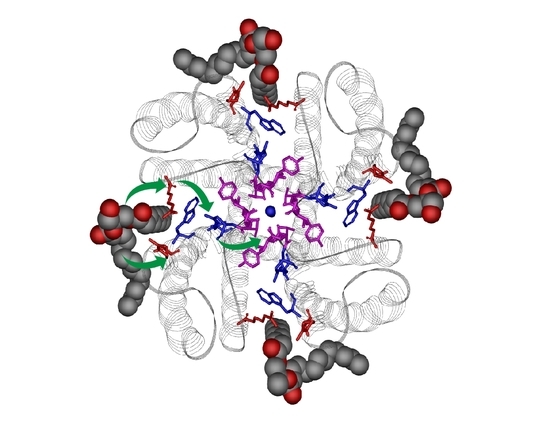
Modulation of Function, Structure and Clustering of K+ Channels by Lipids: Lessons Learnt from KcsA
Abstract
1. Introduction
2. Lipids as Modulators of KcsA Function
3. Lipids as Structural Effectors of KcsA
4. Ion Binding to the KcsA Selectivity Filter and Modulation by Lipids
5. Lipid Modulation of Protein Assembly Processes in KcsA
5.1. Influence of Lipids on Folding and Tetramerization of KcsA
5.2. Effects of Lipids on the Formation of KcsA Clusters and Their Gating Properties
6. Concluding Remark
Author Contributions
Funding
Conflicts of Interest
References
- Lehmann-Horn, F.; Jurkat-Rott, K. Channelopathies; Lehmann-Horn, F., Jurkat-Rott, K., Eds.; Elsevier: Ulm, Germany, 2000; Volume 1, ISBN 978-0-444-50489-0. [Google Scholar]
- Kumar, P.; Kumar, D.; Jha, S.K.; Jha, N.K.; Ambasta, R.K. Ion Channels in Neurological Disorders. In Proceedings of the Advances in Protein Chemistry and Structural Biology; Academic Press Inc.: Cambridge, MA, USA, 2016; Volume 103, pp. 97–136. [Google Scholar]
- Demirbilek, H.; Galcheva, S.; Vuralli, D.; Al-Khawaga, S.; Hussain, K. Ion transporters, channelopathies, and glucose disorders. Int. J. Mol. Sci. 2019, 20, 2590. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Morais-Cabral, J.H.; Kaufman, A.; Mackinnon, R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 Å resolution. Nature 2001, 414, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Morais-Cabral, J.H.; Zhou, Y.; MacKinnon, R. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature 2001, 414, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; MacKinnon, R. A mutant KcsA K(+) channel with altered conduction properties and selectivity filter ion distribution. J. Mol. Biol. 2004, 338, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Morales, J.F.; Cuello, L.G.; Zhao, Y.; Jogini, V.; Cortes, D.M.; Roux, B.; Perozo, E. Molecular determinants of gating at the potassium-channel selectivity filter. Nat. Struct. Mol. Biol. 2006, 13, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Morales, J.F.; Jogini, V.; Lewis, A.; Vasquez, V.; Cortes, D.M.; Roux, B.; Perozo, E. Molecular driving forces determining potassium channel slow inactivation. Nat. Struct. Mol. Biol. 2007, 14, 1062–1069. [Google Scholar] [CrossRef]
- Cordero-Morales, J.F.; Jogini, V.; Chakrapani, S.; Perozo, E. A multipoint hydrogen-bond network underlying KcsA C-type inactivation. Biophys. J. 2011, 100, 2387–2393. [Google Scholar] [CrossRef]
- Cuello, L.G.; Cortes, D.M.; Perozo, E. The gating cycle of a K+ channel at atomic resolution. Elife 2017, 6, e28032. [Google Scholar] [CrossRef]
- Allen, T.W.; Kuyucak, S.; Chung, S.H. Molecular dynamics study of the KcsA potassium channel. Biophys. J. 1999, 77, 2502–2516. [Google Scholar] [CrossRef]
- Bernèche, S.; Roux, B. Molecular dynamics of the KcsA K(+) channel in a bilayer membrane. Biophys. J. 2000, 78, 2900–2917. [Google Scholar] [CrossRef]
- Öster, C.; Hendriks, K.; Kopec, W.; Chevelkov, V.; Shi, C.; Michl, D.; Lange, S.; Sun, H.; de Groot, B.L.; Lange, A. The conduction pathway of potassium channels is water free under physiological conditions. Sci. Adv. 2019, 5, eaaw6756. [Google Scholar] [CrossRef] [PubMed]
- Cuello, L.G.; Jogini, V.; Cortes, D.M.; Perozo, E. Structural mechanism of C-type inactivation in K(+) channels. Nature 2010, 466, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ostmeyer, J.; Boulanger, E.; Rui, H.; Perozo, E.; Roux, B. Chemical substitutions in the selectivity filter of potassium channels do not rule out constricted-like conformations for C-type inactivation. Proc. Natl. Acad. Sci. USA 2017, 114, 11145–11150. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ostmeyer, J.; Cuello, L.G.; Perozo, E.; Roux, B. Rapid constriction of the selectivity filter underlies C-type inactivation in the KcsA potassium channel. J. Gen. Physiol. 2018, 150, 1408–1420. [Google Scholar] [CrossRef]
- Liu, S.; Focke, P.J.; Matulef, K.; Bian, X.; Moënne-Loccoz, P.; Valiyaveetil, F.I.; Lockless, S.W. Ion-binding properties of a K+ channel selectivity filter in different conformations. Proc. Natl. Acad. Sci. USA 2015, 112, 15096–15100. [Google Scholar] [CrossRef]
- Devaraneni, P.K.; Komarov, A.G.; Costantino, C.A.; Devereaux, J.J.; Matulef, K.; Valiyaveetil, F.I. Semisynthetic K+ channels show that the constricted conformation of the selectivity filter is not the C-type inactivated state. Proc. Natl. Acad. Sci. USA 2013, 110, 15698–15703. [Google Scholar] [CrossRef]
- Giudici, A.M.; Renart, M.L.; Díaz-García, C.; Morales, A.; Poveda, J.A.; González-Ros, J.M. Accessibility of Cations to the Selectivity Filter of KcsA in the Inactivated State: An Equilibrium Binding Study. Int. J. Mol. Sci. 2019, 20, 689. [Google Scholar] [CrossRef]
- Lockless, S.W.; Zhou, M.; MacKinnon, R. Structural and thermodynamic properties of selective ion binding in a K+ channel. PLoS Biol. 2007, 5, e121. [Google Scholar] [CrossRef]
- Zhou, Y.; MacKinnon, R. The occupancy of ions in the K+ selectivity filter: Charge balance and coupling of ion binding to a protein conformational change underlie high conduction rates. J. Mol. Biol. 2003, 333, 965–975. [Google Scholar] [CrossRef]
- Montoya, E.; Lourdes Renart, M.; Marcela Giudici, A.; Poveda, J.A.; Fernández, A.M.; Morales, A.; González-Ros, J.M. Differential binding of monovalent cations to KcsA: Deciphering the mechanisms of potassium channel selectivity. Biochim. Biophys. Acta—Biomembr. 2017, 1859, 779–788. [Google Scholar] [CrossRef]
- Matulef, K.; Annen, A.W.; Nix, J.C.; Valiyaveetil, F.I. Individual Ion Binding Sites in the K(+) Channel Play Distinct Roles in C-type Inactivation and in Recovery from Inactivation. Structure 2016, 24, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.A.; Marcela Giudici, A.; Lourdes Renart, M.; Morales, A.; González-Ros, J.M. Towards understanding the molecular basis of ion channel modulation by lipids: Mechanistic models and current paradigms. Biochim. Biophys. Acta—Biomembr. 2017, 1859, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.S.; Koeppe, R.E. Bilayer thickness and membrane protein function: An energetic perspective. Annu. Rev. Biophys. Biomol. Struct. 2007, 36, 107–130. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.G. How lipids affect the activities of integral membrane proteins. Biochim. Biophys. Acta—Biomembr. 2004, 1666, 62–87. [Google Scholar] [CrossRef]
- Marsh, D. Protein modulation of lipids, and vice-versa, in membranes. Biochim. Biophys. Acta—Biomembr. 2008, 1778, 1545–1575. [Google Scholar] [CrossRef]
- Poveda, J.A.; Giudici, A.M.; Renart, M.L.; Molina, M.L.; Montoya, E.; Fernández-Carvajal, A.; Fernández-Ballester, G.; Encinar, J.A.; González-Ros, J.M. Lipid modulation of ion channels through specific binding sites. Biochim. Biophys. Acta—Biomembr. 2014, 1838, 1560–1567. [Google Scholar] [CrossRef]
- Heginbotham, L.; Kolmakova-Partensky, L.; Miller, C. Functional reconstitution of a prokaryotic K+ channel. J. Gen. Physiol. 1998, 111, 741–749. [Google Scholar] [CrossRef]
- Rusinova, R.; Kim, D.M.; Nimigean, C.M.; Andersen, O.S. Regulation of ion channel function by the host lipid bilayer examined by a stopped-flow spectrofluorometric assay. Biophys. J. 2014, 106, 1070–1078. [Google Scholar] [CrossRef]
- Raja, M.; Spelbrink, R.E.J.; de Kruijff, B.; Killian, J.A. Phosphatidic acid plays a special role in stabilizing and folding of the tetrameric potassium channel KcsA. FEBS Lett. 2007, 581, 5715–5722. [Google Scholar] [CrossRef]
- Triano, I.; Barrera, F.N.; Renart, M.L.; Molina, M.L.; Fernández-Ballester, G.; Poveda, J.A.; Fernández, A.M.; Encinar, J.A.; Ferrer-Montiel, A.V.; Otzen, D.; et al. Occupancy of nonannular lipid binding sites on KcsA greatly increases the stability of the tetrameric protein. Biochemistry 2010, 49, 5397–5404. [Google Scholar] [CrossRef]
- Barrera, F.N.; Renart, M.L.; Poveda, J.A.; De Kruijff, B.; Killian, J.A.; González-Ros, J.M. Protein self-assembly and lipid binding in the folding of the potassium channel KcsA. Biochemistry 2008, 47, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Oiki, S. Amphipathic antenna of an inward rectifier K+ channel responds to changes in the inner membrane leaflet. Proc. Natl. Acad. Sci. USA 2013, 110, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Barrantes, F.J. Structural basis for lipid modulation of nicotinic acetylcholine receptor function. Brain Res. Rev. 2004, 47, 71–95. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.G. Biological membranes: The importance of molecular detail. Trends Biochem. Sci. 2011, 36, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Yeagle, P.L. Non-covalent binding of membrane lipids to membrane proteins. Biochim. Biophys. Acta—Biomembr. 2014, 1838, 1548–1559. [Google Scholar] [CrossRef]
- Valiyaveetil, F.I.; Zhou, Y.; MacKinnon, R. Lipids in the structure, folding, and function of the KcsA K+ channel. Biochemistry 2002, 41, 10771–10777. [Google Scholar] [CrossRef]
- Marius, P.; Zagnoni, M.; Sandison, M.E.; Malcolm East, J.; Morgan, H.; Lee, A.G. Binding of anionic lipids to at least three nonannular sites on the potassium channel KcsA is required for channel opening. Biophys. J. 2008, 94, 1689–1698. [Google Scholar] [CrossRef]
- Deol, S.S.; Domene, C.; Bond, P.J.; Sansom, M.S.P. Anionic phospholipid interactions with the potassium channel KcsA: Simulation studies. Biophys. J. 2006, 90, 822–830. [Google Scholar] [CrossRef]
- Weingarth, M.; Prokofyev, A.; Van Der Cruijsen, E.A.W.; Nand, D.; Bonvin, A.M.J.J.; Pongs, O.; Baldus, M. Structural determinants of specific lipid binding to potassium channels. J. Am. Chem. Soc. 2013, 135, 3983–3988. [Google Scholar] [CrossRef]
- Poveda, J.A.; Giudici, A.M.; Renart, M.L.; Millet, O.; Morales, A.; González-Ros, J.M.; Oakes, V.; Furini, S.; Domene, C. Modulation of the potassium channel KcsA by anionic phospholipids: Role of arginines at the non-annular lipid binding sites. Biochim. Biophys. Acta—Biomembr. 2019, 1861, 183029–183043. [Google Scholar] [CrossRef]
- East, J.M.; Melville, D.; Lee, A.G. Exchange rates and numbers of annular lipids for the calcium and magnesium ion dependent adenosinetriphosphatase. Biochemistry 1985, 24, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Marsh, D.; Horváth, L.I. Structure, dynamics and composition of the lipid-protein interface. Perspectives from spin-labelling. Biochim. Biophys. Acta—Rev. Biomembr. 1998, 1376, 267–296. [Google Scholar] [CrossRef]
- Carney, J.; East, J.M.; Mall, S.; Marius, P.; Powl, A.M.; Wright, J.N.; Lee, A.G. Fluorescence Quenching Methods to Study Lipid-Protein Interactions. Curr. Protoc. Protein Sci. 2006, 45, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Alvis, S.J.; Williamson, I.M.; East, J.M.; Lee, A.G. Interactions of Anionic Phospholipids and Phosphatidylethanolamine with the Potassium Channel KcsA. Biophys. J. 2003, 85, 3828–3838. [Google Scholar] [CrossRef]
- Williamson, I.M.; Alvis, S.J.; Malcolm East, J.; Lee, A.G. Interactions of phospholipids with the potassium channel KcsA. Biophys. J. 2002, 83, 2026–2038. [Google Scholar] [CrossRef]
- Sehgal, P.; Otzen, D.E. Thermodynamics of unfolding of an integral membrane protein in mixed micelles. Protein Sci. 2006, 15, 890–899. [Google Scholar] [CrossRef]
- Sturtevant, J.M. Biochemical Applications of Differential Scanning Calorimetry. Annu. Rev. Phys. Chem. 1987, 38, 463–488. [Google Scholar] [CrossRef]
- Cooper, A.; Nutley, M.A.; Wadood, A. Differential scanning microcalorimetry. In Protein-Ligand Interactions: Hydrodynamics and Calorimetry; Harding, S.E., Chowdhry, B.Z., Eds.; Oxford University Press: Oxford, UK, 2000; pp. 287–318. [Google Scholar]
- Kooijman, E.E.; Tieleman, D.P.; Testerink, C.; Munnik, T.; Rijkers, D.T.S.; Burger, K.N.J.; de Kruijff, B. An electrostatic/hydrogen bond switch as the basis for the specific interaction of phosphatidic acid with proteins. J. Biol. Chem. 2007, 282, 11356–11364. [Google Scholar] [CrossRef]
- Callahan, K.M.; Mondou, B.; Sasseville, L.; Schwartz, J.L.; D’Avanzo, N. The influence of membrane bilayer thickness on KcsA channel activity. Channels 2019, 13, 424–439. [Google Scholar] [CrossRef]
- Liu, S.; Bian, X.; Lockless, S.W. Preferential binding of K+ ions in the selectivity filter at equilibrium explains high selectivity of K+ channels. J. Gen. Physiol. 2012, 140, 671–679. [Google Scholar] [CrossRef]
- Liu, S.; Lockless, S.W. Equilibrium selectivity alone does not create K + -selective ion conduction in K + channels. Nat. Commun. 2013, 4, 2746–2753. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.A.; Tzitzilonis, C.; Kwiatkowski, W.; Choe, S.; Riek, R. Conformational dynamics of the KcsA potassium channel governs gating properties. Nat. Struct. Mol. Biol. 2007, 14, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Chill, J.H. NMR study of the tetrameric KcsA potassium channel in detergent micelles. Protein Sci. 2006, 15, 684–698. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Osawa, M.; Takeuchi, K.; Shimada, I. Structural basis underlying the dual gate properties of KcsA. Proc. Natl. Acad. Sci. USA 2010, 107, 6216–6221. [Google Scholar] [CrossRef] [PubMed]
- Renart, M.L.; Barrera, F.N.; Molina, M.L.; Encinar, J.A.; Poveda, J.A.; Fernández, A.M.; Gómez, J.; González-Ros, J.M. Effects of conducting and blocking ions on the structure and stability of the potassium channel KcsA. J. Biol. Chem. 2006, 281, 29905–29915. [Google Scholar] [CrossRef]
- Renart, M.L.; Triano, I.; Poveda, J.A.; Encinar, J.A.; Fernández, A.M.; Ferrer-Montiel, A.V.; Gómez, J.; González Ros, J.M. Ion binding to KcsA: Implications in ion selectivity and channel gating. Biochemistry 2010, 49, 9480–9487. [Google Scholar] [CrossRef]
- Renart, M.L.; Montoya, E.; Fernández, A.M.; Molina, M.L.; Poveda, J.A.; Encinar, J.A.; Ayala, J.L.; Ferrer-Montiel, A.V.; Gómez, J.; Morales, A.; et al. Contribution of ion binding affinity to ion selectivity and permeation in KcsA, a model potassium channel. Biochemistry 2012, 51, 3891–3900. [Google Scholar] [CrossRef]
- Renart, M.L.; Giudici, A.M.; Poveda, J.A.; Fedorov, A.; Berberan-Santos, M.N.; Prieto, M.; Díaz-García, C.; González-Ros, J.M.; Coutinho, A. Conformational plasticity in the KcsA potassium channel pore helix revealed by homo-FRET studies. Sci. Rep. 2019, 9, 6215–6228. [Google Scholar] [CrossRef]
- Renart, M.L.; Montoya, E.; Giudici, A.M.; Poveda, J.A.; Fernández, A.M.; Morales, A.; González-Ros, J.M. Selective exclusion and selective binding both contribute to ion selectivity in KcsA, a model potassium channel. J. Biol. Chem. 2017, 292, 15552–15560. [Google Scholar] [CrossRef]
- Kratochvil, H.T.; Maj, M.; Matulef, K.; Annen, A.W.; Ostmeyer, J.; Perozo, E.; Roux, B.; Valiyaveetil, F.I.; Zanni, M.T. Probing the Effects of Gating on the Ion Occupancy of the K+ Channel Selectivity Filter Using Two-Dimensional Infrared Spectroscopy. J. Am. Chem. Soc. 2017, 139, 8837–8845. [Google Scholar] [CrossRef]
- Encinar, J.A.; Molina, M.L.; Poveda, J.A.; Barrera, F.N.; Renart, M.L.; Fernández, A.M.; González-Ros, J.M. The influence of a membrane environment on the structure and stability of a prokaryotic potassium channel, KcsA. FEBS Lett. 2005, 579, 5199–5204. [Google Scholar] [CrossRef] [PubMed]
- Chipot, C.; Dehez, F.; Schnell, J.R.; Zitzmann, N.; Pebay-Peyroula, E.; Catoire, L.J.; Miroux, B.; Kunji, E.R.S.; Veglia, G.; Cross, T.A.; et al. Perturbations of Native Membrane Protein Structure in Alkyl Phosphocholine Detergents: A Critical Assessment of NMR and Biophysical Studies. Chem. Rev. 2018, 118, 3559–3607. [Google Scholar] [CrossRef] [PubMed]
- Wylie, B.J.; Bhate, M.P.; McDermott, A.E. Transmembrane allosteric coupling of the gates in a potassium channel. Proc. Natl. Acad. Sci. USA 2014, 111, 185–190. [Google Scholar] [CrossRef]
- Bhate, M.P.; Wylie, B.J.; Tian, L.; McDermott, A.E. Conformational dynamics in the selectivity filter of KcsA in response to potassium ion concentration. J. Mol. Biol. 2010, 401, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Ader, C.; Schneider, R.; Hornig, S.; Velisetty, P.; Vardanyan, V.; Giller, K.; Ohmert, I.; Becker, S.; Pongs, O.; Baldus, M. Coupling of activation and inactivation gate in a K-channel: Potassium and ligand sensitivity. EMBO J. 2009, 28, 2825–2834. [Google Scholar] [CrossRef]
- Xu, Y.; Bhate, M.P.; McDermott, A.E. Transmembrane allosteric energetics characterization for strong coupling between proton and potassium ion binding in the KcsA channel. Proc. Natl. Acad. Sci. USA 2017, 114, 8788–8793. [Google Scholar] [CrossRef]
- Qasim, A.; Sher, I.; Hirschhorn, O.; Shaked, H.; Qasem, Z.; Ruthstein, S.; Chill, J.H. Investigation of a KcsA Cytoplasmic pH Gate in Lipoprotein Nanodiscs. Chembiochem 2019, 20, 813–821. [Google Scholar] [CrossRef]
- Dörr, J.M.; Koorengevel, M.C.; Schäfer, M.; Prokofyev, A.V.; Scheidelaar, S.; van der Cruijsen, E.A.W.; Dafforn, T.R.; Baldus, M.; Killian, J.A. Detergent-free isolation, characterization, and functional reconstitution of a tetrameric K+ channel: The power of native nanodiscs. Proc. Natl. Acad. Sci. USA 2014, 111, 18607–18612. [Google Scholar] [CrossRef]
- Shenkarev, Z.O.; Lyukmanova, E.N.; Solozhenkin, O.I.; Gagnidze, I.E.; Nekrasova, O.V.; Chupin, V.V.; Tagaev, A.A.; Yakimenko, Z.A.; Ovchinnikova, T.V.; Kirpichnikov, M.P.; et al. Lipid-protein nanodiscs: Possible application in high-resolution NMR investigations of membrane proteins and membrane-active peptides. Biochemistry. (Mosc.) 2009, 74, 756–765. [Google Scholar] [CrossRef]
- van Dalen, A.; Schrempf, H.; Killian, J.A.; de Kruijff, B. Efficient membrane assembly of the KcsA potassium channel in Escherichia coli requires the protonmotive force. EMBO Rep. 2000, 1, 340–346. [Google Scholar] [CrossRef]
- Van Dalen, A.; Hegger, S.; Killian, J.A.; De Kruijff, B. Influence of lipids on membrane assembly and stability of the potassium channel KcsA. FEBS Lett. 2002, 525, 33–38. [Google Scholar] [CrossRef]
- Ando, M.; Akiyama, M.; Okuno, D.; Hirano, M.; Ide, T.; Sawada, S.; Sasaki, Y.; Akiyoshi, K. Liposome chaperon in cell-free membrane protein synthesis: One-step preparation of KcsA-integrated liposomes and electrophysiological analysis by the planar bilayer method. Biomater. Sci. 2016, 4, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Focke, P.J.; Hein, C.; Hoffmann, B.; Matulef, K.; Bernhard, F.; Dötsch, V.; Valiyaveetil, F.I. Combining in Vitro Folding with Cell Free Protein Synthesis for Membrane Protein Expression. Biochemistry 2016, 55, 4212–4219. [Google Scholar] [CrossRef] [PubMed]
- Vaish, A.; Guo, S.; Murray, R.M.; Grandsard, P.J.; Chen, Q. On-chip membrane protein cell-free expression enables development of a direct binding assay: A curious case of potassium channel KcsA-Kv1.3. Anal. Biochem. 2018, 556, 70–77. [Google Scholar] [CrossRef]
- Barrera, F.N.; Renart, M.L.; Molina, M.L.; Poveda, J.A.; Encinar, J.A.; Fernández, A.M.; Neira, J.L.; González-Ros, J.M. Unfolding and refolding in vitro of a tetrameric, alpha-helical membrane protein: The prokaryotic potassium channel KcsA. Biochemistry 2005, 44, 14344–14352. [Google Scholar] [CrossRef]
- van den Brink-van der Laan, E.; Chupin, V.; Killian, J.A.; de Kruijff, B. Stability of KcsA tetramer depends on membrane lateral pressure. Biochemistry 2004, 43, 4240–4250. [Google Scholar] [CrossRef]
- Raja, M. The role of extramembranous cytoplasmic termini in assembly and stability of the tetrameric K(+)-channel KcsA. J. Membr. Biol. 2010, 235, 51–61. [Google Scholar] [CrossRef][Green Version]
- Molina, M.L.; Barrera, F.N.; Fernandez, A.M.; Poveda, J.A.; Renart, M.L.; Encinar, J.A.; Riquelme, G.; Gonzalez-Ros, J.M. Clustering and coupled gating modulate the activity in KcsA, a potassium channel model. J. Biol. Chem. 2006, 281, 18837–18848. [Google Scholar] [CrossRef]
- Molina, M.L.; Encinar, J.A.; Barrera, F.N.; Fernandez-Ballester, G.; Riquelme, G.; Gonzalez-Ros, J.M. Influence of C-terminal protein domains and protein-lipid interactions on tetramerization and stability of the potassium channel KcsA. Biochemistry 2004, 43, 14924–14931. [Google Scholar] [CrossRef]
- Seeger, H.M.; Bortolotti, C.A.; Alessandrini, A.; Facci, P. Phase-transition-induced protein redistribution in lipid bilayers. J. Phys. Chem. B 2009, 113, 16654–16659. [Google Scholar] [CrossRef]
- Giudici, A.M.; Molina, M.L.; Ayala, J.L.; Montoya, E.; Renart, M.L.; Fernández, A.M.; Encinar, J.A.; Ferrer-Montiel, A.V.; Poveda, J.A.; González-Ros, J.M. Detergent-labile, supramolecular assemblies of KcsA: Relative abundance and interactions involved. Biochim. Biophys. Acta 2013, 1828, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Hegermann, J.; Overbeck, J.; Schrempf, H. In vivo monitoring of the potassium channel KcsA in Streptomyces lividans hyphae using immuno-electron microscopy and energy-filtering transmission electron microscopy. Microbiology 2006, 152, 2831–2841. [Google Scholar] [CrossRef] [PubMed]
- Raja, M.; Vales, E. Effects of sodium chloride on membrane fusion and on the formation of aggregates of potassium channel KcsA in Escherichia coli membrane. Biophys. Chem. 2009, 142, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Ujwal, R.; Cascio, D.; Chaptal, V.; Ping, P.; Abramson, J. Crystal packing analysis of murine VDAC1 crystals in a lipidic environment reveals novel insights on oligomerization and orientation. Channels (Austin) 2009, 3, 167–170. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Molina, M.L.; Giudici, A.M.; Poveda, J.A.; Fernández-Ballester, G.; Montoya, E.; Renart, M.L.; Fernández, A.M.; Encinar, J.A.; Riquelme, G.; Morales, A.; et al. Competing Lipid-Protein and Protein-Protein Interactions Determine Clustering and Gating Patterns in the Potassium Channel from Streptomyces lividans (KcsA). J. Biol. Chem. 2015, 290, 25745–25755. [Google Scholar] [CrossRef]
- Schrempf, H.; Schmidt, O.; Kümmerlen, R.; Hinnah, S.; Müller, D.; Betzler, M.; Steinkamp, T.; Wagner, R. A prokaryotic potassium ion channel with two predicted transmembrane segments from Streptomyces lividans. EMBO J. 1995, 14, 5170–5178. [Google Scholar] [CrossRef]
- Cuello, L.G.; Romero, J.G.; Cortes, D.M.; Perozo, E. pH-dependent gating in the Streptomyces lividans K+ channel. Biochemistry 1998, 37, 3229–3236. [Google Scholar] [CrossRef]
- Meuser, D.; Splitt, H.; Wagner, R.; Schrempf, H. Exploring the open pore of the potassium channel from Streptomyces lividans. FEBS Lett. 1999, 462, 447–452. [Google Scholar] [CrossRef]
- Kazuteru, D.; Tatsunori, O.; Tan, S.H.; Nanda, V. Mutations stabilizing an open conformation within the external region of the permeation pathway of the potassium channel KcsA. Eur. Biophys. J. 2001, 30, 385–391. [Google Scholar]
- Navedo, M.F.; Cheng, E.P.; Yuan, C.; Votaw, S.; Molkentin, J.D.; Scott, J.D.; Santana, L.F. Increased coupled gating of L-type Ca2+ channels during hypertension and Timothy syndrome. Circ. Res. 2010, 106, 748–756. [Google Scholar] [CrossRef]
- Grage, S.L.; Keleshian, A.M.; Turdzeladze, T.; Battle, A.R.; Tay, W.C.; May, R.P.; Holt, S.A.; Contera, S.A.; Haertlein, M.; Moulin, M.; et al. Bilayer-mediated clustering and functional interaction of MscL channels. Biophys. J. 2011, 100, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Vivas, O.; Moreno, C.M.; Santana, L.F.; Hille, B. Proximal clustering between BK and CaV1.3 channels promotes functional coupling and BK channel activation at low voltage. Elife 2017, 6, e28029. [Google Scholar] [CrossRef] [PubMed]
- Spira, F.; Mueller, N.S.; Beck, G.; Von Olshausen, P.; Beig, J.; Wedlich-Söldner, R. Patchwork organization of the yeast plasma membrane into numerous coexisting domains. Nat. Cell Biol. 2012, 14, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.S.; Wedlich-Söldner, R.; Spira, F. From mosaic to patchwork: Matching lipids and proteins in membrane organization. Mol. Membr. Biol. 2012, 29, 186–196. [Google Scholar] [CrossRef]
- Choi, K.-H. Cooperative gating between ion channels. Gen. Physiol. Biophys. 2014, 33, 1–12. [Google Scholar] [CrossRef]
- Kim, G.E.; Kronengold, J.; Barcia, G.; Quraishi, I.H.; Martin, H.C.; Blair, E.; Taylor, J.C.; Dulac, O.; Colleaux, L.; Nabbout, R.; et al. Human slack potassium channel mutations increase positive cooperativity between individual channels. Cell Rep. 2014, 9, 1661–1672. [Google Scholar] [CrossRef]
- Dixon, R.E.; Moreno, C.M.; Yuan, C.; Opitz-Araya, X.; Binder, M.D.; Navedo, M.F.; Santana, L.F. Graded Ca2+/calmodulin-dependent coupling of voltage-gated CaV1.2 channels. Elife 2015, 4, e05608. [Google Scholar] [CrossRef]
- Moreno, C.M.; Dixon, R.E.; Tajada, S.; Yuan, C.; Opitz-Araya, X.; Binder, M.D.; Santana, L.F. Ca(2+) entry into neurons is facilitated by cooperative gating of clustered CaV1.3 channels. Elife 2016, 5, e15744. [Google Scholar] [CrossRef]
- Gianoli, F.; Risler, T.; Kozlov, A.S. Lipid bilayer mediates ion-channel cooperativity in a model of hair-cell mechanotransduction. Proc. Natl. Acad. Sci. USA 2017, 114, E11010–E11019. [Google Scholar] [CrossRef]
- Clatot, J.; Hoshi, M.; Wan, X.; Liu, H.; Jain, A.; Shinlapawittayatorn, K.; Marionneau, C.; Ficker, E.; Ha, T.; Deschênes, I. Voltage-gated sodium channels assemble and gate as dimers. Nat. Commun. 2017, 8, 2077–2091. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renart, M.L.; Giudici, A.M.; Díaz-García, C.; Molina, M.L.; Morales, A.; González-Ros, J.M.; Poveda, J.A. Modulation of Function, Structure and Clustering of K+ Channels by Lipids: Lessons Learnt from KcsA. Int. J. Mol. Sci. 2020, 21, 2554. https://doi.org/10.3390/ijms21072554
Renart ML, Giudici AM, Díaz-García C, Molina ML, Morales A, González-Ros JM, Poveda JA. Modulation of Function, Structure and Clustering of K+ Channels by Lipids: Lessons Learnt from KcsA. International Journal of Molecular Sciences. 2020; 21(7):2554. https://doi.org/10.3390/ijms21072554
Chicago/Turabian StyleRenart, María Lourdes, Ana Marcela Giudici, Clara Díaz-García, María Luisa Molina, Andrés Morales, José M. González-Ros, and José Antonio Poveda. 2020. "Modulation of Function, Structure and Clustering of K+ Channels by Lipids: Lessons Learnt from KcsA" International Journal of Molecular Sciences 21, no. 7: 2554. https://doi.org/10.3390/ijms21072554
APA StyleRenart, M. L., Giudici, A. M., Díaz-García, C., Molina, M. L., Morales, A., González-Ros, J. M., & Poveda, J. A. (2020). Modulation of Function, Structure and Clustering of K+ Channels by Lipids: Lessons Learnt from KcsA. International Journal of Molecular Sciences, 21(7), 2554. https://doi.org/10.3390/ijms21072554