Role of Somatostatin in the Regulation of Central and Peripheral Factors of Satiety and Obesity
Abstract
:1. Introduction
2. Obesity and Satiety
2.1. Role of Hypothalamus in Obesity and Satiety
2.2. Role of Peripheral Hormones on Obesity and Satiety
2.3. Link between the Hypothalamus and Peripheral Hormones in the Regulation of Obesity and Satiety
3. Distribution of Somatostatin and Somatostatin Receptors
3.1. Somatostatin Receptors
3.2. Role of Somatostatin and Its Receptors in Satiety
3.3. Effect of Somatostatin and Its Analogues on Satiety
3.4. Somatostatin and Obesity
3.5. Role of Somatostatin Receptor in Regulation of Food Intake Behaviour
4. Role of Somatostatin in Regulation of Brain-Derived Neurotrophic Factor Induced Appetite
5. Role of Somatostatin and Somatostatin Receptors in Stress and Anxiety Mediated Food Intake Behaviour
6. Somatostatin, Glucocorticoids and Food-Seeking Behaviour
7. Role of Somatostatin in Regulation of Insulin, Leptin, POMC, AgRP and Ghrelin Induced Signaling on Food Intake
8. Role of Somatostatin, nNOS and Cannabinoid Receptor in the Regulation of Appetite
9. Functional Cross-talk Between Receptor Proteins: A Mode for the Promotion of Satiety or Obesity: Future Perspective
10. Somatostatin, Satiety, and Obesity—What Is the Link?
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACTH | Adrenocorticotropic Hormone |
| AgRP | Agouti-Related Peptide |
| ARC | Arcuate Nucleus |
| BBB | Blood Brain Barrier |
| BDNF | Brain-Derived Neurotrophic Factor |
| CART | Cocaine- And Amphetamine-Regulated Transcript |
| CB1R | Endocannabinoids Receptor 1 |
| CCK | Cholecystokinin |
| CCK | Cholecystokinin |
| CNS | Central Nervous System |
| CNS | Central Nervous System |
| CRH | Corticotropin-releasing Hormone |
| CRH | Corticotropin- Releasing Hormone |
| DA | Dopamine |
| DMN | Dorsomedial Nucleus |
| GCs | Glucocorticoids |
| GH | Growth Hormone |
| GHRH | Growth Hormone-Releasing Hormone |
| GLP-1 | Glucagon-like Peptide 1 |
| GIT | Gastrointestinal tract |
| GPCR | G-protein Coupled Receptor |
| HPA | Hypothalamic-Pituitary-Adrenal Axis |
| ICV | Intracerebroventricular injection |
| LHA | Lateral Hypothalamic Area |
| MCR | Melanocortin Receptor |
| MSH | Melanocyte Stimulating Hormone |
| NAc | Nucleus Accumbens |
| nNOS | neuronal Nitric Oxide Synthase |
| NPY | Neuropeptide Y |
| NTS | Nucleus Tractus Solitarius |
| OCT | Octreotide |
| OX | Orexin |
| PeVN | Periventicular Nucleus |
| POMC | Proopiomelanocortin |
| PVN | Paraventicular Nucleus |
| RCT | Randomized Controlled Trial |
| SST | Somatostatin |
| SST-14 | Somatostatin-14 |
| SST-28 | Somatostatin-28 |
| SSTR | Somatostatin Receptors |
| TrkB | Tyrosine Kinase Receptor B |
| VMN | Ventromedial Nucleus |
| VTA | Ventral Tegmental Area |
References
- Brazeau, P.; Vale, W.; Burgus, R.; Ling, N.; Butcher, M.; Rivier, J.; Guillemin, R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 1973, 179, 77–79. [Google Scholar] [CrossRef]
- Kumar, U.; Grant, M. Somatostatin and somatostatin receptors. Results Probl. Cell Differ. 2010, 50, 137–184. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.C. Somatostatin and its receptor family. Frontiers Neuroendocrinol. 1999, 20, 157–198. [Google Scholar] [CrossRef]
- Reichlin, S. Somatostatin and its receptor. Introduction. Ciba Found. Symp. 1995, 190, 1–6. [Google Scholar] [PubMed]
- Goo, T.; Akiba, Y.; Kaunitz, J.D. Mechanisms of intragastric pH sensing. Curr. Gastroenterol. Rep. 2010, 12, 465–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucey, M.R. Endogenous Somatostatin and the Gut. Gut 1986, 27, 457–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reisine, T.; Woulfe, D.; Raynor, K.; Kong, H.; Heerding, J.; Hines, J.; Tallent, M.; Law, S. Interaction of somatostatin receptors with G proteins and cellular effector systems. Ciba Found. Symp. 1995, 190, 160–167. [Google Scholar] [CrossRef]
- Benuck, M.; Marks, N. Differences in the degradation of hypothalamic releasing factors by rat and human serum. Life Sci. 1976, 19, 1271–1276. [Google Scholar] [CrossRef]
- Boehm, B.O.; Lustig, R.H. Use of somatostatin receptor ligands in obesity and diabetic complications. Best Pract. Res. Clin. Gastroenterol. 2002, 16, 493–509. [Google Scholar] [CrossRef]
- Hernandez, C.; Simo-Servat, O.; Simo, R. Somatostatin and diabetic retinopathy: Current concepts and new therapeutic perspectives. Endocrine 2014, 46, 209–214. [Google Scholar] [CrossRef]
- Pinter, E.; Helyes, Z.; Szolcsanyi, J. Inhibitory effect of somatostatin on inflammation and nociception. Pharmacol. Ther. 2006, 112, 440–456. [Google Scholar] [CrossRef] [PubMed]
- Pyronnet, S.; Bousquet, C.; Najib, S.; Azar, R.; Laklai, H.; Susini, C. Antitumor effects of somatostatin. Mol. Cell. Endocrinol. 2008, 286, 230–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, U.; Thrimawithana, T.R.; Valery, C.; Young, S.A. Therapeutic uses of somatostatin and its analogues: Current view and potential applications. Pharmacol. Ther. 2015, 152, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Haffer, K.N. Effects of novel vaccines on weight loss in diet-induced-obese (DIO) mice. J. Anim. Sci. Biotechnol. 2012, 3, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, J.; Marks, D. Hormonal regulators of appetite. Int. J. Pediatric Endocrinol. 2009, 2009, 141753. [Google Scholar] [CrossRef]
- Rutter, G.A. Regulating glucagon secretion: Somatostatin in the spotlight. Diabetes 2009, 58, 299–301. [Google Scholar] [CrossRef] [Green Version]
- Tagi, V.M.; Giannini, C.; Chiarelli, F. Insulin Resistance in Children. Front. Endocrinol. 2019, 10, 342. [Google Scholar] [CrossRef] [Green Version]
- Medina-Urrutia, A.; Posadas-Romero, C.; Posadas-Sanchez, R.; Jorge-Galarza, E.; Villarreal-Molina, T.; Gonzalez-Salazar Mdel, C.; Cardoso-Saldana, G.; Vargas-Alarcon, G.; Torres-Tamayo, M.; Juarez-Rojas, J.G. Role of adiponectin and free fatty acids on the association between abdominal visceral fat and insulin resistance. Cardiovasc. Diabetol. 2015, 14, 20. [Google Scholar] [CrossRef] [Green Version]
- Sears, B.; Perry, M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015, 14, 121. [Google Scholar] [CrossRef] [Green Version]
- Tzotzas, T.; Papazisis, K.; Perros, P.; Krassas, G.E. Use of Somatostatin Analogues in Obesity. Drugs 2008, 68, 1963–1973. [Google Scholar] [CrossRef]
- Foxx-Orenstein, A.; Camilleri, M.; Stephens, D.; Burton, D. Effect of a somatostatin analogue on gastric motor and sensory functions in healthy humans. Gut 2003, 52, 1555–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdu, F.; Hicks, G.A.; Hennig, G.; Allen, J.P.; Grundy, D. Somatostatin sst(2) receptors inhibit peristalsis in the rat and mouse jejunum. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G624–G633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, P.; Rabi, S.; Kulothungan, P. Aminoguanidine, selective nitric oxide synthase inhibitor, ameliorates cyclophosphamide-induced hemorrhagic cystitis by inhibiting protein nitration and PARS activation. Urology 2009, 73, 1402–1406. [Google Scholar] [CrossRef] [PubMed]
- Gerich, J.E.; Lorenzi, M.; Schneider, V.; Karam, J.H.; Rivier, J.; Guillemin, R.; Forsham, P.H. Effects of Somatostatin on Plasma Glucose and Glucagon Levels in Human Diabetes-Mellitus-Pathophysiologic and Therapeutic Implications. New Engl. J. Med. 1974, 291, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Lieverse, R.J.; Jansen, J.B.; Masclee, A.M.; Lamers, C.B. Effects of somatostatin on human satiety. Neuroendocrinology 1995, 61, 112–116. [Google Scholar] [CrossRef]
- Stengel, A.; Karasawa, H.; Tache, Y. The role of brain somatostatin receptor 2 in the regulation of feeding and drinking behavior. Horm. Behav. 2015, 73, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef]
- Arroyo-Johnson, C.; Mincey, K.D. Obesity Epidemiology Worldwide. Gastroenterol. Clin. North. Am. 2016, 45, 571–579. [Google Scholar] [CrossRef] [Green Version]
- Volkow, N.D.; Wang, G.J.; Baler, R.D. Reward, dopamine and the control of food intake: Implications for obesity. Trends Cogn. Sci. 2011, 15, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Wolfenden, L.; Ezzati, M.; Larijani, B.; Dietz, W. The challenge for global health systems in preventing and managing obesity. Obes. Rev. 2019. [Google Scholar] [CrossRef]
- Finucane, M.M.; Stevens, G.A.; Cowan, M.J.; Danaei, G.; Lin, J.K.; Paciorek, C.J.; Singh, G.M.; Gutierrez, H.R.; Lu, Y.A.; Bahalim, A.N.; et al. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011, 377, 557–567. [Google Scholar] [CrossRef] [Green Version]
- Rossi, M.A.; Basiri, M.L.; McHenry, J.A.; Kosyk, O.; Otis, J.M.; van den Munkhof, H.E.; Bryois, J.; Hubel, C.; Breen, G.; Guo, W.; et al. Obesity remodels activity and transcriptional state of a lateral hypothalamic brake on feeding. Science 2019, 364, 1271–1274. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.; Yang, W.; Chen, C.S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437. [Google Scholar] [CrossRef] [Green Version]
- Ajeganova, S.; Andersson, M.L.; Hafstrom, I.; Grp, B.S. Association of obesity with worse disease severity in rheumatoid arthritis as well as with comorbidities: A long-term followup from disease onset. Arthrit Care Res. 2013, 65, 78–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aleksandrova, K.; Stelmach-Mardas, M.; Schlesinger, S. Obesity and Liver Cancer. Recent Results Cancer Res. 2016, 208, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Ashwell, M.; Gunn, P.; Gibson, S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: Systematic review and meta-analysis. Obes. Rev. 2012, 13, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Giovannucci, E. Obesity and Prostate Cancer. Recent Results Cancer Res. 2016, 208, 137–153. [Google Scholar] [CrossRef]
- Clark, M.; Hoenig, M. Metabolic Effects of Obesity and Its Interaction with Endocrine Diseases. Vet. Clin. North Am. Small Anim. Pract. 2016, 46, 797–815. [Google Scholar] [CrossRef]
- Frayn, K.N. Obesity and metabolic disease: Is adipose tissue the culprit? Proc. Nutr. Soc. 2005, 64, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Golay, A.; Ybarra, J. Link between obesity and type 2 diabetes. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 649–663. [Google Scholar] [CrossRef]
- Jochem, C.; Leitzmann, M. Obesity and Colorectal Cancer. Recent Results Cancer Res. 2016, 208, 17–41. [Google Scholar] [CrossRef] [PubMed]
- Kokkoris, P.; Pi-Sunyer, F.X. Obesity and endocrine disease. Endocrinol. Metab. Clin. North Am. 2003, 32, 895–914. [Google Scholar] [CrossRef]
- Le Magueresse-Battistoni, B.; Labaronne, E.; Vidal, H.; Naville, D. Endocrine disrupting chemicals in mixture and obesity, diabetes and related metabolic disorders. World J. Biol. Chem. 2017, 8, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Onstad, M.A.; Schmandt, R.E.; Lu, K.H. Addressing the Role of Obesity in Endometrial Cancer Risk, Prevention, and Treatment. J. Clin. Oncol. 2016, 34, 4225. [Google Scholar] [CrossRef] [PubMed]
- Picon-Ruiz, M.; Morata-Tarifa, C.; Valle-Goffin, J.J.; Friedman, E.R.; Slingerland, J.M. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J. Clin. 2017, 67, 378–397. [Google Scholar] [CrossRef]
- Tworoger, S.S.; Huang, T. Obesity and Ovarian Cancer. Recent Results Cancer Res. 2016, 208, 155–176. [Google Scholar] [CrossRef]
- Wilson, K.M.; Cho, E. Obesity and Kidney Cancer. Recent Results Cancer Res. 2016, 208, 81–93. [Google Scholar] [CrossRef]
- Albuquerque, D.; Nobrega, C.; Manco, L.; Padez, C. The contribution of genetics and environment to obesity. Br. Med. Bull. 2017, 123, 159–173. [Google Scholar] [CrossRef]
- Keesey, R.E.; Powley, T.L. Body energy homeostasis. Appetite 2008, 51, 442–445. [Google Scholar] [CrossRef] [Green Version]
- Hagan, S.; Niswender, K.D. Neuroendocrine regulation of food intake. Pediatr. Blood Cancer 2012, 58, 149–153. [Google Scholar] [CrossRef]
- Rodgers, R.J.; Tschop, M.H.; Wilding, J.P. Anti-obesity drugs: Past, present and future. Dis. Model. Mech. 2012, 5, 621–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coll, A.P.; Farooqi, I.S.; O’Rahilly, S. The hormonal control of food intake. Cell 2007, 129, 251–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swinburn, B.A.; Caterson, I.; Seidell, J.C.; James, W.P. Diet, nutrition and the prevention of excess weight gain and obesity. Public Health Nutr. 2004, 7, 123–146. [Google Scholar] [CrossRef]
- Strubbe, J.H.; Woods, S.C. The timing of meals. Psychol Rev. 2004, 111, 128–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central nervous system control of food intake and body weight. Nature 2006, 443, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Overduin, J. Gastrointestinal regulation of food intake. J. Clin. Invest. 2007, 117, 13–23. [Google Scholar] [CrossRef]
- Elmquist, J.K.; Bjorbaek, C.; Ahima, R.S.; Flier, J.S.; Saper, C.B. Distributions of leptin receptor mRNA isoforms in the rat brain. J. Comp. Neurol 1998, 395, 535–547. [Google Scholar] [CrossRef]
- Morton, G.J.; Blevins, J.E.; Williams, D.L.; Niswender, K.D.; Gelling, R.W.; Rhodes, C.J.; Baskin, D.G.; Schwartz, M.W. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J. Clin. Invest. 2005, 115, 703–710. [Google Scholar] [CrossRef] [Green Version]
- Emond, M.; Schwartz, G.J.; Ladenheim, E.E.; Moran, T.H. Central leptin modulates behavioral and neural responsivity to CCK. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1999, 276, R1545–R1549. [Google Scholar] [CrossRef]
- Blevins, J.E.; Schwartz, M.W.; Baskin, D.G. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R87–R96. [Google Scholar] [CrossRef] [Green Version]
- Grill, H.J.; Schwartz, M.W.; Kaplan, J.M.; Foxhall, J.S.; Breininger, J.; Baskin, D.G. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology 2002, 143, 239–246. [Google Scholar] [CrossRef]
- MacLean, P.S.; Blundell, J.E.; Mennella, J.A.; Batterham, R.L. Biological control of appetite: A daunting complexity. Obesity 2017, 25 (Suppl. 1), S8–S16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Graaf, C.; Blom, W.A.; Smeets, P.A.; Stafleu, A.; Hendriks, H.F. Biomarkers of satiation and satiety. Am. J. Clin. Nutr. 2004, 79, 946–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, M.W.; Woods, S.C.; Porte, D., Jr.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S.; Antwi, D.A. Brain regulation of appetite and satiety. Endocrinol. Metab. Clin. North Am. 2008, 37, 811–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qualls-Creekmore, E.; Munzberg, H. Modulation of Feeding and Associated Behaviors by Lateral Hypothalamic Circuits. Endocrinology 2018, 159, 3631–3642. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, G.C. The Role of Depot Fat in the Hypothalamic Control of Food Intake in the Rat. Proc. R. Soc. Ser. B Biol. Sci. 1953, 140, 578–592. [Google Scholar] [CrossRef]
- Roh, E.; Song, D.K.; Kim, M.S. Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp. Mol. Med. 2016, 48. [Google Scholar] [CrossRef] [Green Version]
- Timper, K.; Bruning, J.C. Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Models Mech. 2017, 10, 679–689. [Google Scholar] [CrossRef] [Green Version]
- Bouret, S.G.; Draper, S.J.; Simerly, R.B. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J. Neurosci. 2004, 24, 2797–2805. [Google Scholar] [CrossRef]
- Simpson, K.A.; Martin, N.M.; Bloom, S.R. Hypothalamic regulation of food intake and clinical therapeutic applications. Arq. Bras. Endocrinol. Metabol. 2009, 53, 120–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leibowitz, S.F.; Hammer, N.J.; Chang, K. Hypothalamic Paraventricular Nucleus Lesions Produce Overeating and Obesity in the Rat. Physiol. Behav. 1981, 27, 1031–1040. [Google Scholar] [CrossRef]
- Xu, B.; Xie, X. Neurotrophic factor control of satiety and body weight. Nat. Rev. Neurosci. 2016, 17, 282–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoebel, B.G.; Teitelbaum, P. Hypothalamic Control of Feeding and Self-Stimulation. Science 1962, 135, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Petrovich, G.D. Lateral Hypothalamus as a Motivation-Cognition Interface in the Control of Feeding Behavior. Front. Syst. Neurosci. 2018, 12. [Google Scholar] [CrossRef]
- Heisler, L.K.; Lam, D.D. An appetite for life: Brain regulation of hunger and satiety. Curr. Opin. Pharmacol. 2017, 37, 100–106. [Google Scholar] [CrossRef]
- Moriguchi, T.; Sakurai, T.; Nambu, T.; Yanagisawa, M.; Goto, K. Neurons containing orexin in the lateral hypothalamic area of the adult rat brain are activated by insulin-induced acute hypoglycemia. Neurosci. Lett. 1999, 264, 101–104. [Google Scholar] [CrossRef]
- Milbank, E.; Lopez, M. Orexins/Hypocretins: Key Regulators of Energy Homeostasis. Frontiers Endocrinol. 2019, 10, 830. [Google Scholar] [CrossRef]
- De Silva, A.; Bloom, S.R. Gut Hormones and Appetite Control: A Focus on PYY and GLP-1 as Therapeutic Targets in Obesity. Gut Liver 2012, 6, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Beck, B. Neuropeptide Y in normal eating and in genetic and dietary-induced obesity. Philos. Trans. R Soc. Lond. B Biol. Sci. 2006, 361, 1159–1185. [Google Scholar] [CrossRef] [Green Version]
- Millington, G.W. The role of proopiomelanocortin (POMC) neurones in feeding behaviour. Nutr. Metab. 2007, 4, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Agostino, G.; Diano, S. Alpha-melanocyte stimulating hormone: Production and degradation. J. Mol. Med. 2010, 88, 1195–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakhate, K.T.; Kokare, D.M.; Singru, P.S.; Taksande, A.G.; Kotwal, S.D.; Subhedar, N.K. Hypothalamic cocaine- and amphetamine-regulated transcript peptide is reduced and fails to modulate feeding behavior in rats with chemically-induced mammary carcinogenesis. Pharmacol. Biochem. Behav. 2010, 97, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Merlino, D.J.; Blomain, E.S.; Aing, A.S.; Waldman, S.A. Gut-Brain Endocrine Axes in Weight Regulation and Obesity Pharmacotherapy. J. Clin. Med. 2014, 3, 763–794. [Google Scholar] [CrossRef] [PubMed]
- Denroche, H.C.; Huynh, F.K.; Kieffer, T.J. The role of leptin in glucose homeostasis. J. Diabetes Investig. 2012, 3, 115–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Souza A., M.; Neumann, U.H.; Glavas, M.M.; Kieffer, T.J. The glucoregulatory actions of leptin. Mol. Metab. 2017, 6, 1052–1065. [Google Scholar] [CrossRef]
- Perianes-Cachero, A.; Burgos-Ramos, E.; Puebla-Jimenez, L.; Canelles, S.; Viveros, M.P.; Mela, V.; Chowen, J.A.; Argente, J.; Arilla-Ferreiro, E.; Barrios, V. Leptin-induced downregulation of the rat hippocampal somatostatinergic system may potentiate its anorexigenic effects. Neurochem. Int. 2012, 61, 1385–1396. [Google Scholar] [CrossRef]
- Davidson, T.L.; Chan, K.; Jarrard, L.E.; Kanoski, S.E.; Clegg, D.J.; Benoit, S.C. Contributions of the Hippocampus and Medial Prefrontal Cortex to Energy and Body Weight Regulation. Hippocampus 2009, 19, 235–252. [Google Scholar] [CrossRef] [Green Version]
- Aponte, Y.; Atasoy, D.; Sternson, S.M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 2011, 14, 351–355. [Google Scholar] [CrossRef]
- Atasoy, D. Deconstruction of a neural circuit for hunger. Acta. Physiol. 2016, 217, 10. [Google Scholar] [CrossRef]
- Betley, J.N.; Xu, S.J.; Cao, Z.F.H.; Gong, R.; Magnus, C.J.; Yu, Y.; Sternson, S.M. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 2015, 521, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Krashes, M.J.; Koda, S.; Ye, C.; Rogan, S.C.; Adams, A.C.; Cusher, D.S.; Maratos-Flier, E.; Roth, B.L.; Lowell, B.B. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Investig. 2011, 121, 1424–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luquet, S.; Perez, F.A.; Hnasko, T.S.; Palmiter, R.D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 2005, 310, 683–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohn, J.W. Network of hypothalamic neurons that control appetite. Bmb Rep. 2015, 48, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging Roles for Serotonin in Regulating Metabolism: New Implications for an Ancient Molecule. Endocr. Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef]
- Schneeberger, M.; Parolari, L.; Das Banerjee, T.; Bhave, V.; Wang, P.; Patel, B.; Topilko, T.; Wu, Z.; Choi, C.H.J.; Yu, X.; et al. Regulation of Energy Expenditure by Brainstem GABA Neurons. Cell 2019, 178, 672–685. [Google Scholar] [CrossRef]
- Breisch, S.T.; Zemlan, F.P.; Hoebel, B.G. Hyperphagia and obesity following serotonin depletion by intraventricular p-chlorophenylalanine. Science 1976, 192, 382–385. [Google Scholar] [CrossRef]
- Heisler, L.K.; Jobst, E.E.; Sutton, G.M.; Zhou, L.; Borok, E.; Thornton-Jones, Z.; Liu, H.Y.; Zigman, J.M.; Balthasar, N.; Kishi, T.; et al. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron 2006, 51, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Kwon, O.; Yu, J.H.; Jeong, E.; Yoo, H.J.; Kim, M.S. Meal-related oscillations in the serum serotonin levels in healthy young men. Clin. Endocrinol. 2018, 88, 549–555. [Google Scholar] [CrossRef]
- Heisler, L.K.; Cowley, M.A.; Tecott, L.H.; Fan, W.; Low, M.J.; Smart, J.L.; Rubinstein, M.; Tatro, J.B.; Marcus, J.N.; Holstege, H.; et al. Activation of central melanocortin pathways by fenfluramine. Science 2002, 297, 609–611. [Google Scholar] [CrossRef]
- Sohn, J.W.; Xu, Y.; Jones, J.E.; Wickman, K.; Williams, K.W.; Elmquist, J.K. Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron 2011, 71, 488–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finley, J.C.; Maderdrut, J.L.; Roger, L.J.; Petrusz, P. The immunocytochemical localization of somatostatin-containing neurons in the rat central nervous system. Neuroscience 1981, 6, 2173–2192. [Google Scholar] [CrossRef] [PubMed]
- Johansson, O.; Hokfelt, T.; Elde, R.P. Immunohistochemical distribution of somatostatin-like immunoreactivity in the central nervous system of the adult rat. Neuroscience 1984, 13, 265–339. [Google Scholar] [CrossRef]
- Zou, S.L.; Somvanshi, R.K.; Paik, S.; Kumar, U. Colocalization of Cannabinoid Receptor 1 with Somatostatin and Neuronal Nitric Oxide Synthase in Rat Brain Hypothalamus. J. Mol. Neurosci. 2015, 55, 480–491. [Google Scholar] [CrossRef]
- Seoane, L.M.; Al-Massadi, O.; Barreiro, F.; Dieguez, C.; Casanueva, F.F. Growth hormone and somatostatin directly inhibit gastric ghrelin secretion. An in vitro organ culture system. J. Endocrinol. Investig. 2007, 30, RC22–RC25. [Google Scholar] [CrossRef]
- Silva, A.P.; Bethmann, K.; Raulf, F.; Schmid, H.A. Regulation of ghrelin secretion by somatostatin analogs in rats. Eur. J. Endocrinol. 2005, 152, 887–894. [Google Scholar] [CrossRef] [Green Version]
- Bellocchio, L.; Cervino, C.; Pasquali, R.; Pagotto, U. The endocannabinoid system and energy metabolism. J. Neuroendocrinol. 2008, 20, 850–857. [Google Scholar] [CrossRef]
- Di Marzo, V.; Matias, I. Endocannabinoid control of food intake and energy balance. Nat. Neurosci. 2005, 8, 585–589. [Google Scholar] [CrossRef]
- Silvestri, C.; Di Marzo, V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013, 17, 475–490. [Google Scholar] [CrossRef] [Green Version]
- Adamo, M.; Raizada, M.K.; LeRoith, D. Insulin and insulin-like growth factor receptors in the nervous system. Mol. Neurobiol. 1989, 3, 71–100. [Google Scholar] [CrossRef]
- Chambers, A.P.; Sandoval, D.A.; Seeley, R.J. Integration of satiety signals by the central nervous system. Curr. Biol. 2013, 23, R379–R388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moran, T.H.; Kinzig, K.P. Gastrointestinal satiety signals II. Cholecystokinin. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G183–R188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moran, T.H.; Ladenheim, E.E.; Schwartz, G.J. Within-meal gut feedback signaling. Int. J. Obes. Relat. Metab. Disord. 2001, 25 (Suppl. 5), S39–S41. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.; Young, R.C.; Smith, G.P. Cholecystokinin decreases food intake in rats. J. Comp. Physiol. Psychol. 1973, 84, 488–495. [Google Scholar] [CrossRef]
- Gutzwiller, J.P.; Goke, B.; Drewe, J.; Hildebrand, P.; Ketterer, S.; Handschin, D.; Winterhalder, R.; Conen, D.; Beglinger, C. Glucagon-like peptide-1: A potent regulator of food intake in humans. Gut 1999, 44, 81–86. [Google Scholar] [CrossRef]
- Batterham, R.L.; Le Roux, C.W.; Cohen, M.A.; Park, A.J.; Ellis, S.M.; Patterson, M.; Frost, G.S.; Ghatei, M.A.; Bloom, S.R. Pancreatic polypeptide reduces appetite and food intake in humans. J. Clin. Endocrinol. Metab. 2003, 88, 3989–3992. [Google Scholar] [CrossRef]
- Koda, S.; Date, Y.; Murakami, N.; Shimbara, T.; Hanada, T.; Toshinai, K.; Niijima, A.; Furuya, M.; Inomata, N.; Osuye, K.; et al. The role of the vagal nerve in peripheral PYY3-36-induced feeding reduction in rats. Endocrinology 2005, 146, 2369–2375. [Google Scholar] [CrossRef] [Green Version]
- Dakin, C.L.; Small, C.J.; Batterham, R.L.; Neary, N.M.; Cohen, M.A.; Patterson, M.; Ghatei, M.A.; Bloom, S.R. Peripheral oxyntomodulin reduces food intake and body weight gain in rats. Endocrinology 2004, 145, 2687–2695. [Google Scholar] [CrossRef] [Green Version]
- Mani, B.K.; Zigman, J.M. A Strong Stomach for Somatostatin. Endocrinology 2015, 156, 3876–3879. [Google Scholar] [CrossRef] [Green Version]
- Cremonini, F.; Camilleri, M.; Gonenne, J.; Stephens, D.; Oenning, L.; Baxter, K.; Foxx-Orenstein, A.; Burton, D. Effect of somatostatin analog on postprandial satiation in obesity. Obes Res. 2005, 13, 1572–1579. [Google Scholar] [CrossRef] [Green Version]
- Stengel, A.; Goebel, M.; Wang, L.; Rivier, J.; Kobelt, P.; Monnikes, H.; Tache, Y. Selective central activation of somatostatin receptor 2 increases food intake, grooming behavior and rectal temperature in rats. J. Physiol. Pharmacol. 2010, 61, 399–407. [Google Scholar] [PubMed]
- Stengel, A.; Goebel-Stengel, M.; Wang, L.; Luckey, A.; Hu, E.; Rivier, J.; Tache, Y. Central administration of pan-somatostatin agonist ODT8-SST prevents abdominal surgery-induced inhibition of circulating ghrelin, food intake and gastric emptying in rats. Neurogastroenterol. Motil. 2011, 23, e294–e308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wren, A.M.; Seal, L.J.; Cohen, M.A.; Brynes, A.E.; Frost, G.S.; Murphy, K.G.; Dhillo, W.S.; Ghatei, M.A.; Bloom, S.R. Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endocrinol. Metab. 2001, 86, 5992. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Khan, J.A. Food intake regulation by leptin: Mechanisms mediating gluconeogenesis and energy expenditure. Asian Pac. J. Trop. Med. 2017, 10, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Bassi, M.; do Carmo, J.M.; Hall, J.E.; da Silva, A.A. Chronic effects of centrally administered adiponectin on appetite, metabolism and blood pressure regulation in normotensive and hypertensive rats. Peptides 2012, 37, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Air, E.L.; Benoit, S.C.; Blake Smith, K.A.; Clegg, D.J.; Woods, S.C. Acute third ventricular administration of insulin decreases food intake in two paradigms. Pharmacol. Biochem. Behav. 2002, 72, 423–429. [Google Scholar] [CrossRef]
- Lutz, T.A. Control of food intake and energy expenditure by amylin-therapeutic implications. Int. J. Obes. 2009, 33 (Suppl 1), S24–S27. [Google Scholar] [CrossRef] [Green Version]
- Camilleri, M. Peripheral Mechanisms in Appetite Regulation. Gastroenterology 2015, 148, 1219–1233. [Google Scholar] [CrossRef] [Green Version]
- Bauer, S.; Hay, M.; Amilhon, B.; Jean, A.; Moyse, E. In vivo neurogenesis in the dorsal vagal complex of the adult rat brainstem. Neuroscience 2005, 130, 75–90. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Smith, R.J.; Moorman, D.E.; Richardson, K.A. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology 2009, 56 (Suppl 1), 112. [Google Scholar] [CrossRef] [Green Version]
- Jennings, J.H.; Ung, R.L.; Resendez, S.L.; Stamatakis, A.M.; Taylor, J.G.; Huang, J.; Veleta, K.; Kantak, P.A.; Aita, M.; Shilling-Scrivo, K.; et al. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell 2015, 160, 516–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutter, M.; Nestler, E.J. Homeostatic and hedonic signals interact in the regulation of food intake. J. Nutr. 2009, 139, 629–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varela, L.; Horvath, T.L. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. Embo Rep. 2012, 13, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.; Halaas, J.; Ravussin, E.; Pratley, R.E.; Lee, G.H.; Zhang, Y.; Fei, H.; Kim, S.; Lallone, R.; Ranganathan, S.; et al. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1995, 1, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Watterson, K.R.; Bestow, D.; Gallagher, J.; Hamilton, D.L.; Ashford, F.B.; Meakin, P.J.; Ashford, M.L. Anorexigenic and orexigenic hormone modulation of mammalian target of rapamycin complex 1 activity and the regulation of hypothalamic agouti-related protein mRNA expression. Neurosignals 2013, 21, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Msaouel, P.; Galanis, E.; Koutsilieris, M. Somatostatin and somatostatin receptors: Implications for neoplastic growth and cancer biology. Expert Opin. Investig. Drugs 2009, 18, 1297–1316. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.P.; Pictet, R.L.; Rutter, W.J. Human somatostatin I: Sequence of the cDNA. Proc. Natl. Acad. Sci. USA 1982, 79, 4575–4579. [Google Scholar] [CrossRef] [Green Version]
- Gahete, M.D.; Cordoba-Chacon, J.; Duran-Prado, M.; Malagon, M.M.; Martinez-Fuentes, A.J.; Gracia-Navarro, F.; Luque, R.M.; Castano, J.P. Somatostatin and its receptors from fish to mammals. Ann. NY Acad. Sci. 2010, 1200, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Pradayrol, L.; Jornvall, H.; Mutt, V.; Ribet, A. N-terminally extended somatostatin: The primary structure of somatostatin-28. FEBS Lett. 1980, 109, 55–58. [Google Scholar] [CrossRef] [Green Version]
- van der Hoek, J.; Hofland, L.J.; Lamberts, S.W. Novel subtype specific and universal somatostatin analogues: Clinical potential and pitfalls. Curr. Pharm. Des. 2005, 11, 1573–1592. [Google Scholar] [CrossRef]
- Hernandez, C.; Carrasco, E.; Casamitjana, R.; Deulofeu, R.; Garcia-Arumi, J.; Simo, R. Somatostatin molecular variants in the vitreous fluid - A comparative study between diabetic patients with proliferative diabetic retinopathy and nondiabetic control subjects. Diabetes Care 2005, 28, 1941–1947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stengel, A.; Tache, Y. Activation of somatostatin 2 receptors in the brain and the periphery induces opposite changes in circulating ghrelin levels: Functional implications. Frontiers in endocrinology 2012, 3, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katakami, H.; Downs, T.R.; Frohman, L.A. Inhibitory effect of hypothalamic medial preoptic area somatostatin on growth hormone-releasing factor in the rat. Endocrinology 1988, 123, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Tannenbaum, G.S.; Turner, J.; Guo, F.; Videau, C.; Epelbaum, J.; Beaudet, A. Homologous upregulation of sst2 somatostatin receptor expression in the rat arcuate nucleus in vivo. Neuroendocrinology 2001, 74, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Berelowitz, M.; Dudlak, D.; Frohman, L.A. Release of somatostatin-like immunoreactivity from incubated rat hypothalamus and cerebral cortex. Effects of glucose and glucoregulatory hormones. J. Clin. Invest. 1982, 69, 1293–1301. [Google Scholar] [CrossRef]
- Chihara, K.; Arimura, A.; Schally, A.V. Effect of intraventricular injection of dopamine, noreprinephrine, acetylcholine, and 5-hydroxytryptamine on immunoreactive somatostatin release into rat hypophyseal portal blood. Endocrinology 1979, 104, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Reichlin, S. Somatostatin (second of two parts). N. Engl. J. Med. 1983, 309, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Reichlin, S. Somatostatin. N. Engl. J. Med. 1983, 309, 1495–1501. [Google Scholar] [CrossRef]
- Sharma, K.; Patel, Y.C.; Srikant, C.B. Subtype-selective induction of wild-type p53 and apoptosis, but not cell cycle arrest, by human somatostatin receptor 3. Molec. Endocrinol. 1996, 10, 1688–1696. [Google Scholar]
- Hoyer, D.; Bell, G.I.; Berelowitz, M.; Epelbaum, J.; Feniuk, W.; Humphrey, P.P.; O’Carroll, A.M.; Patel, Y.C.; Schonbrunn, A.; Taylor, J.E.; et al. Classification and nomenclature of somatostatin receptors. Trends Pharmacol. Sci. 1995, 16, 86–88. [Google Scholar] [CrossRef]
- Kreienkamp, H.J.; Liew, C.W.; Bächner, D.; Mameza, M.G.; Soltau, M.; Quitsch, A.; Richter, D. Physiology of Somatostatin Receptors: From Genetics to Molecular Analysis. In Somatostatin; Srikant, C.B., Ed.; Springer: Boston, MA, USA, 2004; Volume 24, pp. 185–202. [Google Scholar]
- Lamberts, S.W.; Krenning, E.P.; Reubi, J.C. The role of somatostatin and its analogs in the diagnosis and treatment of tumors. Endocr. Rev. 1991, 12, 450–482. [Google Scholar] [CrossRef] [PubMed]
- Weckbecker, G.; Lewis, I.; Albert, R.; Schmid, H.A.; Hoyer, D.; Bruns, C. Opportunities in somatostatin research: Biological, chemical and therapeutic aspects. Nat. Rev. Drug Discov. 2003, 2, 999–1017. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.; Sasi, R.; Suresh, S.; Patel, A.; Thangaraju, M.; Metrakos, P.; Patel, S.C.; Patel, Y.C. Subtype-selective expression of the five somatostatin receptors (hSSTR1-5) in human pancreatic islet cells: A quantitative double-label immunohistochemical analysis. Diabetes 1999, 48, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Barnett, P. Somatostatin and somatostatin receptor physiology. Endocrine 2003, 20, 255–264. [Google Scholar] [CrossRef]
- Lamberts, S.W.J.; van der Lely, A.J.; Hofland, L.J. New somatostatin analogs: Will they fulfil old promises? Eur. J. Endocrinol. 2002, 146, 701–705. [Google Scholar] [CrossRef] [Green Version]
- Tulipano, G.; Schulz, S. Novel insights in somatostatin receptor physiology. Eur. J. Endocrinol. 2007, 156 (Suppl 1), S3–S11. [Google Scholar] [CrossRef] [Green Version]
- Cejvan, K.; Coy, D.H.; Efendic, S. Intra-islet somatostatin regulates glucagon release via type 2 somatostatin receptors in rats. Diabetes 2003, 52, 1176–1181. [Google Scholar] [CrossRef] [Green Version]
- Cervia, D.; Casini, G.; Bagnoli, P. Physiology and pathology of somatostatin in the mammalian retina: A current view. Mol. Cell. Endocrinol. 2008, 286, 112–122. [Google Scholar] [CrossRef] [Green Version]
- War, S.A.; Somvanshi, R.K.; Kumar, U. Somatostatin receptor-3 mediated intracellular signaling and apoptosis is regulated by its cytoplasmic terminal. Bba-Mol. Cell Res. 2011, 1813, 390–402. [Google Scholar] [CrossRef] [Green Version]
- Chisholm, C.; Greenberg, G.R. Somatostatin-28 regulates GLP-1 secretion via somatostatin receptor subtype 5 in rat intestinal cultures. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E311–E317. [Google Scholar] [CrossRef] [Green Version]
- Scheich, B.; Gaszner, B.; Kormos, V.; Laszlo, K.; Adori, C.; Borbely, E.; Hajna, Z.; Tekus, V.; Bolcskei, K.; Abraham, I.; et al. Somatostatin receptor subtype 4 activation is involved in anxiety and depression-like behavior in mouse models. Neuropharmacology 2016, 101, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Stengel, A.; Coskun, T.; Goebel, M.; Wang, L.; Craft, L.; Alsina-Fernandez, J.; Rivier, J.; Tache, Y. Central injection of the stable somatostatin analog ODT8-SST induces a somatostatin2 receptor-mediated orexigenic effect: Role of neuropeptide Y and opioid signaling pathways in rats. Endocrinology 2010, 151, 4224–4235. [Google Scholar] [CrossRef] [PubMed]
- Stengel, A.; Goebel, M.; Wang, L.; Rivier, J.; Kobelt, P.; Monnikes, H.; Tache, Y. Activation of brain somatostatin 2 receptors stimulates feeding in mice: Analysis of food intake microstructure. Physiol. Behav. 2010, 101, 614–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stengel, A.; Goebel-Stengel, M.; Wang, L.; Larauche, M.; Rivier, J.; Tache, Y. Central somatostatin receptor 1 activation reverses acute stress-related alterations of gastric and colonic motor function in mice. Neurogastroenterol. Motil. 2011, 23, e223–e236. [Google Scholar] [CrossRef] [Green Version]
- Kumar, U. Colocalization of somatostatin receptor subtypes (SSTR1-5) with somatostatin, NADPH-diaphorase (NADPH-d), and tyrosine hydroxylase in the rat hypothalamus. J. Comp. Neurol. 2007, 504, 185–205. [Google Scholar] [CrossRef]
- Rajput, P.S.; Billova, S.; Patel, S.C.; Kharmate, G.; Somvanshi, R.K.; Kumar, U. Expression of somatostatin and somatostatin receptor subtypes in Apolipoprotein D (ApoD) knockout mouse brain: An immunohistochemical analysis. J. Chem. Neuroanat. 2009, 38, 20–33. [Google Scholar] [CrossRef]
- Karasawa, H.; Yakabi, S.; Wang, L.X.; Tache, Y. Orexin-1 receptor mediates the increased food and water intake induced by intracerebroventricular injection of the stable somatostatin pan-agonist, ODT8-SST in rats. Neurosci. Lett. 2014, 576, 88–92. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Fiori, J.L.; Shin, Y.K.; Okun, E.; Kim, J.S.; Rapp, P.R.; Egan, J.M. Pancreatic polypeptide inhibits somatostatin secretion. FEBS Lett. 2014, 588, 3233–3239. [Google Scholar] [CrossRef] [Green Version]
- van Liessum, P.A.; Hopman, W.P.; Pieters, G.F.; Jansen, J.B.; Smals, A.G.; Rosenbusch, G.; Kloppenborg, P.W.; Lamers, C.B. Postprandial gallbladder motility during long term treatment with the long-acting somatostatin analog SMS 201-995 in acromegaly. J. Clin. Endocrinol. Metab. 1989, 69, 557–562. [Google Scholar] [CrossRef]
- Reisine, T.; Kong, H.; Raynor, K.; Yano, H.; Takeda, J.; Yasuda, K.; Bell, G.I. Splice variant of the somatostatin receptor 2 subtype, somatostatin receptor 2B, couples to adenylyl cyclase. Mol. Pharmacol. 1993, 44, 1016–1020. [Google Scholar]
- Campbell, R.M.; Scanes, C.G. Inhibition of growth hormone-stimulated lipolysis by somatostatin, insulin, and insulin-like growth factors (somatomedins) in vitro. Proc. Soc. Exp. Biol. Med. 1988, 189, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.A.; Romero, B.; Calle, C. Characterization of somatostatin binding sites in isolated rat adipocytes. Regul. Pept. 1988, 23, 261–270. [Google Scholar] [CrossRef]
- Levine, A.S.; Morley, J.E. Peripherally administered somatostatin reduces feeding by a vagal mediated mechanism. Pharmacol. Biochem. Behav. 1982, 16, 897–902. [Google Scholar] [CrossRef]
- Rocca, A.S.; Brubaker, P.L. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology 1999, 140, 1687–1694. [Google Scholar] [CrossRef]
- Simsolo, R.B.; Ezzat, S.; Ong, J.M.; Saghizadeh, M.; Kern, P.A. Effects of acromegaly treatment and growth hormone on adipose tissue lipoprotein lipase. J. Clin. Endocrinol. Metab. 1995, 80, 3233–3238. [Google Scholar] [CrossRef]
- Corleto, V.D. Somatostatin and the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 63–68. [Google Scholar] [CrossRef]
- Tulassay, Z. Somatostatin and the gastrointestinal tract. Scand. J. Gastroenterol. Suppl. 1998, 228, 115–121. [Google Scholar] [CrossRef]
- Fuessl, H.S.; Carolan, G.; Williams, G.; Bloom, S.R. Effect of a Long-Acting Somatostatin Analog (Sms 201-995) on Postprandial Gastric-Emptying of Tc-99m-Tin Colloid and Mouth-to-Cecum Transit-Time in Man. Digestion 1987, 36, 101–107. [Google Scholar] [CrossRef]
- Heini, A.F.; Kirk, K.A.; Lara-Castro, C.; Weinsier, R.L. Relationship between hunger-satiety feelings and various metabolic parameters in women with obesity during controlled weight loss. Obes. Res. 1998, 6, 225–230. [Google Scholar] [CrossRef]
- Johansson, C.; Wisen, O.; Efendic, S.; Uvnaswallensten, K. Effects of Somatostatin on Gastrointestinal Propagation and Absorption of Oral Glucose in Man. Digestion 1981, 22, 126–137. [Google Scholar] [CrossRef]
- Lembcke, B.; Creutzfeldt, W.; Schleser, S.; Ebert, R.; Shaw, C.; Koop, I. Effect of the Somatostatin Analog Sandostatin (Sms 201-995) on Gastrointestinal, Pancreatic and Biliary Function and Hormone-Release in Normal Men. Digestion 1987, 36, 108–124. [Google Scholar] [CrossRef] [PubMed]
- Lotter, E.C.; Krinsky, R.; McKay, J.M.; Treneer, C.M.; Porter, D., Jr.; Woods, S.C. Somatostatin decreases food intake of rats and baboons. J. Comp. Physiol. Psychol. 1981, 95, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Danguir, J. Food-Intake in Rats Is Increased by Intracerebroventricular Infusion of the Somatostatin Analog Sms-201-995 and Is Decreased by Somatostatin Antiserum. Peptides 1988, 9, 211–213. [Google Scholar] [CrossRef]
- Beranek, L.; Hajdu, I.; Gardi, J.; Taishi, P.; Obal, F., Jr.; Krueger, J.M. Central administration of the somatostatin analog octreotide induces captopril-insensitive sleep responses. Am. J. Physiol. 1999, 277, R1297–R1304. [Google Scholar] [CrossRef]
- Tachibana, T.; Cline, M.A.; Sugahara, K.; Ueda, H.; Hiramatsu, K. Central administration of somatostatin stimulates feeding behavior in chicks. Gen. Comp. Endocrinol. 2009, 161, 354–359. [Google Scholar] [CrossRef]
- Feifel, D.; Vaccarino, F.J.; Rivier, J.; Vale, W.W. Evidence for a common neural mechanism mediating growth hormone-releasing factor-induced and somatostatin-induced feeding. Neuroendocrinology 1993, 57, 299–305. [Google Scholar] [CrossRef]
- Karasawa, H.; Yakabi, S.; Wang, L.; Stengel, A.; Rivier, J.; Tache, Y. Brain somatostatin receptor 2 mediates the dipsogenic effect of central somatostatin and cortistatin in rats: Role in drinking behavior. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R793–R801. [Google Scholar] [CrossRef] [Green Version]
- Rezek, M.; Havlicek, V.; Hughes, K.R.; Friesen, H. Central Site of Action of Somatostatin (Srif) - Role of Hippocampus. Neuropharmacology 1976, 15, 499–504. [Google Scholar] [CrossRef]
- Cummings, S.L.; Truong, B.G.; Gietzen, D.W. Neuropeptide Y and somatostatin in the anterior piriform cortex alter intake of amino acid-deficient diets. Peptides 1998, 19, 527–535. [Google Scholar] [CrossRef]
- Feifel, D.; Vaccarino, F.J. Central somatostatin: A re-examination of its effects on feeding. Brain Res. 1990, 535, 189–194. [Google Scholar] [CrossRef]
- Lin, M.T.; Chen, J.J.; Ho, L.T. Hypothalamic involvement in the hyperglycemia and satiety actions of somatostatin in rats. Neuroendocrinology 1987, 45, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, K.; Takata, S.; Ishii, A.; Nagao, K.; Bannai, M.; Takahashi, M.; Murakami, N. Somatostatin is involved in anorexia in mice fed a valine-deficient diet. Amino Acids 2012, 42, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, E.; Mccann, S.M. Suppression of Feeding and Drinking Activity in Rats Following Intraventricular-Injection of Thyrotropin Releasing Hormone (Trh). Endocrinology 1977, 100, 1727–1730. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A. Nutrient intake is modulated by peripheral peptide administration. Obes. Res. 1995, 3, S569–S572. [Google Scholar] [CrossRef]
- Tannenbaum, G.S.; Patel, Y.C. On the Fate of Centrally Administered Somatostatin in the Rat - Massive Hypersomatostatinemia Resulting from Leakage into the Peripheral-Circulation Has Effects on Growth-Hormone Secretion and Glucoregulation. Endocrinology 1986, 118, 2137–2143. [Google Scholar] [CrossRef]
- Vecsei, L.; Pavo, I.; Zsigo, J.; Penke, B.; Widerlov, E. Comparative Studies of Somatostatin-14 and Some of Its Fragments on Passive-Avoidance Behavior, Open-Field Activity and on Barrel Rotation Phenomenon in Rats. Peptides 1989, 10, 1153–1157. [Google Scholar] [CrossRef]
- Roth, C.L.; von Schnurbein, J.; Elfers, C.; Moss, A.; Wabitsch, M. Changes in Satiety Hormones in Response to Leptin Treatment in a Patient with Leptin Deficiency. Horm. Res. Paediat. 2018, 90, 424–430. [Google Scholar] [CrossRef]
- Roth, C.L. Hypothalamic Obesity in Craniopharyngioma Patients: Disturbed Energy Homeostasis Related to Extent of Hypothalamic Damage and Its Implication for Obesity Intervention. J. Clin. Med. 2015, 4, 1774–1797. [Google Scholar] [CrossRef] [Green Version]
- Czech, M.P.; Tencerova, M.; Pedersen, D.J.; Aouadi, M. Insulin signalling mechanisms for triacylglycerol storage. Diabetologia 2013, 56, 949–964. [Google Scholar] [CrossRef] [Green Version]
- Hansen, J.B.; Arkhammar, P.O.G.; Bodvarsdottir, T.B.; Wahl, P. Inhibition of insulin secretion as a new drug target in the treatment of metabolic disorders. Curr. Med. Chem. 2004, 11, 1595–1615. [Google Scholar] [CrossRef]
- Pliquett, R.U.; Fuhrer, D.; Falk, S.; Zysset, S.; von Cramon, D.Y.; Stumvoll, M. The effects of insulin on the central nervous system--focus on appetite regulation. Horm. Metab. Res. 2006, 38, 442–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Iersel, L.; Brokke, K.E.; Adan, R.A.H.; Bulthuis, L.C.M.; van den Akker, E.L.T.; van Santen, H.M. Pathophysiology and Individualized Treatment of Hypothalamic Obesity Following Craniopharyngioma and Other Suprasellar Tumors: A Systematic Review. Endocr. Rev. 2019, 40, 193–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fung, L.C.; Greenberg, G.R. Characterization of somatostatin receptor subtypes mediating inhibition of nutrient-stimulated gastric acid and gastrin in dogs. Regul. Pept. 1997, 68, 197–203. [Google Scholar] [CrossRef]
- Shimada, M.; Date, Y.; Mondal, M.S.; Toshinai, K.; Shimbara, T.; Fukunaga, K.; Murakami, N.; Miyazato, M.; Kangawa, K.; Yoshimatsu, H.; et al. Somatostatin suppresses ghrelin secretion from the rat stomach. Biochem. Biophys. Res. Commun. 2003, 302, 520–525. [Google Scholar] [CrossRef]
- Riechelmann, R.P.; Pereira, A.A.; Rego, J.F.M.; Costa, F.P. Refractory carcinoid syndrome: A review of treatment options. Ther. Adv. Med. Oncol. 2017, 9, 127–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.P.H.; Keating, G.M. Octreotide Long-Acting Release (LAR) A Review of its Use in the Management of Acromegaly. Drugs 2010, 70, 1745–1769. [Google Scholar] [CrossRef]
- Krassas, G.E.; Tzotzas, T.; Papazisis, K.; Pazaitou-Panayiotou, K.; Boboridis, K. The efficacy of somatostatin analogues in the treatment of diabetic retinopathy and thyroid eye disease. Clin. Ophthalmol. 2007, 1, 209–215. [Google Scholar]
- Lustig, R.H.; Hinds, P.S.; Ringwald-Smith, K.; Christensen, R.K.; Kaste, S.C.; Schreiber, R.E.; Rai, S.N.; Lensing, S.Y.; Wu, S.J.; Xiong, X.P. Octreotide therapy of pediatric hypothalamic obesity: A double-blind, placebo-controlled trial. J. Clin. Endocr. Metab. 2003, 88, 2586–2592. [Google Scholar] [CrossRef] [Green Version]
- Boehm, B.O. The therapeutic potential of somatostatin receptor ligands in the treatment of obesity and diabetes. Expert Opin. Investig. Drugs 2003, 12, 1501–1509. [Google Scholar] [CrossRef]
- Srivastava, G.; Apovian, C. Future Pharmacotherapy for Obesity: New Anti-obesity Drugs on the Horizon. Curr. Obes. Rep. 2018, 7, 147–161. [Google Scholar] [CrossRef]
- Zhu, C.; Yao, Y.; Xiong, Y.; Cheng, M.; Chen, J.; Zhao, R.; Liao, F.; Shi, R.; Song, S. Somatostatin Neurons in the Basal Forebrain Promote High-Calorie Food Intake. Cell Rep. 2017, 20, 112–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, J.N.; Macosko, E.Z.; Fenselau, H.; Pers, T.H.; Lyubetskaya, A.; Tenen, D.; Goldman, M.; Verstegen, A.M.; Resch, J.M.; McCarroll, S.A.; et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat. Neurosci. 2017, 20, 484–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, W.H.; Xiang, H.D.; Rajan, A.S.; Kunze, D.L.; Boyd, A.E. Somatostatin Inhibits Insulin-Secretion by a G-Protein-Mediated Decrease in Ca2+ Entry through Voltage-Dependent Ca2+ Channels in the Beta-Cell. J. Biol. Chem. 1991, 266, 837–843. [Google Scholar] [PubMed]
- Lustig, R.H.; Rose, S.R.; Burghen, G.A.; Velasquez-Mieyer, P.; Broome, D.C.; Smith, K.; Li, H.; Hudson, M.M.; Heideman, R.L.; Kun, L.E. Hypothalamic obesity caused by cranial insult in children: Altered glucose and insulin dynamics and reversal by a somatostatin agonist. J. Pediatr. 1999, 135, 162–168. [Google Scholar] [CrossRef]
- Haqq, A.M.; Stadler, D.D.; Rosenfeld, R.G.; Pratt, K.L.; Weigle, D.S.; Frayo, R.S.; Lafranchi, S.H.; Cummings, D.E.; Purnell, J.Q. Circulating ghrelin levels are suppressed by meals and octreotide therapy in children with Prader-Willi syndrome. J. Clin. Endocr. Metab. 2003, 88, 3573–3576. [Google Scholar] [CrossRef] [Green Version]
- Velasquez-Mieyer, P.A.; Cowan, P.A.; Arheart, K.L.; Buffington, C.K.; Spencer, K.A.; Connelly, B.E.; Cowan, G.W.; Lustig, R.H. Suppression of insulin secretion is associated with weight loss and altered macronutrient intake and preference in a subset of obese adults. Int J. Obes. 2003, 27, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Velasquez-Mieyer, P.A.; Umpierrez, G.E.; Lustig, R.H.; Cashion, A.K.; Cowan, P.A.; Christensen, M.; Spencer, K.A.; Burghen, G.A. Race affects insulin and GLP-1 secretion and response to a long-acting somatostatin analogue in obese adults. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 330–333. [Google Scholar] [CrossRef] [Green Version]
- Gambineri, A.; Patton, L.; De Iasio, R.; Cantelli, B.; Cognini, G.E.; Filicori, M.; Barreca, A.; Diamanti-Kandarakis, E.; Pagotto, U.; Pasquali, R. Efficacy of octreotide-LAR in dieting women with abdominal obesity and polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2005, 90, 3854–3862. [Google Scholar] [CrossRef] [Green Version]
- Lustig, R.H.; Greenway, F.; Velasquez-Mieyer, P.; Heimburger, D.; Schumacher, D.; Smith, D.; Smith, W.; Soler, N.; Warsi, G.; Berg, W.; et al. A multicenter, randomized, double-blind, placebo-controlled, dose-finding trial of a long-acting formulation of octreotide in promoting weight loss in obese adults with insulin hypersecretion. Int J. Obes. 2006, 30, 331–341. [Google Scholar] [CrossRef] [Green Version]
- Memmert, S.; Damanaki, A.; Nokhbehsaim, M.; Nogueira, A.V.B.; Eick, S.; Cirelli, J.A.; Jager, A.; Deschner, J. Regulation of somatostatin receptor 2 by proinflammatory, microbial and obesity-related signals in periodontal cells and tissues. Head Face Med. 2019, 15, S13005–S13018. [Google Scholar] [CrossRef]
- Sotos-Prieto, M.; Guillen, M.; Guillem-Saiz, P.; Portoles, O.; Corella, D. The rs1466113 polymorphism in the somatostatin receptor 2 gene is associated with obesity and food intake in a Mediterranean population. Ann. Nutr. Metab. 2010, 57, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Givalois, L.; Naert, G.; Tapia-Arancibia, L.; Arancibia, S. Involvement of brain-derived neurotrophic factor in the regulation of hypothalamic somatostatin in vivo. J. Endocrinol. 2006, 188, 425–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Munoz, I.; Sanchez-Franco, F.; Vallejo, M.; Fernandez, A.; Palacios, N.; Fernandez, M.; Sanchez-Grande, M.; Cacicedo, L. Regulation of somatostatin gene expression by brain derived neurotrophic factor in fetal rat cerebrocortical cells. Brain Res. 2011, 1375, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Vargas, H.; Martinez-Ezquerro, J.D.; Bienvenu, T. Brain-derived neurotrophic factor, food intake regulation, and obesity. Arch. Med Res. 2011, 42, 482–494. [Google Scholar] [CrossRef]
- Xu, B.; Goulding, E.H.; Zang, K.; Cepoi, D.; Cone, R.D.; Jones, K.R.; Tecott, L.H.; Reichardt, L.F. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat. Neurosci. 2003, 6, 736–742. [Google Scholar] [CrossRef] [Green Version]
- Kernie, S.G.; Liebl, D.J.; Parada, L.F. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000, 19, 1290–1300. [Google Scholar] [CrossRef] [Green Version]
- Krabbe, K.S.; Nielsen, A.R.; Krogh-Madsen, R.; Plomgaard, P.; Rasmussen, P.; Erikstrup, C.; Fischer, C.P.; Lindegaard, B.; Petersen, A.M.; Taudorf, S.; et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 2007, 50, 431–438. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Pedersen, M.; Krabbe, K.S.; Bruunsgaard, H.; Matthews, V.B.; Febbraio, M.A. Role of exercise-induced brain-derived neurotrophic factor production in the regulation of energy homeostasis in mammals. Exp. Physiol 2009, 94, 1153–1160. [Google Scholar] [CrossRef]
- Gray, J.; Yeo, G.S.; Cox, J.J.; Morton, J.; Adlam, A.L.; Keogh, J.M.; Yanovski, J.A.; El Gharbawy, A.; Han, J.C.; Tung, Y.C.; et al. Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes 2006, 55, 3366–3371. [Google Scholar] [CrossRef] [Green Version]
- Yeo, G.S.; Connie Hung, C.C.; Rochford, J.; Keogh, J.; Gray, J.; Sivaramakrishnan, S.; O’Rahilly, S.; Farooqi, I.S. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat. Neurosci 2004, 7, 1187–1189. [Google Scholar] [CrossRef]
- Fox, E.A.; Byerly, M.S. A mechanism underlying mature-onset obesity: Evidence from the hyperphagic phenotype of brain-derived neurotrophic factor mutants. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R994–R1004. [Google Scholar] [CrossRef] [PubMed]
- Lyons, W.E.; Mamounas, L.A.; Ricaurte, G.A.; Coppola, V.; Reid, S.W.; Bora, S.H.; Wihler, C.; Koliatsos, V.E.; Tessarollo, L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc. Natl. Acad. Sci. USA 1999, 96, 15239–15244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rios, M.; Fan, G.; Fekete, C.; Kelly, J.; Bates, B.; Kuehn, R.; Lechan, R.M.; Jaenisch, R. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol. Endocrinol. 2001, 15, 1748–1757. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Morikawa, Y.; Nanjo, K.; Senba, E. Induction of brain-derived neurotrophic factor by leptin in the ventromedial hypothalamus. Neuroscience 2006, 139, 1107–1115. [Google Scholar] [CrossRef]
- Conner, J.M.; Lauterborn, J.C.; Yan, Q.; Gall, C.M.; Varon, S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: Evidence for anterograde axonal transport. J. Neurosci. 1997, 17, 2295–2313. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, N.; Kanba, S.; Arita, J. Temporal changes in the expression of brain-derived neurotrophic factor mRNA in the ventromedial nucleus of the hypothalamus of the developing rat brain. Brain Res. Mol. Brain Res. 2003, 115, 69–77. [Google Scholar] [CrossRef]
- Unger, T.J.; Calderon, G.A.; Bradley, L.C.; Sena-Esteves, M.; Rios, M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J. Neurosci. 2007, 27, 14265–14274. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.; Radeke, M.J.; Matheson, C.R.; Talvenheimo, J.; Welcher, A.A.; Feinstein, S.C. Immunocytochemical localization of TrkB in the central nervous system of the adult rat. J. Comp. Neurol. 1997, 378, 135–157. [Google Scholar] [CrossRef]
- Rios, M. BDNF and the central control of feeding: Accidental bystander or essential player? Trends Neurosci. 2013, 36, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Gelfo, F.; De Bartolo, P.; Tirassa, P.; Croce, N.; Caltagirone, C.; Petrosini, L.; Angelucci, F. Intraperitoneal injection of neuropeptide Y (NPY) alters neurotrophin rat hypothalamic levels: Implications for NPY potential role in stress-related disorders. Peptides 2011, 32, 1320–1323. [Google Scholar] [CrossRef]
- Pelleymounter, M.A.; Cullen, M.J.; Wellman, C.L. Characteristics of BDNF-induced weight loss. Exp. Neurol. 1995, 131, 229–238. [Google Scholar] [CrossRef]
- Feijo Fde, M.; Bertoluci, M.C.; Reis, C. Serotonin and hypothalamic control of hunger: A review. Rev. Assoc. Med. Bras. (1992) 2011, 57, 74–77. [Google Scholar] [CrossRef]
- Cordeira, J.W.; Frank, L.; Sena-Esteves, M.; Pothos, E.N.; Rios, M. Brain-derived neurotrophic factor regulates hedonic feeding by acting on the mesolimbic dopamine system. J. Neurosci. 2010, 30, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Fulton, S.; Pissios, P.; Manchon, R.P.; Stiles, L.; Frank, L.; Pothos, E.N.; Maratos-Flier, E.; Flier, J.S. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 2006, 51, 811–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glorioso, C.; Sabatini, M.; Unger, T.; Hashimoto, T.; Monteggia, L.M.; Lewis, D.A.; Mirnics, K. Specificity and timing of neocortical transcriptome changes in response to BDNF gene ablation during embryogenesis or adulthood. Mol. Psychiatry 2006, 11, 633–648. [Google Scholar] [CrossRef] [PubMed]
- Martinowich, K.; Schloesser, R.J.; Jimenez, D.V.; Weinberger, D.R.; Lu, B. Activity-dependent brain-derived neurotrophic factor expression regulates cortistatin-interneurons and sleep behavior. Mol. Brain 2011, 4, 11. [Google Scholar] [CrossRef] [Green Version]
- Guilloux, J.P.; Douillard-Guilloux, G.; Kota, R.; Wang, X.; Gardier, A.M.; Martinowich, K.; Tseng, G.C.; Lewis, D.A.; Sibille, E. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol. Psychiatry 2012, 17, 1130–1142. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.C.; Sibille, E. Reduced brain somatostatin in mood disorders: A common pathophysiological substrate and drug target? Front. Pharmacol. 2013, 4, 110. [Google Scholar] [CrossRef] [Green Version]
- Rage, F.; Riteau, B.; Alonso, G.; Tapia-Arancibia, L. Brain-Derived Neurotrophic Factor and Neurotrophin-3 Enhance Somatostatin Gene Expression through a Likely Direct Effect on Hypothalamic Somatostatin Neurons. Endocrinology 1999, 140, 909–916. [Google Scholar] [CrossRef]
- Yau, Y.H.; Potenza, M.N. Stress and eating behaviors. Minerva Endocrinol. 2013, 38, 255–267. [Google Scholar] [PubMed]
- Stengel, A.; Tache, Y. Gut-Brain Neuroendocrine Signaling Under Conditions of Stress-Focus on Food Intake-Regulatory Mediators. Front. Endocrinol. 2018, 9, 498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, S.C.; Cox, H.M. Peptide YY, neuropeptide Y and corticotrophin-releasing factor modulate gastrointestinal motility and food intake during acute stress. Neurogastroenterol. Motil. 2014, 26, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Sominsky, L.; Spencer, S.J. Eating behavior and stress: A pathway to obesity. Front. Psychol. 2014, 5, 434. [Google Scholar] [CrossRef] [PubMed]
- Stengel, A.; Tache, Y. Neuroendocrine control of the gut during stress: Corticotropin-releasing factor signaling pathways in the spotlight. Annu. Rev. Physiol. 2009, 71, 219–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stengel, A.; Tache, Y.F. Activation of Brain Somatostatin Signaling Suppresses CRF Receptor-Mediated Stress Response. Front. Neurosci. 2017, 11, 231. [Google Scholar] [CrossRef]
- Tringali, G.; Greco, M.C.; Lisi, L.; Pozzoli, G.; Navarra, P. Cortistatin modulates the expression and release of corticotrophin releasing hormone in rat brain. Comparison with somatostatin and octreotide. Peptides 2012, 34, 353–359. [Google Scholar] [CrossRef]
- Stengel, A.; Tache, Y. Corticotropin-releasing factor signaling and visceral response to stress. Exp. Biol. Med. 2010, 235, 1168–1178. [Google Scholar] [CrossRef] [Green Version]
- Martinez, V.; Rivier, J.; Coy, D.; Tache, Y. Intracisternal injection of somatostatin receptor 5-preferring agonists induces a vagal cholinergic stimulation of gastric emptying in rats. J. Pharmacol. Exp. Ther. 2000, 293, 1099–1105. [Google Scholar]
- Brown, M.; Rivier, J.; Vale, W. Somatostatin-28: Selective action on the pancreatic beta-cell and brain. Endocrinology 1981, 108, 2391–2396. [Google Scholar] [CrossRef]
- Brown, M.R.; Rivier, C.; Vale, W. Central nervous system regulation of adrenocorticotropin secretion: Role of somatostatins. Endocrinology 1984, 114, 1546–1549. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.A.; Brown, M.R. Somatostatin analog: Plasma catecholamine suppression mediated by the central nervous system. Endocrinology 1980, 107, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Stengel, A.; Rivier, J.; Tache, Y. Modulation of the adaptive response to stress by brain activation of selective somatostatin receptor subtypes. Peptides 2013, 42, 70–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viollet, C.; Vaillend, C.; Videau, C.; Bluet-Pajot, M.T.; Ungerer, A.; L’Heritier, A.; Kopp, C.; Potier, B.; Billard, J.; Schaeffer, J.; et al. Involvement of sst2 somatostatin receptor in locomotor, exploratory activity and emotional reactivity in mice. Eur. J. Neurosci. 2000, 12, 3761–3770. [Google Scholar] [CrossRef]
- McEwen, B.S. Glucocorticoids, depression, and mood disorders: Structural remodeling in the brain. Metabolism 2005, 54, 20–23. [Google Scholar] [CrossRef]
- Kohler, O.; Benros, M.E.; Nordentoft, M.; Farkouh, M.E.; Iyengar, R.L.; Mors, O.; Krogh, J. Effect of Anti-inflammatory Treatment on Depression, Depressive Symptoms, and Adverse Effects A Systematic Review and Meta-analysis of Randomized Clinical Trials. Jama Psychiat 2014, 71, 1381–1391. [Google Scholar] [CrossRef]
- Raglan, G.B.; Schmidt, L.A.; Schulkin, J. The role of glucocorticoids and corticotropin-releasing hormone regulation on anxiety symptoms and response to treatment. Endocr. Connect. 2017, 6, R1–R7. [Google Scholar] [CrossRef]
- Asensio, C.; Muzzin, P.; Rohner-Jeanrenaud, F. Role of glucocorticoids in the physiopathology of excessive fat deposition and insulin resistance. Int. J. Obes. 2004, 28, S45–S52. [Google Scholar] [CrossRef] [Green Version]
- Cavagnini, F.; Croci, M.; Putignano, P.; Petroni, M.L.; Invitti, C. Glucocorticoids and neuroendocrine function. Int. J. Obes. 2000, 24, S77–S79. [Google Scholar] [CrossRef] [Green Version]
- Zou, S.L.; Somvanshi, R.K.; Kumar, U. Somatostatin Receptor 5 Is a Prominent Regulator of Signaling Pathways in Cells with Coexpression of Cannabinoid Receptors 1. Neuroscience 2017, 340, 218–231. [Google Scholar] [CrossRef]
- Lenard, N.R.; Berthoud, H.R. Central and peripheral regulation of food intake and physical activity: Pathways and genes. Obesity 2008, 16 (Suppl 3), S11–S22. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.L.; Wang, Q.; Zigman, J.M. The central nervous system sites mediating the orexigenic actions of ghrelin. Annu. Rev. Physiol. 2014, 76, 519–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummings, D.E.; Purnell, J.Q.; Frayo, R.S.; Schmidova, K.; Wisse, B.E.; Weigle, D.S. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001, 50, 1714–1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, J.W. PVN pathways controlling energy homeostasis. Indian J. Endocrinol. Metab. 2012, 16, S627–S636. [Google Scholar] [CrossRef] [PubMed]
- Otto, B.; Cuntz, U.; Fruehauf, E.; Wawarta, R.; Folwaczny, C.; Riepl, R.L.; Heiman, M.L.; Lehnert, P.; Fichter, M.; Tschop, M. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur. J. Endocrinol. 2001, 145, 669–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, M.W.; Figlewicz, D.P.; Baskin, D.G.; Woods, S.C.; Porte, D. Insulin in the Brain - a Hormonal Regulator of Energy-Balance. Endocr. Rev. 1992, 13, 387–414. [Google Scholar] [CrossRef]
- Duarte, A.I.; Moreira, P.I.; Oliveira, C.R. Insulin in Central Nervous System: More than Just a Peripheral Hormone. J. Aging Res. 2012. [Google Scholar] [CrossRef] [Green Version]
- Stockhorst, U.; de Fries, D.; Steingrueber, H.J.; Scherbaum, W.A. Insulin and the CNS: Effects on food intake, memory, and endocrine parameters and the role of intranasal insulin administration in humans. Physiol. Behav. 2004, 83, 47–54. [Google Scholar] [CrossRef]
- Baura, G.D.; Foster, D.M.; Porte, D.; Kahn, S.E.; Bergman, R.N.; Cobelli, C.; Schwartz, M.W. Saturable Transport of Insulin from Plasma into the Central-Nervous-System of Dogs in-Vivo - a Mechanism for Regulated Insulin Delivery to the Brain. J. Clin. Investig. 1993, 92, 1824–1830. [Google Scholar] [CrossRef] [Green Version]
- Woods, S.C.; Lotter, E.C.; Mckay, L.D.; Porte, D. Chronic Intracerebroventricular Infusion of Insulin Reduces Food-Intake and Body-Weight of Baboons. Nature 1979, 282, 503–505. [Google Scholar] [CrossRef]
- Obici, S.; Feng, Z.; Karkanias, G.; Baskin, D.G.; Rossetti, L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat. Neurosci. 2002, 5, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.I.; Heffron, H.; Smith, M.A.; Al-Qassab, H.; Xu, A.W.; Selman, C.; Simmgen, M.; Clements, M.; Claret, M.; Maccoll, G.; et al. The role of insulin receptor substrate 2 in hypothalamic and beta cell function. J. Clin. Invest. 2005, 115, 940–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polonsky, K.S.; Given, B.D.; Van Cauter, E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J. Clin. Invest. 1988, 81, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Jequier, E. Leptin signaling, adiposity, and energy balance. Ann. NY Acad. Sci. 2002, 967, 379–388. [Google Scholar] [CrossRef]
- Rigamonti, A.E.; Sartorio, A.; Bonomo, S.M.; Giunta, M.; Grassi, G.; Perotti, M.; Cella, S.G.; Muller, E.E.; Pincelli, A.I. Effect of a somatostatin infusion on circulating levels of adipokines in obese women. Metabolism 2012, 61, 1797–1802. [Google Scholar] [CrossRef]
- Seboek, D.; Linscheid, P.; Zulewski, H.; Langer, I.; Christ-Crain, M.; Keller, U.; Muller, B. Somatostatin is expressed and secreted by human adipose tissue upon infection and inflammation. J. Clin. Endocr. Metab. 2004, 89, 4833–4839. [Google Scholar] [CrossRef] [Green Version]
- Stepanyan, Z.; Kocharyan, A.; Behrens, M.; Koebnick, C.; Pyrski, M.; Meyerhof, W. Somatostatin, a negative-regulator of central leptin action in the rat hypothalamus. J. Neurochem. 2007, 100, 468–478. [Google Scholar] [CrossRef]
- Quintela, M.; Senaris, R.; Heiman, M.L.; Casanueva, F.F.; Dieguez, C. Leptin inhibits in vitro hypothalamic somatostatin secretion and somatostatin mRNA levels. Endocrinology 1997, 138, 5641–5644. [Google Scholar] [CrossRef]
- Perianes-Cachero, A.; Burgos-Ramos, E.; Puebla-Jimenez, L.; Canelles, S.; Frago, L.M.; Hervas-Aguilar, A.; De Frutos, S.; Toledo-Lobo, M.V.; Mela, V.; Viveros, M.P.; et al. Acute up-Regulation of the Rat Brain Somatostatin Receptor-Effector System by Leptin Is Related to Activation of Insulin Signaling and May Counteract Central Leptin Actions. Neuroscience 2013, 252, 289–301. [Google Scholar] [CrossRef]
- Bolanowski, M.; Milewicz, A.; Bidzinska, B.; Jedrzejuk, D.; Daroszewski, J.; Mikulski, E. Serum leptin levels in acromegaly--a significant role for adipose tissue and fasting insulin/glucose ratio. Med Sci. Monit. 2002, 8, CR685–CR689. [Google Scholar]
- Donahoo, W.T.; Jensen, D.R.; Yost, T.J.; Eckel, R.H. Isoproterenol and somatostatin decrease plasma leptin in humans: A novel mechanism regulating leptin secretion. J. Clin. Endocr. Metab. 1997, 82, 4139–4143. [Google Scholar] [CrossRef]
- Tan, K.C.B.; Tso, A.W.K.; Lam, K.S.L. Effect of Sandostatin((R)) LAR((R)) on serum leptin levels in patients with acromegaly. Clin. Endocrinol. 2001, 54, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, D.; De Gennaro Colonna, V.; Bagnasco, M.; Bonacci, D.; Muller, E.E. Leptin regulates GH secretion in the rat by acting on GHRH and somatostatinergic functions. J. Endocrinol. 1999, 162, 95–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmquist, J.K.; Ahima, R.S.; Elias, C.F.; Flier, J.S.; Saper, C.B. Leptin activates distinct projections from the dorsomedial and ventromedial hypothalamic nuclei. Proc. Natl. Acad. Sci. USA 1998, 95, 741–746. [Google Scholar] [CrossRef] [Green Version]
- Elmquist, J.K.; Ahima, R.S.; Maratos-Flier, E.; Flier, J.S.; Saper, C.B. Leptin activates neurons in ventrobasal hypothalamus and brainstem. Endocrinology 1997, 138, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.W.; Seeley, R.J.; Campfield, L.A.; Burn, P.; Baskin, D.G. Identification of targets of leptin action in rat hypothalamus. J. Clin. Invest. 1996, 98, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.; Okano, H.J.; Li, C.; Lee, G.H.; Zhao, C.; Darnell, R.; Friedman, J.M. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc. Natl. Acad. Sci. USA 1997, 94, 7001–7005. [Google Scholar] [CrossRef] [Green Version]
- Atasoy, D.; Betley, J.N.; Su, H.H.; Sternson, S.M. Deconstruction of a neural circuit for hunger. Nature 2012, 488, 172–177. [Google Scholar] [CrossRef]
- Balthasar, N.; Dalgaard, L.T.; Lee, C.E.; Yu, J.; Funahashi, H.; Williams, T.; Ferreira, M.; Tang, V.; McGovern, R.A.; Kenny, C.D.; et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 2005, 123, 493–505. [Google Scholar] [CrossRef]
- Gropp, E.; Shanabrough, M.; Borok, E.; Xu, A.W.; Janoschek, R.; Buch, T.; Plum, L.; Balthasar, N.; Hampel, B.; Waisman, A.; et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat. Neurosci. 2005, 8, 1289–1291. [Google Scholar] [CrossRef]
- Lohr, H.; Hess, S.; Pereira, M.M.A.; Reinoss, P.; Leibold, S.; Schenkel, C.; Wunderlich, C.M.; Kloppenburg, P.; Bruning, J.C.; Hammerschmidt, M. Diet-Induced Growth Is Regulated via Acquired Leptin Resistance and Engages a Pomc-Somatostatin-Growth Hormone Circuit. Cell Rep. 2018, 23, 1728–1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Lin, Y.C.; Kuo, T.W.; Knight, Z.A. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell 2015, 160, 829–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowley, M.A.; Smart, J.L.; Rubinstein, M.; Cordan, M.G.; Diano, S.; Horvath, T.L.; Cone, R.D.; Low, M.J. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 2001, 411, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Cowley, M.A.; Smith, R.G.; Diano, S.; Tschop, M.; Pronchuk, N.; Grove, K.L.; Strasburger, C.J.; Bidlingmaier, M.; Esterman, M.; Heiman, M.L.; et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 2003, 37, 649–661. [Google Scholar] [CrossRef] [Green Version]
- Munzberg, H.; Jobst, E.E.; Bates, S.H.; Jones, J.; Villanueva, E.; Leshan, R.; Bjornholm, M.; Elmquist, J.; Sleeman, M.; Cowley, M.A.; et al. Appropriate inhibition of orexigenic hypothalamic arcuate nucleus neurons independently of leptin receptor/STAT3 signaling. J. Neurosci. 2007, 27, 69–74. [Google Scholar] [CrossRef]
- Spanswick, D.; Smith, M.A.; Groppi, V.E.; Logan, S.D.; Ashford, M.L. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature 1997, 390, 521–525. [Google Scholar] [CrossRef]
- Gaykema, R.P.; Nguyen, X.M.; Boehret, J.M.; Lambeth, P.S.; Joy-Gaba, J.; Warthen, D.M.; Scott, M.M. Characterization of excitatory and inhibitory neuron activation in the mouse medial prefrontal cortex following palatable food ingestion and food driven exploratory behavior. Front. Neuroanat. 2014, 8, 60. [Google Scholar] [CrossRef] [Green Version]
- Dodd, G.T.; Decherf, S.; Loh, K.; Simonds, S.E.; Wiede, F.; Balland, E.; Merry, T.L.; Munzberg, H.; Zhang, Z.Y.; Kahn, B.B.; et al. Leptin and Insulin Act on POMC Neurons to Promote the Browning of White Fat. Cell 2015, 160, 88–104. [Google Scholar] [CrossRef] [Green Version]
- Muller, T.D.; Nogueiras, R.; Andermann, M.L.; Andrews, Z.B.; Anker, S.D.; Argente, J.; Batterham, R.L.; Benoit, S.C.; Bowers, C.Y.; Broglio, F.; et al. Ghrelin. Mol. Metab. 2015, 4, 437–460. [Google Scholar] [CrossRef]
- Di Vito, L.; Broglio, F.; Benso, A.; Gottero, C.; Prodam, F.; Papotti, M.; Muccioli, G.; Dieguez, C.; Casanueva, F.F.; Deghenghi, R.; et al. The GH-releasing effect of ghrelin, a natural GH secretagogue, is only blunted by the infusion of exogenous somatostatin in humans. Clin. Endocrinol. 2002, 56, 643–648. [Google Scholar] [CrossRef]
- Malagon, M.M.; Luque, R.M.; Ruiz-Guerrero, E.; Rodriguez-Pacheco, F.; Garcia-Navarro, S.; Casanueva, F.F.; Gracia-Navarro, F.; Castano, J.P. Intracellular signaling mechanisms mediating ghrelin-stimulated growth hormone release in somatotropes. Endocrinology 2003, 144, 5372–5380. [Google Scholar] [CrossRef] [PubMed]
- Korbonits, M.; Goldstone, A.P.; Gueorguiev, M.; Grossman, A.B. Ghrelin - a hormone with multiple functions. Front. Neuroendocrinol. 2004, 25, 27–68. [Google Scholar] [CrossRef] [PubMed]
- Broglio, F.; Papotti, M.; Muccioli, G.; Ghigo, E. Brain-gut communication: Cortistatin, somatostatin and ghrelin. Trends Endocrin. Met. 2007, 18, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Gahete, M.D.; Cordoba-Chacon, J.; Salvatori, R.; Castano, J.P.; Kineman, R.D.; Luque, R.M. Metabolic regulation of ghrelin O-acyl transferase (GOAT) expression in the mouse hypothalamus, pituitary, and stomach. Mol. Cell. Endocrinol. 2010, 317, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Luque, R.M.; Gahete, M.D.; Hochgeschwender, U.; Kineman, R.D. Evidence that endogenous SST inhibits ACTH and ghrelin expression by independent pathways. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E395–E403. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.; Zhang, L.; Brandon, A.; Wang, Q.P.; Begg, D.; Qi, Y.; Fu, M.; Kulkarni, R.; Teo, J.; Baldock, P.; et al. Insulin controls food intake and energy balance via NPY neurons. Mol. Metab. 2017, 6, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Toshinai, K.; Date, Y.; Murakami, N.; Shimada, M.; Mondal, M.S.; Shimbara, T.; Guan, J.L.; Wang, Q.P.; Funahashi, H.; Sakurai, T.; et al. Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology 2003, 144, 1506–1512. [Google Scholar] [CrossRef] [Green Version]
- Pucci, A.; Cheung, W.H.; Jones, J.; Manning, S.; Kingett, H.; Adamo, M.; Elkalaawy, M.; Jenkinson, A.; Finer, N.; Doyle, J.; et al. A case of severe anorexia, excessive weight loss and high peptide YY levels after sleeve gastrectomy. Endocrinol. Diab. Meta. 2015. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef] [Green Version]
- Kirkham, T.C.; Williams, C.M.; Fezza, F.; Di Marzo, V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: Stimulation of eating by 2-arachidonoyl glycerol. Br. J. Pharmacol. 2002, 136, 550–557. [Google Scholar] [CrossRef]
- Soria-Gomez, E.; Bellocchio, L.; Reguero, L.; Lepousez, G.; Martin, C.; Bendahmane, M.; Ruehle, S.; Remmers, F.; Desprez, T.; Matias, I.; et al. The endocannabinoid system controls food intake via olfactory processes. Nat. Neurosci. 2014, 17, 407–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanus, L.; Avraham, Y.; Ben-Shushan, D.; Zolotarev, O.; Berry, E.M.; Mechoulam, R. Short-term fasting and prolonged semistarvation have opposite effects on 2-AG levels in mouse brain. Brain Res. 2003, 983, 144–151. [Google Scholar] [CrossRef]
- Osei-Hyiaman, D.; Depetrillo, M.; Harvey-White, J.; Bannon, A.W.; Cravatt, B.F.; Kuhar, M.J.; Mackie, K.; Palkovits, M.; Kunos, G. Cocaine- and amphetamine-related transcript is involved in the orexigenic effect of endogenous anandamide. Neuroendocrinology 2005, 81, 273–282. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, R.D.; Gundlach, A.L. Food or water deprivation modulate nitric oxide synthase (NOS) activity and gene expression in rat hypothalamic neurones: Correlation with neurosecretory activity? Journal of neuroendocrinology 1996, 8, 417–425. [Google Scholar] [CrossRef]
- Gaskin, F.S.; Farr, S.A.; Banks, W.A.; Kumar, V.B.; Morley, J.E. Ghrelin-induced feeding is dependent on nitric oxide. Peptides 2003, 24, 913–918. [Google Scholar] [CrossRef]
- Morley, J.E.; Alshaher, M.M.; Farrs, S.A.; Flood, J.F.; Kumar, V.B. Leptin and neuropeptide Y (NPY) modulate nitric oxide synthase: Further evidence for a role of nitric oxide in feeding. Peptides 1999, 20, 595–600. [Google Scholar] [CrossRef]
- Leshan, R.L.; Greenwald-Yarnell, M.; Patterson, C.M.; Gonzalez, I.E.; Myers, M.G. Leptin action through hypothalamic nitric oxide synthase-1-expressing neurons controls energy balance. Nat. Med. 2012, 18, 820–823. [Google Scholar] [CrossRef] [Green Version]
- Shao, Z.; Yin, J.; Chapman, K.; Grzemska, M.; Clark, L.; Wang, J.; Rosenbaum, D.M. High-resolution crystal structure of the human CB1 cannabinoid receptor. Nature 2016, 540, 602–606. [Google Scholar] [CrossRef]
- Hua, T.; Vemuri, K.; Pu, M.; Qu, L.; Han, G.W.; Wu, Y.; Zhao, S.; Shui, W.; Li, S.; Korde, A.; et al. Crystal Structure of the Human Cannabinoid Receptor CB1. Cell 2016, 167, 750–762. [Google Scholar] [CrossRef] [Green Version]
- Vallee, M.; Vitiello, S.; Bellocchio, L.; Hebert-Chatelain, E.; Monlezun, S.; Martin-Garcia, E.; Kasanetz, F.; Baillie, G.L.; Panin, F.; Cathala, A.; et al. Pregnenolone Can Protect the Brain from Cannabis Intoxication. Science 2014, 343, 94–98. [Google Scholar] [CrossRef] [Green Version]
- Heimann, A.S.; Gomes, L.; Dale, C.S.; Pagano, R.L.; Gupta, A.; de Souza, L.L.; Luchessi, A.D.; Castro, L.M.; Giorgi, R.; Rioli, V.; et al. Hemopressin is an inverse agonist of CB1 cannabinoid receptors. Proc. Natl. Acad. Sci. USA. 2007, 104, 20588–20593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodd, G.T.; Worth, A.A.; Hodkinson, D.J.; Srivastava, R.K.; Lutz, B.; Williams, S.R.; Luckman, S.M. Central functional response to the novel peptide cannabinoid, hemopressin. Neuropharmacology 2013, 71, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Dodd, G.T.; Mancini, G.; Lutz, B.; Luckman, S.M. The Peptide Hemopressin Acts through CB1 Cannabinoid Receptors to Reduce Food Intake in Rats and Mice. J. Neurosci. 2010, 30, 7369–7376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busquets Garcia, A.; Soria-Gomez, E.; Bellocchio, L.; Marsicano, G. Cannabinoid receptor type-1: Breaking the dogmas. F1000Research 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Nogueiras, R.; Veyrat-Durebex, C.; Suchanek, P.M.; Klein, M.; Tschop, J.; Caldwell, C.; Woods, S.C.; Wittmann, G.; Watanabe, M.; Liposits, Z.; et al. Peripheral, but Not Central, CB1 Antagonism Provides Food Intake-Independent Metabolic Benefits Diet-Induced Obese Rats. Diabetes 2008, 57, 2977–2991. [Google Scholar] [CrossRef] [Green Version]
- Tam, J.; Cinar, R.; Liu, J.; Godlewski, G.; Wesley, D.; Jourdan, T.; Szanda, G.; Mukhopadhyay, B.; Chedester, L.; Liow, J.S.; et al. Peripheral Cannabinoid-1 Receptor Inverse Agonism Reduces Obesity by Reversing Leptin Resistance. Cell Metab. 2012, 16, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Koch, M. Cannabinoid Receptor Signaling in Central Regulation of Feeding Behavior: A Mini-Review. Front. Neurosci. 2017, 11. [Google Scholar] [CrossRef]
- Liu, T.M.; Kong, D.; Shah, B.P.; Ye, C.P.; Koda, S.; Saunders, A.; Ding, J.B.; Yang, Z.F.; Sabatini, B.L.; Lowell, B.B. Fasting Activation of AgRP Neurons Requires NMDA Receptors and Involves Spinogenesis and Increased Excitatory Tone. Neuron 2012, 73, 511–522. [Google Scholar] [CrossRef] [Green Version]
- Albizu, L.; Moreno, J.L.; Gonzalez-Maeso, J.; Sealfon, S.C. Heteromerization of G Protein-Coupled Receptors: Relevance to Neurological Disorders and Neurotherapeutics. Cns Neurol. Disord. Drug Targets 2010, 9, 636–650. [Google Scholar] [CrossRef] [Green Version]
- Befort, K. Interactions of the opioid and cannabinoid systems in reward: Insights from knockout studies. Front. Pharmacol. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Somvanshi, R.K.; Kumar, U. Pathophysiology of GPCR Homo- and Heterodimerization: Special Emphasis on Somatostatin Receptors. Pharmaceuticals 2012, 5, 417–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ugur, M.; Derouiche, L.; Massotte, D. Heteromerization Modulates mu Opioid Receptor Functional Properties in vivo. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamal, M.; Jockers, R. Biological Significance of GPCR Heteromerization in the Neuro-Endocrine System. Front. Endocrinol. 2011, 2, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajput, P.S.; Kharmate, G.; Norman, M.; Liu, S.H.; Sastry, B.R.; Brunicardi, C.F.; Kumar, U. Somatostatin receptor 1 and 5 double knockout mice mimic neurochemical changes of Huntington’s disease transgenic mice. PLoS ONE 2011, 6, e24467. [Google Scholar] [CrossRef]
- Suzuki, K.; Jayasena, C.N.; Bloom, S.R. Obesity and appetite control. Exp. Diabetes. Res. 2012, 2012, 824305. [Google Scholar] [CrossRef] [Green Version]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef]
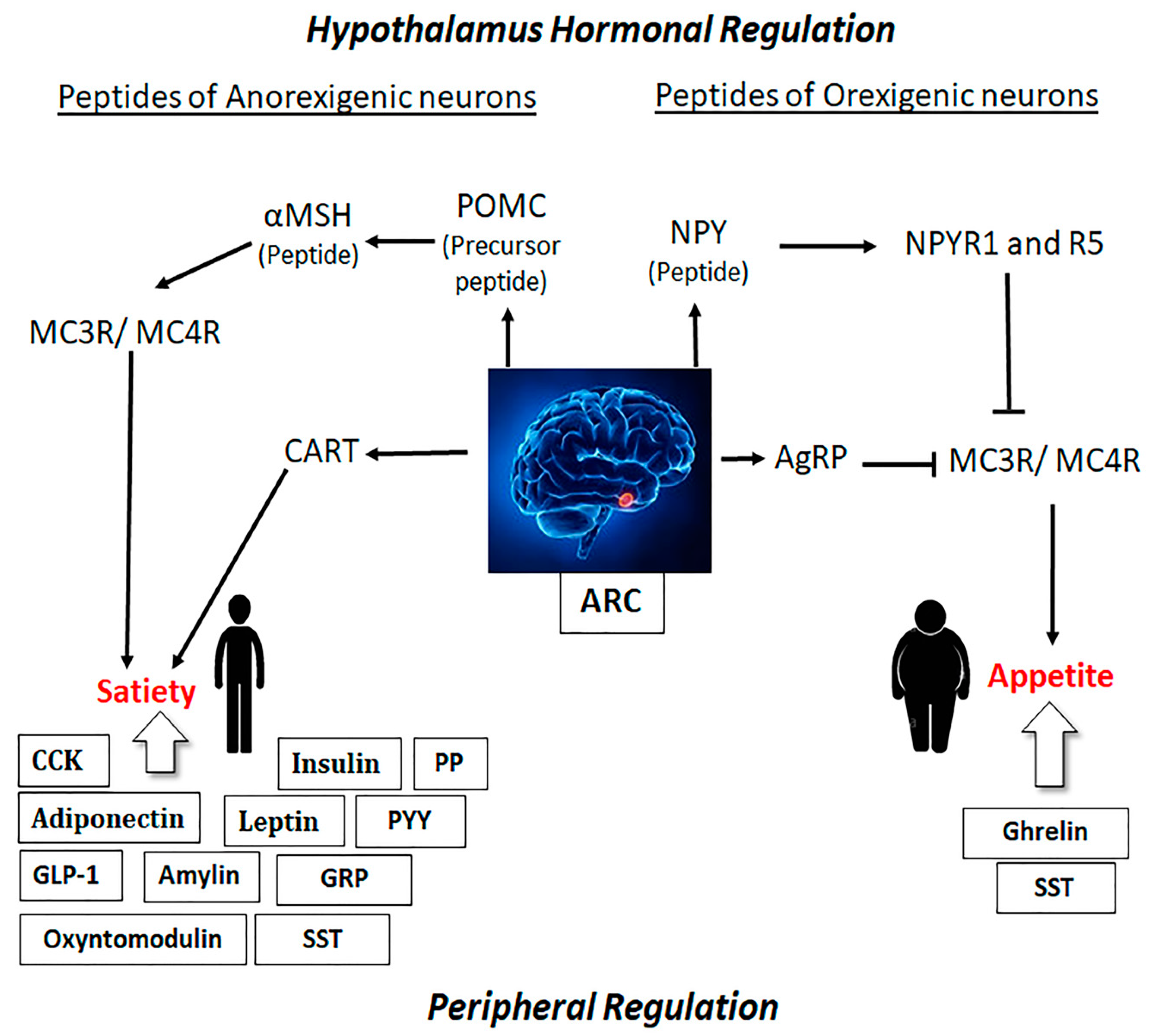
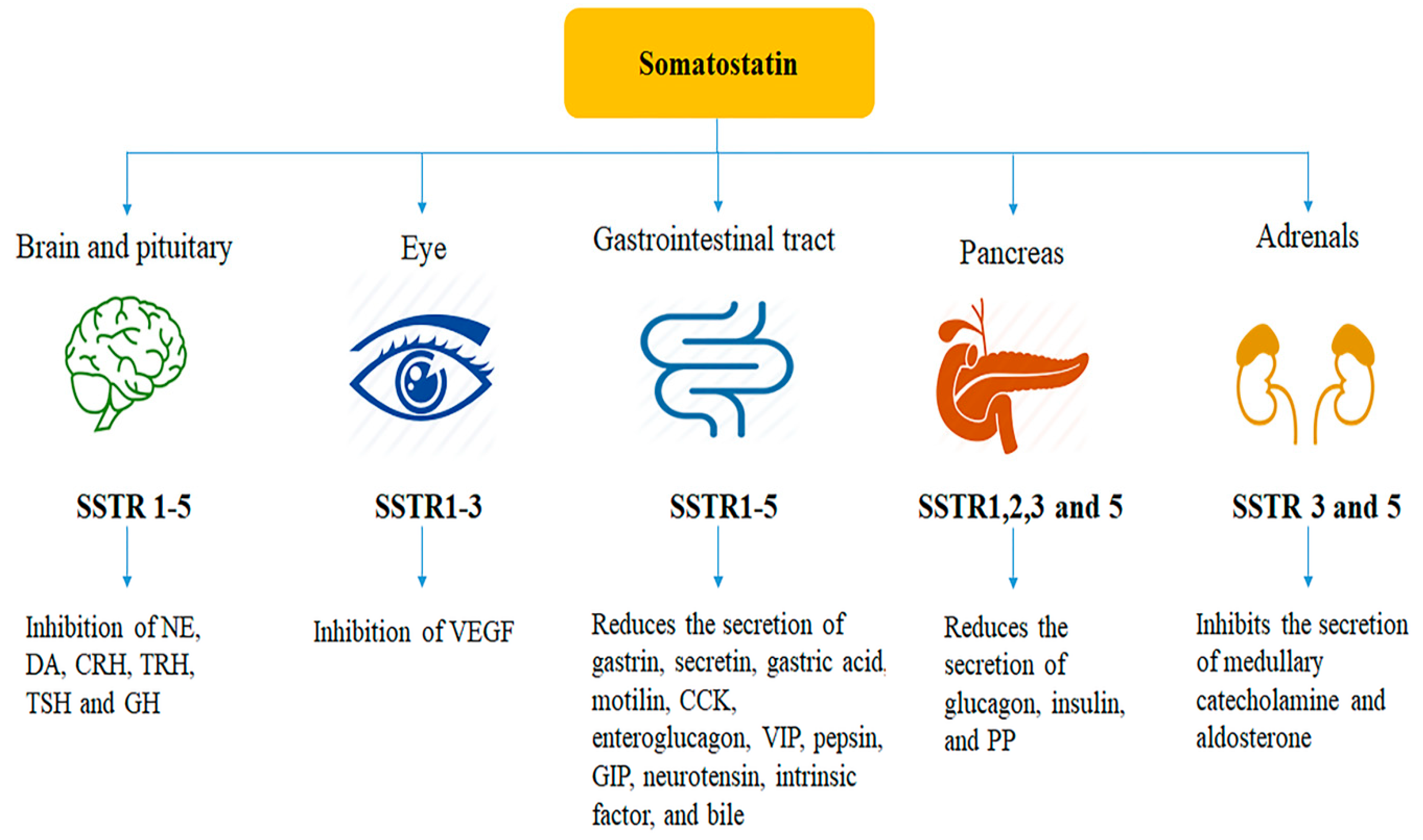

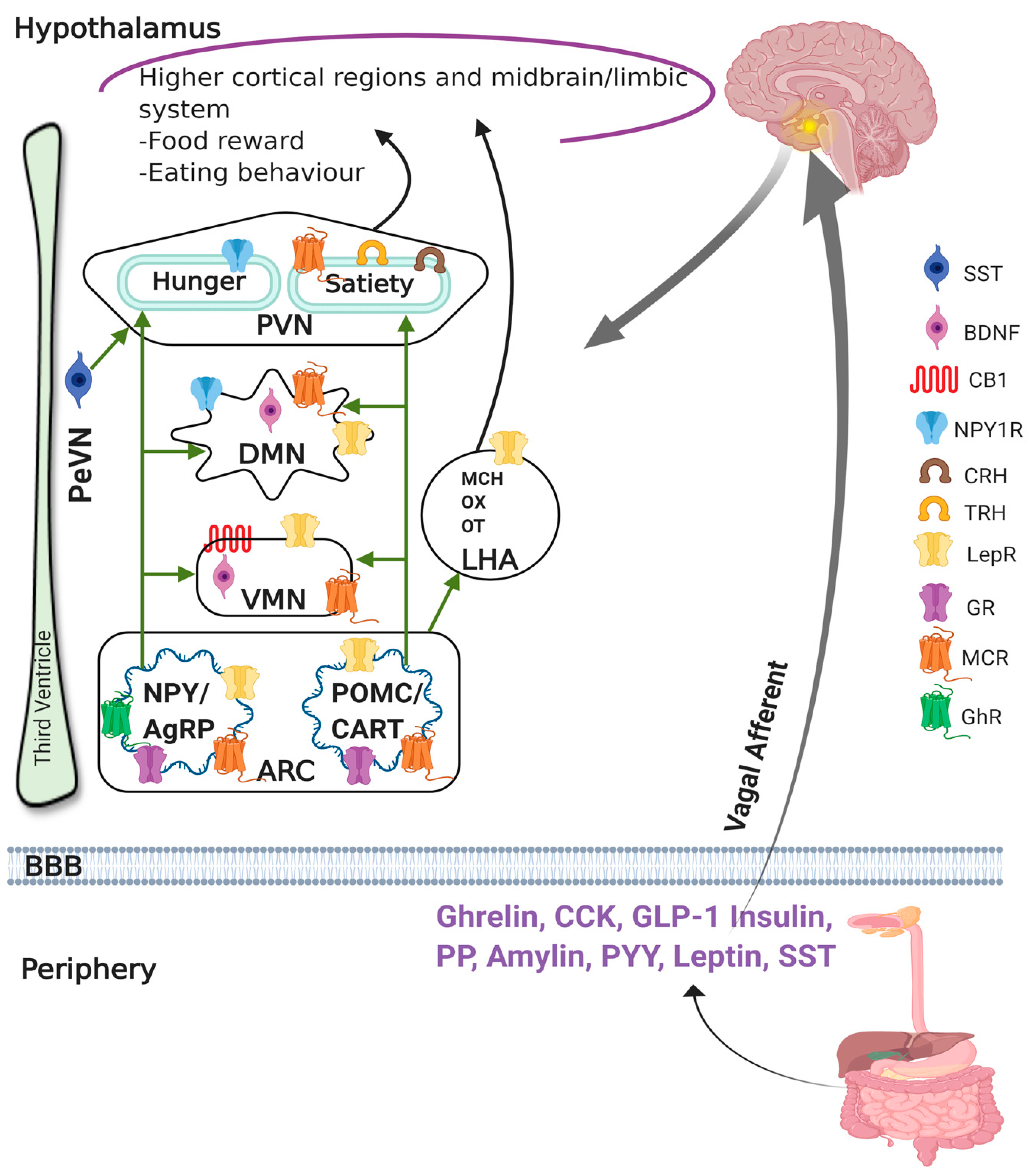
| Peptide Hormones | Origin of Secretion | Binding Receptors | Effect on Food Intake | References |
|---|---|---|---|---|
| Cholecystokinin | I cells | Cholecystokinin receptor subtype A | Decreased | [114] |
| Glucagon-like peptide 1 | L cells | Glucagon-like peptide-1 receptor | [115] | |
| Pancreatic polypeptide | PP cells | Pancreatic polypeptide Y2 & Y4 receptor | [116] | |
| Peptide tyrosine tyrosine | L cells | Peptide tyrosine tyrosine Y2 receptor | [117] | |
| Oxyntomodulin | Oxyntic cells | Oxyntomodulin | [118] | |
| Somatostatin | Delta cells; Neurons | Somatostatin receptors | Increased/Decreased | [26,119,120,121,122] |
| Ghrelin | P/D1 and Epsilon cells | Growth hormone secretagogue receptor | Increased | [123] |
| Leptin | Fat cells | Ob-Rb, Leptin receptor | Decreased | [124] |
| Adiponectin | Adipose tissue | Adiponectin receptor 1 and 2 | [125] | |
| Insulin | Beta cells | Insulin receptors | [126] | |
| Amylin | Beta cells | Amylin-specific receptors | [127] |
| Treatment | Species | Route of Administration | Proposed Mechanism | Effect on Food Intake | References |
|---|---|---|---|---|---|
| SST | Rat and baboon | IP | Vagally mediated mechanism | Decreased | [174,183] |
| OCT | Rat | ICV | Activation of brain SSTR2 | Increased | [184,185] |
| SST | Healthy volunteers | IV infusion | Reduced gastric emptying and gut motility | Initially decreased Increased in presence of low dose of intraduodenal fat Post meal satiety was higher | [25] |
| SST-14 | Chick | ICV | Activation of brain SSTR2 | Increased | [186] |
| SST analogue (ODT8-SST) | Rat | ICV | Activation of brain SSTR2 | Increased | [163] |
| OCT | Mouse | ICV | Activation of brain SSTR2 | Increased | [164] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, U.; Singh, S. Role of Somatostatin in the Regulation of Central and Peripheral Factors of Satiety and Obesity. Int. J. Mol. Sci. 2020, 21, 2568. https://doi.org/10.3390/ijms21072568
Kumar U, Singh S. Role of Somatostatin in the Regulation of Central and Peripheral Factors of Satiety and Obesity. International Journal of Molecular Sciences. 2020; 21(7):2568. https://doi.org/10.3390/ijms21072568
Chicago/Turabian StyleKumar, Ujendra, and Sneha Singh. 2020. "Role of Somatostatin in the Regulation of Central and Peripheral Factors of Satiety and Obesity" International Journal of Molecular Sciences 21, no. 7: 2568. https://doi.org/10.3390/ijms21072568
APA StyleKumar, U., & Singh, S. (2020). Role of Somatostatin in the Regulation of Central and Peripheral Factors of Satiety and Obesity. International Journal of Molecular Sciences, 21(7), 2568. https://doi.org/10.3390/ijms21072568





