Protein Signature in Saliva of Temporomandibular Disorders Myalgia
Abstract
:1. Introduction
2. Results
2.1. Clinical Outcomes
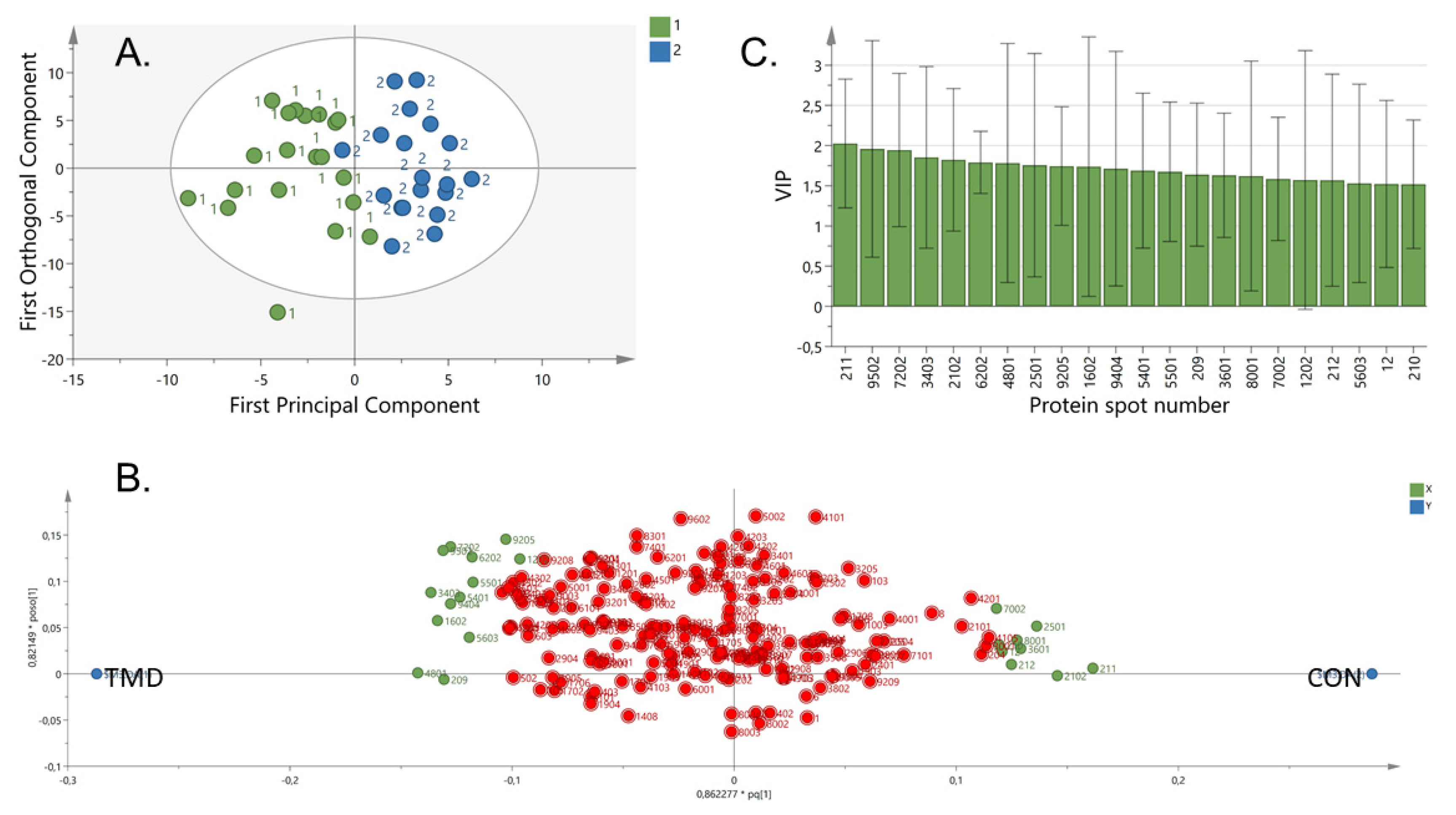
2.2. Proteome Pattern in TMD Myalgia
2.3. Correlation Analysis between Altered Levels of Proteins and Clinical Parameters
3. Discussion
4. Methods and Materials
4.1. Participants
4.2. Questionnaires and Clinical Measurements
4.3. Pressure Pain Threshold
4.4. Sample Collection and Preparation
4.5. Gel Electrophoresis
4.6. Staining and Image Analysis
4.7. Protein Identification by LC–MS/MS
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poveda Roda, R.; Diaz Fernandez, J.M.; Hernandez Bazan, S.; Jimenez Soriano, Y.; Margaix, M.; Sarrion, G. A review of temporomandibular joint disease (TMJD). Part II: Clinical and radiological semiology. Morbidity processes. Med. Oral Patol. Oral Cir. Bucal 2008, 13, 102–109. [Google Scholar]
- Fernandez-de-las-Penas, C.; Svensson, P. Myofascial Temporomandibular Disorder. Curr. Rheumatol. Rev. 2016, 12, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Slade, G.D.; Ohrbach, R.; Greenspan, J.D.; Fillingim, R.B.; Bair, E.; Sanders, A.E.; Dubner, R.; Diatchenko, L.; Meloto, C.B.; Smith, S.; et al. Painful Temporomandibular Disorder: Decade of Discovery from OPPERA Studies. J. Dent. Res. 2016, 95, 1084–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fillingim, R.B.; Ohrbach, R.; Greenspan, J.D.; Knott, C.; Diatchenko, L.; Dubner, R.; Bair, E.; Baraian, C.; Mack, N.; Slade, G.D.; et al. Psychological factors associated with development of TMD: The OPPERA prospective cohort study. J. Pain 2013, 14, T75–T90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasim, H.; Louca, S.; Christidis, N.; Ernberg, M. Salivary cortisol and psychological factors in women with chronic and acute oro-facial pain. J. Oral Rehabil. 2014, 41, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Groupdagger. J. Oral Facial Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef]
- Ernberg, M. Masticatory Muscle Pain Biomarkers. In Orofacial Pain Biomarkers; Goulet, J., Velly, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 79–93. [Google Scholar]
- Yoshizawa, J.M.; Schafer, C.A.; Schafer, J.J.; Farrell, J.J.; Paster, B.J.; Wong, D.T. Salivary biomarkers: Toward future clinical and diagnostic utilities. Clin. Microbiol. Rev. 2013, 26, 781–791. [Google Scholar] [CrossRef] [Green Version]
- Chandramouli, K.; Qian, P.Y. Proteomics: Challenges, techniques and possibilities to overcome biological sample complexity. Hum. Genom. Proteom. HGP 2009, 2009, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Geyer, P.E.; Kulak, N.A.; Pichler, G.; Holdt, L.M.; Teupser, D.; Mann, M. Plasma Proteome Profiling to Assess Human Health and Disease. Cell Syst. 2016, 2, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Ignjatovic, V.; Geyer, P.E.; Palaniappan, K.K.; Chaaban, J.E.; Omenn, G.S.; Baker, M.S.; Deutsch, E.W.; Schwenk, J.M. Mass Spectrometry-Based Plasma Proteomics: Considerations from Sample Collection to Achieving Translational Data. J. Proteome Res. 2019, 18, 4085–4097. [Google Scholar] [CrossRef]
- Olausson, P.; Gerdle, B.; Ghafouri, N.; Sjostrom, D.; Blixt, E.; Ghafouri, B. Protein alterations in women with chronic widespread pain--An explorative proteomic study of the trapezius muscle. Sci. Rep. 2015, 5, 11894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazzichi, L.; Ciregia, F.; Giusti, L.; Baldini, C.; Giannaccini, G.; Giacomelli, C.; Sernissi, F.; Bombardieri, S.; Lucacchini, A. Detection of potential markers of primary fibromyalgia syndrome in human saliva. Proteom. Clin. Appl. 2009, 3, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Giusti, L.; Baldini, C.; Bazzichi, L.; Ciregia, F.; Tonazzini, I.; Mascia, G.; Giannaccini, G.; Bombardieri, S.; Lucacchini, A. Proteome analysis of whole saliva: A new tool for rheumatic diseases--the example of Sjogren’s syndrome. Proteomics 2007, 7, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Olausson, P.; Gerdle, B.; Ghafouri, N.; Larsson, B.; Ghafouri, B. Identification of proteins from interstitium of trapezius muscle in women with chronic myalgia using microdialysis in combination with proteomics. PLoS ONE 2012, 7, e52560. [Google Scholar] [CrossRef] [Green Version]
- Olausson, P.; Ghafouri, B.; Backryd, E.; Gerdle, B. Clear differences in cerebrospinal fluid proteome between women with chronic widespread pain and healthy women - a multivariate explorative cross-sectional study. J. Pain Res. 2017, 10, 575–590. [Google Scholar] [CrossRef] [Green Version]
- Ji, E.H.; Diep, C.; Liu, T.; Li, H.; Merrill, R.; Messadi, D.; Hu, S. Potential protein biomarkers for burning mouth syndrome discovered by quantitative proteomics. Mol. Pain 2017, 13. [Google Scholar] [CrossRef] [Green Version]
- Backryd, E.; Ghafouri, B.; Carlsson, A.K.; Olausson, P.; Gerdle, B. Multivariate proteomic analysis of the cerebrospinal fluid of patients with peripheral neuropathic pain and healthy controls - a hypothesis-generating pilot study. J. Pain Res. 2015, 8, 321–333. [Google Scholar] [CrossRef] [Green Version]
- Krief, G.; Haviv, Y.; Deutsch, O.; Keshet, N.; Almoznino, G.; Zacks, B.; Palmon, A.; Aframian, D.J. Proteomic profiling of whole-saliva reveals correlation between Burning Mouth Syndrome and the neurotrophin signaling pathway. Sci. Rep. 2019, 9, 4794. [Google Scholar] [CrossRef]
- Hadrevi, J.; Ghafouri, B.; Larsson, B.; Gerdle, B.; Hellstrom, F. Multivariate modeling of proteins related to trapezius myalgia, a comparative study of female cleaners with or without pain. PLoS ONE 2013, 8, e73285. [Google Scholar] [CrossRef] [Green Version]
- Ciregia, F.; Giacomelli, C.; Giusti, L.; Boldrini, C.; Piga, I.; Pepe, P.; Consensi, A.; Gori, S.; Lucacchini, A.; Mazzoni, M.R.; et al. Putative salivary biomarkers useful to differentiate patients with fibromyalgia. J. Proteom. 2019, 190, 44–54. [Google Scholar] [CrossRef]
- Jasim, H.; Carlsson, A.; Hedenberg-Magnusson, B.; Ghafouri, B.; Ernberg, M. Saliva as a medium to detect and measure biomarkers related to pain. Sci. Rep. 2018, 8, 3220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasim, H.; Olausson, P.; Hedenberg-Magnusson, B.; Ernberg, M.; Ghafouri, B. The proteomic profile of whole and glandular saliva in healthy pain-free subjects. Sci. Rep. 2016, 6, 39073. [Google Scholar] [CrossRef] [PubMed]
- Grassl, N.; Kulak, N.A.; Pichler, G.; Geyer, P.E.; Jung, J.; Schubert, S.; Sinitcyn, P.; Cox, J.; Mann, M. Ultra-deep and quantitative saliva proteome reveals dynamics of the oral microbiome. Genome Med. 2016, 8, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.; Liang, X.; Zhang, X.; Liu, T.; Shi, Q.; Song, Y.; Jiang, Y.; Wu, H.; Jiang, Y.; Lu, X.; et al. Phosphoglycerate kinase-1 is a predictor of poor survival and a novel prognostic biomarker of chemoresistance to paclitaxel treatment in breast cancer. Br. J. Cancer 2015, 112, 1332–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Yan, X.; Li, X.; Zheng, Y.; Li, S.; Chang, X. PGK1, a glucose metabolism enzyme, may play an important role in rheumatoid arthritis. Inflamm. Res. 2016, 65, 815–825. [Google Scholar] [CrossRef]
- Vissing, J.; Akman, H.O.; Aasly, J.; Kahler, S.G.; Bacino, C.A.; DiMauro, S.; Haller, R.G. Level of residual enzyme activity modulates the phenotype in phosphoglycerate kinase deficiency. Neurology 2018, 91, 1077–1082. [Google Scholar] [CrossRef]
- Wang, D.; Moothart, D.R.; Lowy, D.R.; Qian, X. The expression of glyceraldehyde-3-phosphate dehydrogenase associated cell cycle (GACC) genes correlates with cancer stage and poor survival in patients with solid tumors. PLoS ONE 2013, 8, e61262. [Google Scholar] [CrossRef] [Green Version]
- Tristan, C.; Shahani, N.; Sedlak, T.W.; Sawa, A. The diverse functions of GAPDH: Views from different subcellular compartments. Cell. Signal. 2011, 23, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Giusti, L.; Bazzichi, L.; Baldini, C.; Ciregia, F.; Mascia, G.; Giannaccini, G.; Del Rosso, M.; Bombardieri, S.; Lucacchini, A. Specific proteins identified in whole saliva from patients with diffuse systemic sclerosis. J. Rheumatol. 2007, 34, 2063–2069. [Google Scholar]
- Fatima, G.; Das, S.K.; Mahdi, A.A. Oxidative stress and antioxidative parameters and metal ion content in patients with fibromyalgia syndrome: Implications in the pathogenesis of the disease. Clin. Exp. Rheumatol. 2013, 31, S128–S133. [Google Scholar]
- Van Stegeren, A.; Rohleder, N.; Everaerd, W.; Wolf, O.T. Salivary alpha amylase as marker for adrenergic activity during stress: Effect of betablockade. Psychoneuroendocrinology 2006, 31, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Nater, U.M.; Rohleder, N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology 2009, 34, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Nater, U.M.; Rohleder, N.; Gaab, J.; Berger, S.; Jud, A.; Kirschbaum, C.; Ehlert, U. Human salivary alpha-amylase reactivity in a psychosocial stress paradigm. Int. J. Psychophysiol. 2005, 55, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Bugdayci, G.; Yildiz, S.; Altunrende, B.; Yildiz, N.; Alkoy, S. Salivary alpha amylase activity in migraine patients. Auton. Neurosci. Basic Clin. 2010, 155, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.J.; Raposo, N.R.; Ferreira, A.P.; Vitral, R.W. Salivary alpha-amylase activity: A possible indicator of pain-induced stress in orthodontic patients. Pain Med. 2011, 12, 1162–1166. [Google Scholar] [CrossRef] [Green Version]
- Takai, N.; Yamaguchi, M.; Aragaki, T.; Eto, K.; Uchihashi, K.; Nishikawa, Y. Effect of psychological stress on the salivary cortisol and amylase levels in healthy young adults. Arch. Oral Biol. 2004, 49, 963–968. [Google Scholar] [CrossRef]
- Noto, Y.; Sato, T.; Kudo, M.; Kurata, K.; Hirota, K. The relationship between salivary biomarkers and state-trait anxiety inventory score under mental arithmetic stress: A pilot study. Anesth. Analg. 2005, 101, 1873–1876. [Google Scholar] [CrossRef]
- Chatterton, R.T., Jr.; Vogelsong, K.M.; Lu, Y.C.; Hudgens, G.A. Hormonal responses to psychological stress in men preparing for skydiving. J. Clin. Endocrinol. Metab. 1997, 82, 2503–2509. [Google Scholar] [CrossRef]
- Gilman, S.; Thornton, R.; Miller, D.; Biersner, R. Effects of exercise stress on parotid gland secretion. Horm. Metab. Res. 1979, 11, 454. [Google Scholar] [CrossRef]
- Wittwer, A.; Krummenacher, P.; La Marca, R.; Ehlert, U.; Folkers, G. Salivary Alpha-Amylase Correlates with Subjective Heat Pain Perception. Pain Med. 2016, 17, 1131–1136. [Google Scholar] [CrossRef]
- Shirasaki, S.; Fujii, H.; Takahashi, M.; Sato, T.; Ebina, M.; Noto, Y.; Hirota, K. Correlation between salivary alpha-amylase activity and pain scale in patients with chronic pain. Reg. Anesth. Pain Med. 2007, 32, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi-Motamayel, F.; Shahriari, S.; Goodarzi, M.T.; Moghimbeigi, A.; Jazaeri, M.; Babaei, P. The relationship between the level of salivary alpha amylase activity and pain severity in patients with symptomatic irreversible pulpitis. Restor. Dent. Endod. 2013, 38, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Doerr, J.M.; Strahler, J.; Mewes, R.; Thieme, K.; Nater, U.M. Stress exacerbates pain in the everyday lives of women with fibromyalgia syndrome--The role of cortisol and alpha-amylase. Psychoneuroendocrinology 2016, 63, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Couture-Lalande, M.E.; Narain, T.A.; Lebel, S.; Bielajew, C. Salivary Alpha-Amylase Reactivity in Breast Cancer Survivors. Int. J. Environ. Res. Public Health 2016, 13, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbs, G.M.; Roelants, K.; O’Bryan, M.K. The CAP superfamily: Cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 proteins--roles in reproduction, cancer, and immune defense. Endocr. Rev. 2008, 29, 865–897. [Google Scholar] [CrossRef]
- Grupp, K.; Kohl, S.; Sirma, H.; Simon, R.; Steurer, S.; Becker, A.; Adam, M.; Izbicki, J.; Sauter, G.; Minner, S.; et al. Cysteine-rich secretory protein 3 overexpression is linked to a subset of PTEN-deleted ERG fusion-positive prostate cancers with early biochemical recurrence. Mod. Pathol. 2013, 26, 733–742. [Google Scholar] [CrossRef] [Green Version]
- Laine, M.; Porola, P.; Udby, L.; Kjeldsen, L.; Cowland, J.B.; Borregaard, N.; Hietanen, J.; Stahle, M.; Pihakari, A.; Konttinen, Y.T. Low salivary dehydroepiandrosterone and androgen-regulated cysteine-rich secretory protein 3 levels in Sjogren’s syndrome. Arthritis Rheum. 2007, 56, 2575–2584. [Google Scholar] [CrossRef]
- Ohrfelt, A.; Andreasson, U.; Simon, A.; Zetterberg, H.; Edman, A.; Potter, W.; Holder, D.; Devanarayan, V.; Seeburger, J.; Smith, A.D.; et al. Screening for new biomarkers for subcortical vascular dementia and Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. Extra 2011, 1, 31–42. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, Y.; Shao, X.; Ni, Z.; Mou, S. L-FABP: A novel biomarker of kidney disease. Clin. Chim. Acta 2015, 445, 85–90. [Google Scholar] [CrossRef]
- Shinzawa, M.; Dogru, M.; Den, S.; Ichijima, T.; Higa, K.; Kojima, T.; Seta, N.; Nomura, T.; Tsubota, K.; Shimazaki, J. Epidermal Fatty Acid-Binding Protein: A Novel Marker in the Diagnosis of Dry Eye Disease in Sjögren Syndrome. Int. J. Mol. Sci. 2018, 19, 3463. [Google Scholar] [CrossRef] [Green Version]
- Rezar, R.; Jirak, P.; Gschwandtner, M.; Derler, R.; Felder, T.K.; Haslinger, M.; Kopp, K.; Seelmaier, C.; Granitz, C.; Hoppe, U.C.; et al. Heart-Type Fatty Acid-Binding Protein (H-FABP) and its Role as a Biomarker in Heart Failure: What Do We Know So Far? J. Clin. Med. 2020, 9, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuhashi, M.; Hotamisligil, G.S. Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008, 7, 489–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, X.; Studholme, K.; Kanjiya, M.P.; Luk, J.; Bogdan, D.; Elmes, M.W.; Carbonetti, G.; Tong, S.; Gary Teng, Y.H.; Rizzo, R.C.; et al. Fatty-acid-binding protein inhibition produces analgesic effects through peripheral and central mechanisms. Mol. Pain 2017, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pruenster, M.; Vogl, T.; Roth, J.; Sperandio, M. S100A8/A9: From basic science to clinical application. Pharmacol. Ther. 2016, 167, 120–131. [Google Scholar] [CrossRef]
- Gorg, A.; Drews, O.; Luck, C.; Weiland, F.; Weiss, W. 2-DE with IPGs. Electrophoresis 2009, 1, S122–S132. [Google Scholar] [CrossRef]
- Ghafouri, B.; Tagesson, C.; Lindahl, M. Mapping of proteins in human saliva using two-dimensional gel electrophoresis and peptide mass fingerprinting. Proteomics 2003, 3, 1003–1015. [Google Scholar] [CrossRef]
- Wheelock, A.M.; Wheelock, C.E. Trials and tribulations of ‘omics data analysis: Assessing quality of SIMCA-based multivariate models using examples from pulmonary medicine. Mol. Biosyst. 2013, 9, 2589–2596. [Google Scholar] [CrossRef] [Green Version]

| Variable | Patients | Controls | p-Value |
|---|---|---|---|
| Age (years) | 28.1 ± 8.8 | 28.3 ± 8.4 | p > 0.05 |
| Sex, (n, F/M) | 14/6 | 14/6 | p > 0.05 |
| Body Mass Index (kg/m2) | 24.4 ± 3.8 | 22.7 ± 3.0 | p > 0.05 |
| Number of teeth | 28 (2) | 30 (3) | p > 0.05 |
| Pain-free opening (mm) | 41.4 ± 10.8 | 56.4 ± 5.9 | p < 0.0001 |
| Maximum unassisted opening (mm) | 54.0 ± 6.0 | 57.7 ± 6.1 | p > 0.05 |
| Salivary Flow (ml/min) | 1.57 ± 0.50 | 1.74 ± 1.05 | p > 0.05 |
| Pain duration (years) | 6.3 ± 6.3 | 0 (0) | p < 0.001 |
| Current pain intensity (NRS) | 6 (2.5) | 0 (0) | p < 0.001 |
| CPI | 65 (27) | 0 (0) | p < 0.001 |
| PHQ-9 Score (0–36) | 6.5 (7) | 1 (3.5) | p < 0.001 |
| PHQ-15 Score (0–30) | 11.5 (9) | 2.5 (4) | p < 0.0001 |
| GAD-7 Score (0–28) | 3.5 (8.5) | 1 (2.5) | p < 0.01 |
| PSS-10 Score (0–40) | 15.5 (10) | 10 (8) | p < 0.01 |
| JFLS Score (0–10) | 1.65 (2.0) | 0 (0) | p < 0.0001 |
| PCS Score | 15 (18) | 5 (10) | p < 0.01 |
| ISI Score | 10 (15) | 5 (5) | p < 0.01 |
| PPT reference (kPa) | 382 ± 127 | 437 ±130 | p > 0.05 |
| PPT masseter muscle (kPa) | 179 ± 63 | 272 ± 81 | p < 0.001 |
| Spot No | Protein Name | UniProt ID | VIP | p-Value | Pat vs Con |
|---|---|---|---|---|---|
| 211 | Immunoglobulin J chain | P01591 | 2.026 | 0.005 | ↓ |
| 9502 | Phosphoglycerate kinase 1 | P00558 | 1.959 | 0.056 | ↑ |
| 7202 | Glyceraldehyde-3-phosphate dehydrogenase | P04406 | 1.944 | 0.04 | ↑ |
| 2102 | Fatty acid-binding protein | Q01469 | 1.823 | 0.042 | ↓ |
| 6202 | Immunoglobulin kappa light chain | P0DOX7 | 1.791 | 0.04 | ↑ |
| 4801 | Alpha-amylase 1; Alpha-amylase 2B | P04745/P19961 | 1.783 | 0.213 | ↑ |
| 2501 | Alpha-amylase 1; Alpha-amylase 2B | P04745/P19961 | 1.756 | 0.007 | ↓ |
| 9205 | Cysteine-rich secretory protein 3 | P54108 | 1.745 | 0.053 | ↑ |
| 1602 | Zinc-alpha-2-glycoprotein | P25311 | 1.738 | 0.026 | ↑ |
| 9404 | Chitinase-3-like protein 2 | Q15782 | 1.713 | 0.033 | ↑ |
| 5401 | Alpha-amylase 1; Alpha-amylase 2B | P04745/P19961 | 1.689 | 0.06 | ↑ |
| 5501 | Alpha-amylase 1; Alpha-amylase 2B | P04745/P19961 | 1.674 | 0.027 | ↑ |
| 209 | Interleukin-1 receptor antagonist protein | P18510 | 1.639 | 0.025 | ↑ |
| 3601 | Alpha-amylase 1; Alpha-amylase 2B | P04745/P19961 | 1.630 | 0.168 | ↓ |
| 8001 | Protein S100-A8 | P05109 | 1.622 | 0.004 | ↓ |
| 1202 | Albumin (N terminal fragment) | P02768 | 1.572 | 0.285 | ↑ |
| 212 | Immunoglobulin J chain | P01591 | 1.569 | 0.009 | ↓ |
| 5603 | Alpha-amylase 1; Alpha-amylase 2B | P04745/P19961 | 1.530 | 0.172 | ↑ |
| 12 | Thioredoxin | P10599 | 1.523 | 0.176 | ↓ |
| 210 | Immunoglobulin J chain | P01591 | 1.519 | 0.028 | ↓ |
| Variable | Myalgia | Myofascial Pain | VIP | p-Value |
|---|---|---|---|---|
| Phosphoglycerate kinase 1 | 1 282 ± 519 | 323 ± 441 | 2.090 | 0.001 |
| PPT masseter muscle (kPa) | 227 ± 59 | 141 ± 31 | 1.945 | 0.001 |
| Level of physical activity * | ≥ 3 times/week | 1–2 times/week | 1.639 | 0.039 |
| PHQ-9 SCORE (0–36) | 4 (6) | 8 (8) | 1.571 | 0.121 |
| Alpha-amylase 1; Alpha-amylase 2B | 2 927 ± 1 885 | 1 102 ± 1 624 | 1.569 | 0.017 |
| Current pain intensity (NRS) | 4 (3) | 6 (1) | 1.511 | 0.023 |
| CPI | 53 (20) | 73 (17) | 1.474 | 0.023 |
| GCPS (Grade 0–IV) | 2 (2) | 3 (1) | 1.431 | 0.131 |
| Alpha-amylase 1; Alpha-amylase 2B | 1 785 ± 1 498 | 845 ± 827 | 1.389 | 0.140 |
| Chitinase-3-like protein 2 | 1 679 ± 1 177 | 675 ± 636 | 1.265 | 0.026 |
| Glyceraldehyde-3-phosphate dehydrogenase | 5 862 ± 4 225 | 2 568 ± 4 822 | 1.260 | 0.011 |
| PHQ-15 Score (0–30) | 8 (11) | 12 (5) | 1.210 | 0.273 |
| PPT reference (kPa) | 419 ± 151 | 353 ± 103 | 1.210 | 0.450 |
| ISI Score | 9 (13) | 12 (14) | 1.188 | 0.488 |
| Headache duration (years) | 3 ± 4 | 8 ± 4 | 1.120 | 0.037 |
| PSS Score (0–40) | 13 (11) | 19 (7) | 1.073 | 0.121 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasim, H.; Ernberg, M.; Carlsson, A.; Gerdle, B.; Ghafouri, B. Protein Signature in Saliva of Temporomandibular Disorders Myalgia. Int. J. Mol. Sci. 2020, 21, 2569. https://doi.org/10.3390/ijms21072569
Jasim H, Ernberg M, Carlsson A, Gerdle B, Ghafouri B. Protein Signature in Saliva of Temporomandibular Disorders Myalgia. International Journal of Molecular Sciences. 2020; 21(7):2569. https://doi.org/10.3390/ijms21072569
Chicago/Turabian StyleJasim, Hajer, Malin Ernberg, Anders Carlsson, Björn Gerdle, and Bijar Ghafouri. 2020. "Protein Signature in Saliva of Temporomandibular Disorders Myalgia" International Journal of Molecular Sciences 21, no. 7: 2569. https://doi.org/10.3390/ijms21072569
APA StyleJasim, H., Ernberg, M., Carlsson, A., Gerdle, B., & Ghafouri, B. (2020). Protein Signature in Saliva of Temporomandibular Disorders Myalgia. International Journal of Molecular Sciences, 21(7), 2569. https://doi.org/10.3390/ijms21072569





