Immune Checkpoint Inhibitor-Related Myositis: From Biology to Bedside
Abstract
:1. Introduction
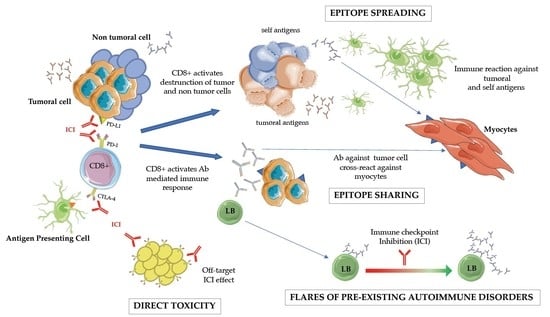
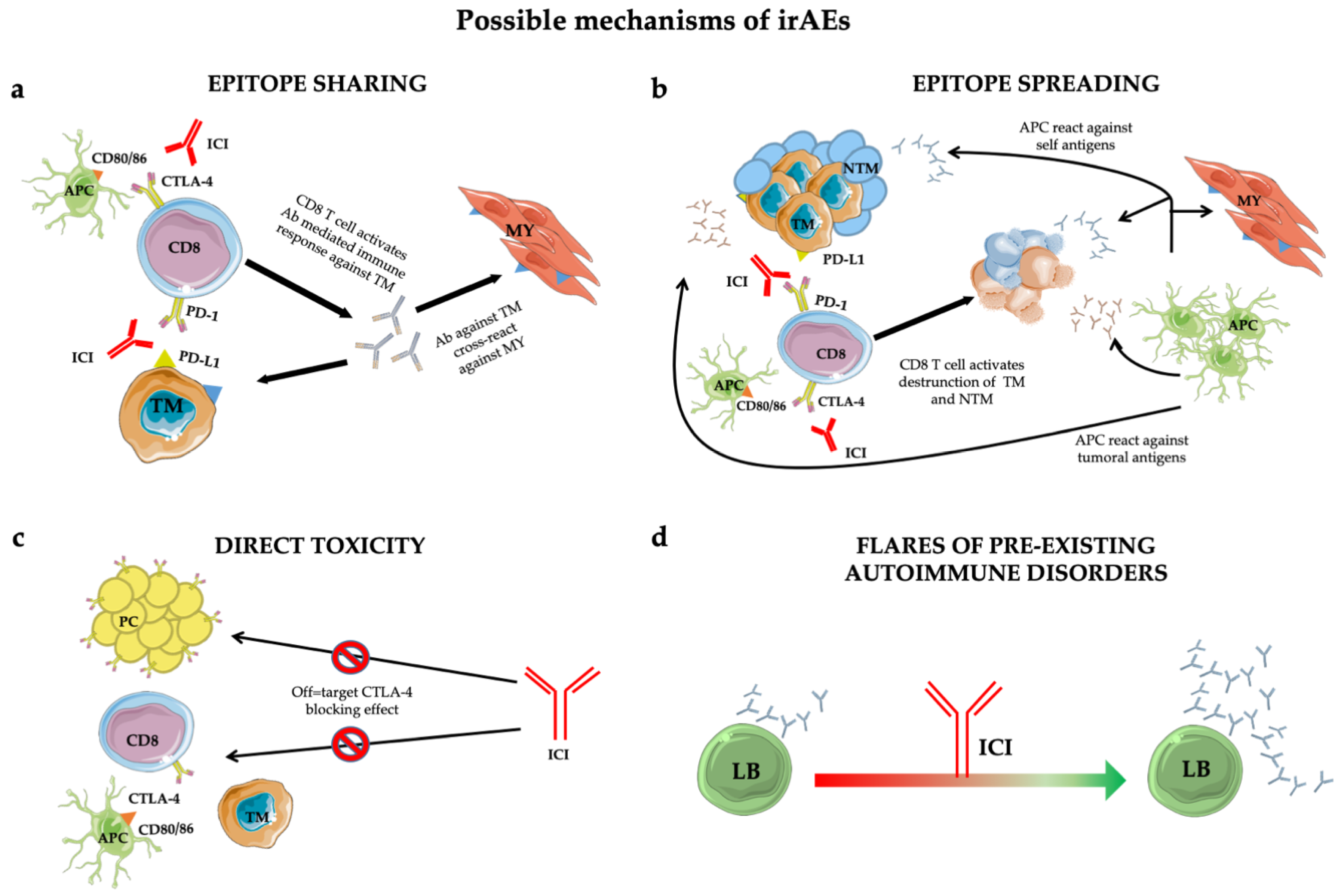
1.1. Biological Background: Bridging the Gaps between Immune Checkpoint Inhibition and Physiopathology ICI-Related Disease
1.2. Characteristic Autoantibody Patterns
2. Clinical Work-Up
2.1. Treatment of ICI-Induced Myositis: Corticosteroids
2.2. Treatment of ICI-Induced Myositis: Immunoglobulins and Plasmapheresis
3. Clinical Outcome and ICI-Related Muscular Involvement
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blomberg, O.S.; Spagnuolo, L.; de Visser, K.E. Immune regulation of metastasis: Mechanistic insights and therapeutic opportunities. Dis. Models Mech. 2018, 11. [Google Scholar] [CrossRef] [Green Version]
- Antonio, G.; Oronzo, B.; Vito, L.; Angela, C.; Antonel-la, A.; Roberto, C.; Giovanni, S.A.; Antonella, L. Immune system and bone microenvironment: Rationale for targeted cancer therapies. Oncotarget 2020, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argentiero, A.; Solimando, A.G.; Brunetti, O.; Calabrese, A.; Pantano, F.; Iuliani, M.; Santini, D.; Silvestris, N.; Vacca, A. Skeletal Metastases of Unknown Primary: Biological Landscape and Clinical Overview. Cancers 2019, 11, 1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azoury, S.C.; Straughan, D.M.; Shukla, V. Immune Checkpoint Inhibitors for Cancer Therapy: Clinical Efficacy and Safety. Curr. Cancer Drug Targets 2015, 15, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.; Brunetti, O.; Gnoni, A.; Licchetta, A.; Delcuratolo, S.; Memeo, R.; Solimando, A.G.; Argentiero, A. Emerging role of Immune Checkpoint Inhibitors in Hepatocellular Carcinoma. Med. Kaunas Lith. 2019, 55, 698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menon, S.; Shin, S.; Dy, G. Advances in Cancer Immunotherapy in Solid Tumors. Cancers 2016, 8, 106. [Google Scholar] [CrossRef] [Green Version]
- Armand, P. Immune checkpoint blockade in hematologic malignancies. Blood 2015, 125, 3393–3400. [Google Scholar] [CrossRef]
- Leone, P.; Di Lernia, G.; Solimando, A.G.; Cicco, S.; Saltarella, I.; Lamanuzzi, A.; Ria, R.; Frassanito, M.A.; Ponzoni, M.; Ditonno, P.; et al. Bone marrow endothelial cells sustain a tumor-specific CD8+ T cell subset with suppressive function in myeloma patients. Oncoimmunology 2019, 8, e1486949. [Google Scholar] [CrossRef] [Green Version]
- Cappelli, L.C.; Gutierrez, A.K.; Bingham, C.O.; Shah, A.A. Rheumatic and Musculoskeletal Immune-Related Adverse Events Due to Immune Checkpoint Inhibitors: A Systematic Review of the Literature. Arthritis Care Res. 2017, 69, 1751–1763. [Google Scholar] [CrossRef] [Green Version]
- Bilen, M.A.; Subudhi, S.K.; Gao, J.; Tannir, N.M.; Tu, S.-M.; Sharma, P. Acute rhabdomyolysis with severe polymyositis following ipilimumab-nivolumab treatment in a cancer patient with elevated anti-striated muscle antibody. J. Immunother. Cancer 2016, 4, 36. [Google Scholar] [CrossRef] [Green Version]
- Touat, M.; Maisonobe, T.; Knauss, S.; Ben Hadj Salem, O.; Hervier, B.; Auré, K.; Szwebel, T.-A.; Kramkimel, N.; Lethrosne, C.; Bruch, J.-F.; et al. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology 2018, 91, e985–e994. [Google Scholar] [CrossRef]
- Seki, M.; Uruha, A.; Ohnuki, Y.; Kamada, S.; Noda, T.; Onda, A.; Ohira, M.; Isami, A.; Hiramatsu, S.; Hibino, M.; et al. Inflammatory myopathy associated with PD-1 inhibitors. J. Autoimmun. 2019, 100, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Touat, M.; Talmasov, D.; Ricard, D.; Psimaras, D. Neurological toxicities associated with immune-checkpoint inhibitors. Curr. Opin. Neurol. 2017, 30, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Kao, J.C.; Liao, B.; Markovic, S.N.; Klein, C.J.; Naddaf, E.; Staff, N.P.; Liewluck, T.; Hammack, J.E.; Sandroni, P.; Finnes, H.; et al. Neurological Complications Associated With Anti-Programmed Death 1 (PD-1) Antibodies. JAMA Neurol. 2017, 74, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Nie, Y.; Yang, Y.; Lu, Y.-T.; Su, Q. Risk of Neurological Toxicities Following the Use of Different Immune Checkpoint Inhibitor Regimens in Solid Tumors: A Systematic Review and Meta-analysis. Neurologist 2019, 24, 75–83. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zak, K.M.; Grudnik, P.; Magiera, K.; Dömling, A.; Dubin, G.; Holak, T.A. Structural Biology of the Immune Checkpoint Receptor PD-1 and Its Ligands PD-L1/PD-L2. Struct. Lond. Engl. 2017, 25, 1163–1174. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [Green Version]
- Chambers, C.A.; Kuhns, M.S.; Egen, J.G.; Allison, J.P. CTLA-4-mediated inhibition in regulation of T cell responses: Mechanisms and manipulation in tumor immunotherapy. Annu. Rev. Immunol. 2001, 19, 565–594. [Google Scholar] [CrossRef] [Green Version]
- Kwiecien, I.; Stelmaszczyk-Emmel, A.; Polubiec-Kownacka, M.; Dziedzic, D.; Domagala-Kulawik, J. Elevated regulatory T cells, surface and intracellular CTLA-4 expression and interleukin-17 in the lung cancer microenvironment in humans. Cancer Immunol. Immunother. 2017, 66, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Bhattacharya, P.; Prabhakar, B.S. A comprehensive review on the role of co-signaling receptors and Treg homeostasis in autoimmunity and tumor immunity. J. Autoimmun. 2018, 95, 77–99. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; Warshauer, J.T.; Bluestone, J.A. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat. Med. 2017, 23, 540–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caturegli, P.; Di Dalmazi, G.; Lombardi, M.; Grosso, F.; Larman, H.B.; Larman, T.; Taverna, G.; Cosottini, M.; Lupi, I. Hypophysitis Secondary to Cytotoxic T-Lymphocyte-Associated Protein 4 Blockade: Insights into Pathogenesis from an Autopsy Series. Am. J. Pathol. 2016, 186, 3225–3235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toi, Y.; Sugawara, S.; Sugisaka, J.; Ono, H.; Kawashima, Y.; Aiba, T.; Kawana, S.; Saito, R.; Aso, M.; Tsurumi, K.; et al. Profiling Preexisting Antibodies in Patients Treated With Anti-PD-1 Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2019, 5, 376–383. [Google Scholar] [CrossRef]
- Johnson, D.B.; Sullivan, R.J.; Ott, P.A.; Carlino, M.S.; Khushalani, N.I.; Ye, F.; Guminski, A.; Puzanov, I.; Lawrence, D.P.; Buchbinder, E.I.; et al. Ipilimumab Therapy in Patients with Advanced Melanoma and Preexisting Autoimmune Disorders. JAMA Oncol. 2016, 2, 234–240. [Google Scholar] [CrossRef]
- Menzies, A.M.; Johnson, D.B.; Ramanujam, S.; Atkinson, V.G.; Wong, A.N.M.; Park, J.J.; McQuade, J.L.; Shoushtari, A.N.; Tsai, K.K.; Eroglu, Z.; et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 368–376. [Google Scholar] [CrossRef]
- Gutzmer, R.; Koop, A.; Meier, F.; Hassel, J.C.; Terheyden, P.; Zimmer, L.; Heinzerling, L.; Ugurel, S.; Pföhler, C.; Gesierich, A.; et al. Programmed cell death protein-1 (PD-1) inhibitor therapy in patients with advanced melanoma and preexisting autoimmunity or ipilimumab-triggered autoimmunity. Eur. J. Cancer Oxf. Engl. 2017, 75, 24–32. [Google Scholar] [CrossRef]
- Leonardi, G.C.; Gainor, J.F.; Altan, M.; Kravets, S.; Dahlberg, S.E.; Gedmintas, L.; Azimi, R.; Rizvi, H.; Riess, J.W.; Hellmann, M.D.; et al. Safety of Programmed Death-1 Pathway Inhibitors Among Patients With Non-Small-Cell Lung Cancer and Preexisting Autoimmune Disorders. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 1905–1912. [Google Scholar] [CrossRef]
- Tison, A.; Quéré, G.; Misery, L.; Funck-Brentano, E.; Danlos, F.-X.; Routier, E.; Robert, C.; Loriot, Y.; Lambotte, O.; Bonniaud, B.; et al. Safety and Efficacy of Immune Checkpoint Inhibitors in Patients With Cancer and Preexisting Autoimmune Disease: A Nationwide, Multicenter Cohort Study. Arthritis Rheumatol. Hoboken N. J. 2019, 71, 2100–2111. [Google Scholar] [CrossRef]
- Limaye, V.S.; Blumbergs, P.; Roberts-Thomson, P.J. Idiopathic inflammatory myopathies. Intern. Med. J. 2009, 39, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Gunawardena, H.; Betteridge, Z.E.; McHugh, N.J. Myositis-specific autoantibodies: Their clinical and pathogenic significance in disease expression. Rheumatol. Oxf. Engl. 2009, 48, 607–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Racanelli, V.; Prete, M.; Musaraj, G.; Dammacco, F.; Perosa, F. Autoantibodies to intracellular antigens: Generation and pathogenetic role. Autoimmun. Rev. 2011, 10, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Suber, T.L.; Casciola-Rosen, L.; Rosen, A. Mechanisms of disease: Autoantigens as clues to the pathogenesis of myositis. Nat. Clin. Pract. Rheumatol. 2008, 4, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Peng, Q.; Yin, L.; Li, S.; Shi, J.; Zhang, Y.; Lu, X.; Shu, X.; Zhang, S.; Wang, G. Identification of multiple cancer-associated myositis-specific autoantibodies in idiopathic inflammatory myopathies: A large longitudinal cohort study. Arthritis Res. Ther. 2017, 19, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, J.C.; Casciola-Rosen, L.; Samedy, L.-A.; Werner, J.; Owoyemi, K.; Danoff, S.K.; Christopher-Stine, L. Anti-melanoma differentiation-associated protein 5-associated dermatomyositis: Expanding the clinical spectrum. Arthritis Care Res. 2013, 65, 1307–1315. [Google Scholar] [CrossRef] [Green Version]
- Fiorentino, D.; Casciola-Rosen, L. Autoantibodies to transcription intermediary factor 1 in dermatomyositis shed insight into the cancer-myositis connection. Arthritis Rheum. 2012, 64, 346–349. [Google Scholar] [CrossRef]
- Gono, T.; Sato, S.; Kawaguchi, Y.; Kuwana, M.; Hanaoka, M.; Katsumata, Y.; Takagi, K.; Baba, S.; Okamoto, Y.; Ota, Y.; et al. Anti-MDA5 antibody, ferritin and IL-18 are useful for the evaluation of response to treatment in interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis. Rheumatol. Oxf. Engl. 2012, 51, 1563–1570. [Google Scholar] [CrossRef] [Green Version]
- Fiorentino, D.F.; Chung, L.S.; Christopher-Stine, L.; Zaba, L.; Li, S.; Mammen, A.L.; Rosen, A.; Casciola-Rosen, L. Most patients with cancer-associated dermatomyositis have antibodies to nuclear matrix protein NXP-2 or transcription intermediary factor 1γ. Arthritis Rheum. 2013, 65, 2954–2962. [Google Scholar] [CrossRef]
- Calabrese, L.H.; Calabrese, C.; Cappelli, L.C. Rheumatic immune-related adverse events from cancer immunotherapy. Nat. Rev. Rheumatol. 2018, 14, 569–579. [Google Scholar] [CrossRef]
- Kadota, H.; Gono, T.; Shirai, Y.; Okazaki, Y.; Takeno, M.; Kuwana, M. Immune Checkpoint Inhibitor-Induced Myositis: A Case Report and Literature Review. Curr. Rheumatol. Rep. 2019, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (accessed on 25 April 2020).
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Tanaka, S.; Ceribelli, A.; Calise, S.J.; Chan, E.K.L. A Comprehensive Overview on Myositis-Specific Antibodies: New and Old Biomarkers in Idiopathic Inflammatory Myopathy. Clin. Rev. Allergy Immunol. 2017, 52, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Carsons, S. The association of malignancy with rheumatic and connective tissue diseases. Semin. Oncol. 1997, 24, 360–372. [Google Scholar] [PubMed]
- Racanelli, V.; Prete, M.; Minoia, C.; Favoino, E.; Perosa, F. Rheumatic disorders as paraneoplastic syndromes. Autoimmun. Rev. 2008, 7, 352–358. [Google Scholar] [CrossRef]
- Moreira, A.; Loquai, C.; Pföhler, C.; Kähler, K.C.; Knauss, S.; Heppt, M.V.; Gutzmer, R.; Dimitriou, F.; Meier, F.; Mitzel-Rink, H.; et al. Myositis and neuromuscular side-effects induced by immune checkpoint inhibitors. Eur. J. Cancer Oxf. Engl. 2019, 106, 12–23. [Google Scholar] [CrossRef]
- Johansen, A.; Christensen, S.J.; Scheie, D.; Højgaard, J.L.S.; Kondziella, D. Neuromuscular adverse events associated with anti-PD-1 monoclonal antibodies: Systematic review. Neurology 2019, 92, 663–674. [Google Scholar] [CrossRef]
- Dalakas, M.C.; Illa, I.; Dambrosia, J.M.; Soueidan, S.A.; Stein, D.P.; Otero, C.; Dinsmore, S.T.; McCrosky, S. A controlled trial of high-dose intravenous immune globulin infusions as treatment for dermatomyositis. N. Engl. J. Med. 1993, 329, 1993–2000. [Google Scholar] [CrossRef]
- Danieli, M.G.; Pettinari, L.; Moretti, R.; Logullo, F.; Gabrielli, A. Subcutaneous immunoglobulin in polymyositis and dermatomyositis: A novel application. Autoimmun. Rev. 2011, 10, 144–149. [Google Scholar] [CrossRef]
- Azizi, G.; Ziaee, V.; Tavakol, M.; Alinia, T.; Yazdai, R.; Mohammadi, H.; Abolhassani, H.; Aghamohammadi, A. Approach to the Management of Autoimmunity in Primary Immunodeficiency. Scand. J. Immunol. 2017, 85, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Jolles, S.; Stein, M.R.; Longhurst, H.J.; Borte, M.; Ritchie, B.; Sturzenegger, M.H.; Berger, M. New Frontiers in Subcutaneous Immunoglobulin Treatment. Biol. Ther. 2011, 1, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vacca, A.; Melaccio, A.; Sportelli, A.; Solimando, A.G.; Dammacco, F.; Ria, R. Subcutaneous immunoglobulins in patients with multiple myeloma and secondary hypogammaglobulinemia: A randomized trial. Clin. Immunol. Orlando Fla. 2018, 191, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Shimanovsky, A.; Alvarez Argote, J.; Murali, S.; Dasanu, C.A. Autoimmune manifestations in patients with multiple myeloma and monoclonal gammopathy of undetermined significance. BBA Clin. 2016, 6, 12–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dau, P.C. Plasmapheresis in idiopathic inflammatory myopathy. Experience with 35 patients. Arch. Neurol. 1981, 38, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.W.; Leitman, S.F.; Cronin, M.E.; Hicks, J.E.; Leff, R.L.; Wesley, R.; Fraser, D.D.; Dalakas, M.; Plotz, P.H. Controlled trial of plasma exchange and leukapheresis in polymyositis and dermatomyositis. N. Engl. J. Med. 1992, 326, 1380–1384. [Google Scholar] [CrossRef] [PubMed]
- Leipe, J.; Mariette, X. Management of rheumatic complications of ICI therapy: A rheumatology viewpoint. Rheumatol. Oxf. Engl. 2019, 58, vii49–vii58. [Google Scholar] [CrossRef] [Green Version]
- Tajiri, K.; Aonuma, K.; Sekine, I. Immune checkpoint inhibitor-related myocarditis. Jpn. J. Clin. Oncol. 2018, 48, 7–12. [Google Scholar] [CrossRef]
- Leone, P.; Cicco, S.; Prete, M.; Solimando, A.G.; Susca, N.; Crudele, L.; Buonavoglia, A.; Colonna, P.; Dammacco, F.; Vacca, A.; et al. Early echocardiographic detection of left ventricular diastolic dysfunction in patients with systemic lupus erythematosus asymptomatic for cardiovascular disease. Clin. Exp. Med. 2020, 20, 11–19. [Google Scholar] [CrossRef]
- Salem, J.-E.; Allenbach, Y.; Vozy, A.; Brechot, N.; Johnson, D.B.; Moslehi, J.J.; Kerneis, M. Abatacept for Severe Immune Checkpoint Inhibitor-Associated Myocarditis. N. Engl. J. Med. 2019, 380, 2377–2379. [Google Scholar] [CrossRef]
- Tjärnlund, A.; Tang, Q.; Wick, C.; Dastmalchi, M.; Mann, H.; Tomasová Studýnková, J.; Chura, R.; Gullick, N.J.; Salerno, R.; Rönnelid, J.; et al. Abatacept in the treatment of adult dermatomyositis and polymyositis: A randomised, phase IIb treatment delayed-start trial. Ann. Rheum. Dis. 2018, 77, 55–62. [Google Scholar] [CrossRef]
- Anquetil, C.; Salem, J.-E.; Lebrun-Vignes, B.; Johnson, D.B.; Mammen, A.L.; Stenzel, W.; Léonard-Louis, S.; Benveniste, O.; Moslehi, J.J.; Allenbach, Y. Immune Checkpoint Inhibitor-Associated Myositis: Expanding the Spectrum of Cardiac Complications of the Immunotherapy Revolution. Circulation 2018, 138, 743–745. [Google Scholar] [CrossRef] [PubMed]
- Perosa, F.; Favoino, E.; Vicenti, C.; Guarnera, A.; Racanelli, V.; De Pinto, V.; Dammacco, F. Two structurally different rituximab-specific CD20 mimotope peptides reveal that rituximab recognizes two different CD20-associated epitopes. J. Immunol. Baltim. Md. 2009, 182, 416–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, E.A.; Ledbetter, J.A. How does B cell depletion therapy work, and how can it be improved? Ann. Rheum. Dis. 2005, 64 (Suppl. 4), iv77–iv80. [Google Scholar] [CrossRef] [PubMed]
- Solimando, A.G.; Ribatti, D.; Vacca, A.; Einsele, H. Targeting B-cell non Hodgkin lymphoma: New and old tricks. Leuk. Res. 2016, 42, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Fasano, S.; Gordon, P.; Hajji, R.; Loyo, E.; Isenberg, D.A. Rituximab in the treatment of inflammatory myopathies: A review. Rheumatol. Oxf. Engl. 2017, 56, 26–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oddis, C.V.; Reed, A.M.; Aggarwal, R.; Rider, L.G.; Ascherman, D.P.; Levesque, M.C.; Barohn, R.J.; Feldman, B.M.; Harris-Love, M.O.; Koontz, D.C.; et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: A randomized, placebo-phase trial. Arthritis Rheum. 2013, 65, 314–324. [Google Scholar] [CrossRef]
- Maher, V.E.; Fernandes, L.L.; Weinstock, C.; Tang, S.; Agarwal, S.; Brave, M.; Ning, Y.-M.; Singh, H.; Suzman, D.; Xu, J.; et al. Analysis of the Association Between Adverse Events and Outcome in Patients Receiving a Programmed Death Protein 1 or Programmed Death Ligand 1 Antibody. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 2730–2737. [Google Scholar] [CrossRef]
- García-Aranda, M.; Redondo, M. Analysis of the association between adverse events and outcome in patients receiving a programmed death protein-1 or programmed death ligand-1 antibody. Chin. Clin. Oncol. 2020. [Google Scholar] [CrossRef]

| G1 | G2 | G3 | G4 |
|---|---|---|---|
| Mild pain | Moderate pain, associated with weakness; pain, that limits age-appropriate activities of daily life | Pain associated with severe weakness, that limits age-appropriate activities of daily life | Life-threatening implications |
| Any Grade |
| Neurological and rheumatological anamnesis, rheumatological (inspection of the skin to identify signs suggestive of dermatomyositis) and neurological (muscle strength determination) objective examinations |
Blood chemistry tests including:
|
| Consider a neurophysiological examination (needle EMG, neuromuscular plaque determination to identify possible concomitant MG, or a nerve conduction study to identify possible concomitant neuropathy) |
| Consider muscle MRI or tissue biopsy if the diagnosis is uncertain |
| CPK, ESR and CRP for follow-up |
| Grade 2 |
| In addition to the above: Rapid rheumatological or neurological evaluation |
| Grade 3 |
| In addition to the above: Urgent rheumatological or neurological evaluation |
| Reference | Type of irAE n. pts | irAE Grade CTCAE | Autoantibody Subtypes n. pts Positive/Tested | Treatment n. pts | Outcome of irAE n.pts (%) |
|---|---|---|---|---|---|
| Touat et al.; 2018 [11] n = 10 pts | PM = 10 DM = 0 | G≥3 = 9 G≤2 = 1 | MSA n = 0/7 MAA n = 0/7 Other Abs: anti- SSA/Ro52 n = 1 * | None n = 1 Prednisone monotherapy n = 4 IVMP monotherapy n = 2 IVMP + IVIG n = 2 IVMP + PLEX n = 1 | Remission n = 10 (100%) Sequele n = 0 (0%) irAE-death n = 0 (0%) All causes-death n = 5 (50%) |
| Moreira et al.; 2019 [47] n = 20 pts | PM = 19 DM = 1 | G≥3 = 12 G≤2 = 8 | MSA n = 4/18 MAA n = 1/18 anti-SRP n = 1 anti-TIF1γ n = 1 EJ + RO52 § n = 1 PL7 + PL12 + anti-SRP n = 1 Other Abs: ANA n = 1 | None n = 4 Steroid monotherapy n = 10 Steroid + IVIG n = 4 Steroid + pyridostigmine n = 1 | Remission n = 11 (55%) Sequele n = 4 (20%) N/A n = 3 (15%) irAE-death n = 2 (10%) All causes-death n = 3 (15%) |
| Seki et al.; 2019 [12] n = 19 pts | PM = 19 DM = 0 | G≥3 = 9 G≤2 = 10 | MSA n = 0/19 MAA n = 0/19 Other Abs: anti-SM n = 11 anti-SM + anti-AChR n = 2 | None n = 2 PSL monotherapy n = 6 IVMP + PSL n = 6 PSL + IVIG n = 1 PSL + PPH n = 1 IVMP + PSL + PPH + IVIG n = 2 IVMP + PSL + PPH + IVIG + tacrolimus n = 1 | Remission n = 10 (53%) Sequele n = 8 (42%) irAE-death n = 1 (5%) All causes-death n = 7 (37%) |
| Kadota et al.; 2019 [41] n = 15 pts | PM = 15 DM = 3 | N/A | MSA n = 3/10 MAA n = 0/10 anti-SRP + anti-ARS n = 1 anti-TIF1-γ n = 1 anti-ARS n = 1 * Other Abs: anti-SM n = 1 + 1 * ANA n = 1 anti-AChR n = 1 + 1 * | PSL monotherapy n = 6 PSL + IVIG n = 2 PSL + PLEX n = 2 PSL + IFX n = 1 PSL + IVIG + PPH n = 1 PSL + IVIG + PPH + IFX n = 1 PS + IVIG + PLEX + pyridostigmine n = 2 | Remission/ Improvement n = 10 (67%) irAE-death n = 5 (33%) All causes-death n = 7 (47%) |
| Johansen et al.; 2019 [48] n = 29 pts | PM = 29 DM = 0 | N/A | MSA = N/A MAA = N/A Other Abs: anti-AChR n = 2/10 | Steroid PO n = 9 Steroid IV n = 16 IVIG n = 7 PLEX n = 6 Pyridostigmine n = 1 Unspecified Immunomodulator n = 4 | Remission/Improvement n = 20 (69%) irAE-death n = N/A All causes-death n = 12 (41%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solimando, A.G.; Crudele, L.; Leone, P.; Argentiero, A.; Guarascio, M.; Silvestris, N.; Vacca, A.; Racanelli, V. Immune Checkpoint Inhibitor-Related Myositis: From Biology to Bedside. Int. J. Mol. Sci. 2020, 21, 3054. https://doi.org/10.3390/ijms21093054
Solimando AG, Crudele L, Leone P, Argentiero A, Guarascio M, Silvestris N, Vacca A, Racanelli V. Immune Checkpoint Inhibitor-Related Myositis: From Biology to Bedside. International Journal of Molecular Sciences. 2020; 21(9):3054. https://doi.org/10.3390/ijms21093054
Chicago/Turabian StyleSolimando, Antonio G., Lucilla Crudele, Patrizia Leone, Antonella Argentiero, Matteo Guarascio, Nicola Silvestris, Angelo Vacca, and Vito Racanelli. 2020. "Immune Checkpoint Inhibitor-Related Myositis: From Biology to Bedside" International Journal of Molecular Sciences 21, no. 9: 3054. https://doi.org/10.3390/ijms21093054
APA StyleSolimando, A. G., Crudele, L., Leone, P., Argentiero, A., Guarascio, M., Silvestris, N., Vacca, A., & Racanelli, V. (2020). Immune Checkpoint Inhibitor-Related Myositis: From Biology to Bedside. International Journal of Molecular Sciences, 21(9), 3054. https://doi.org/10.3390/ijms21093054