Degradation, Bone Regeneration and Tissue Response of an Innovative Volume Stable Magnesium-Supported GBR/GTR Barrier Membrane
Abstract
1. Introduction
2. Results
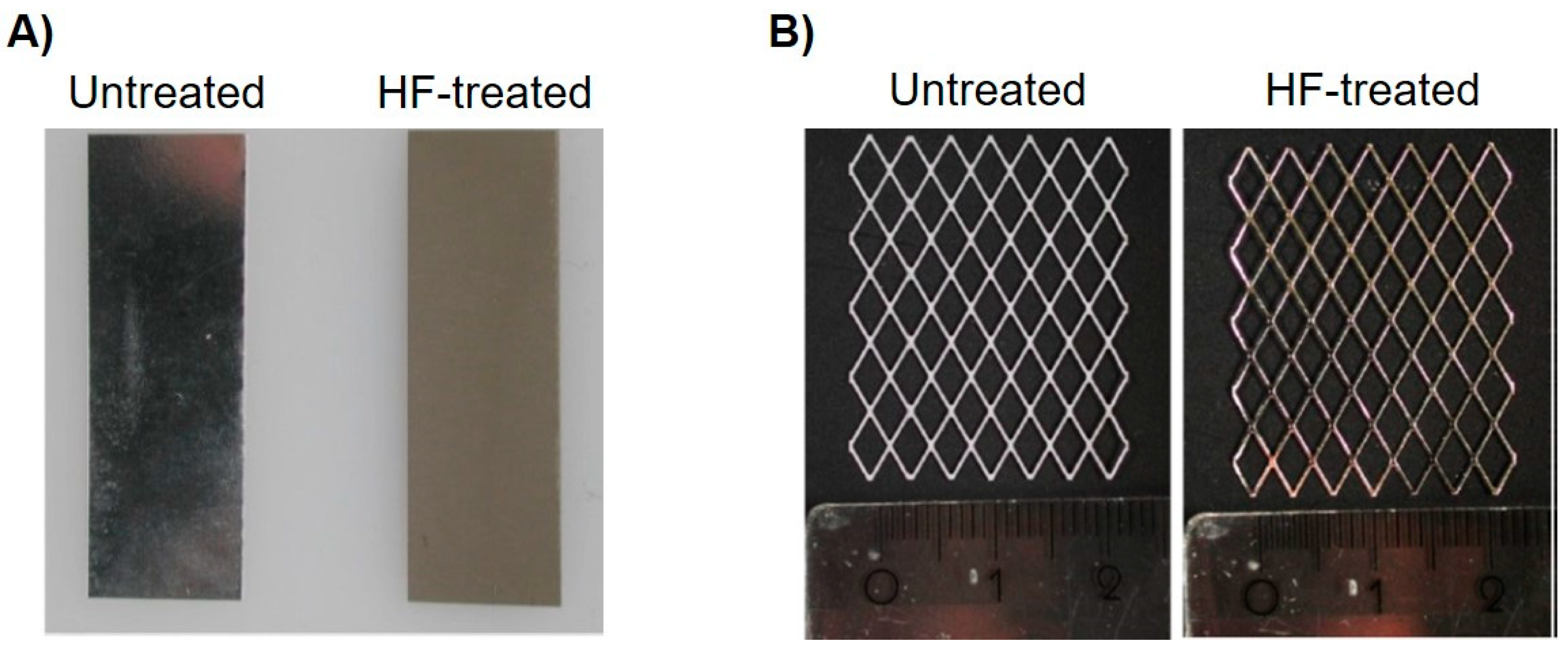
2.1. Manufacturing of a Magnesium Collagen Membrane
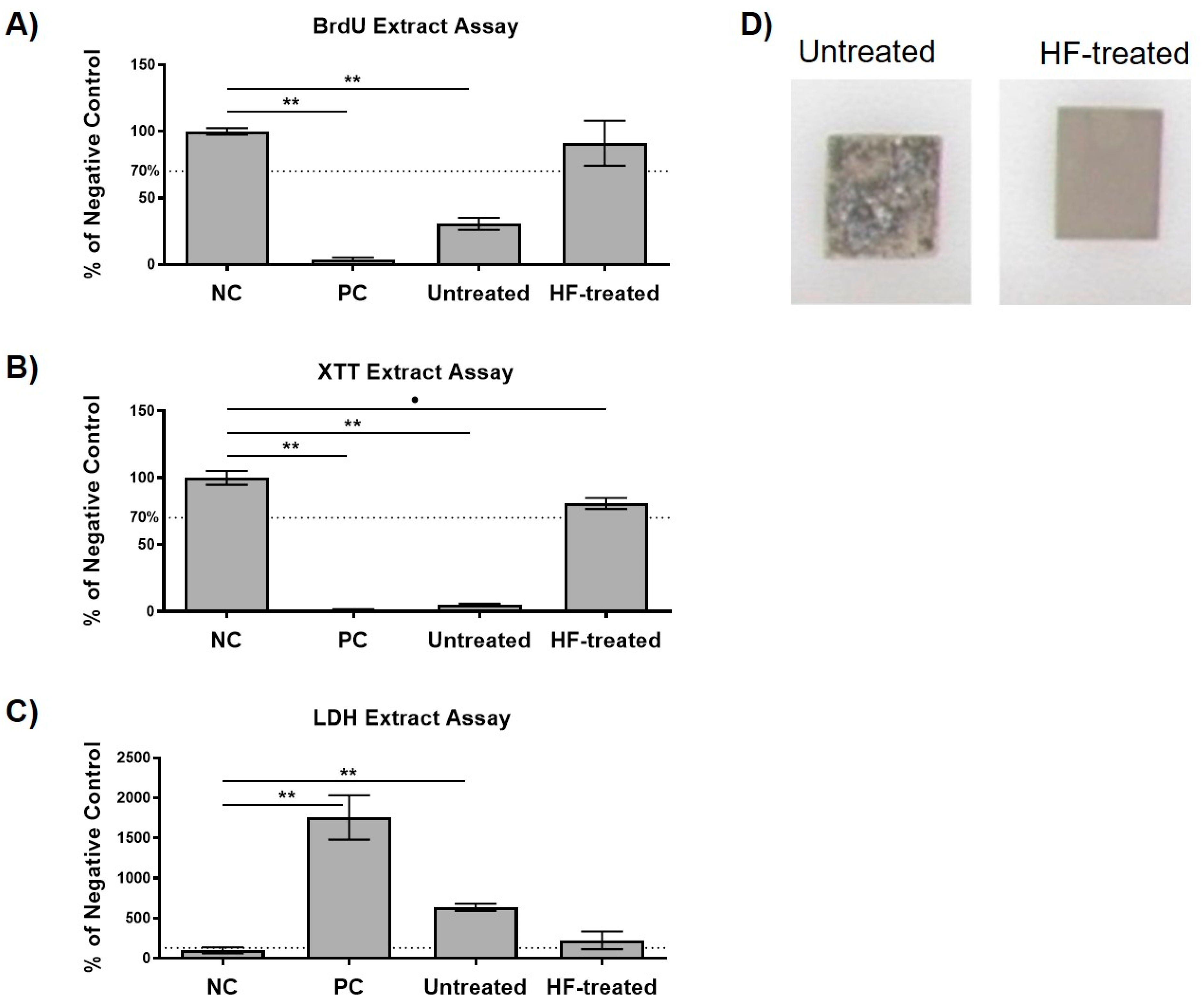
2.2. Cytocompatibility Analysis
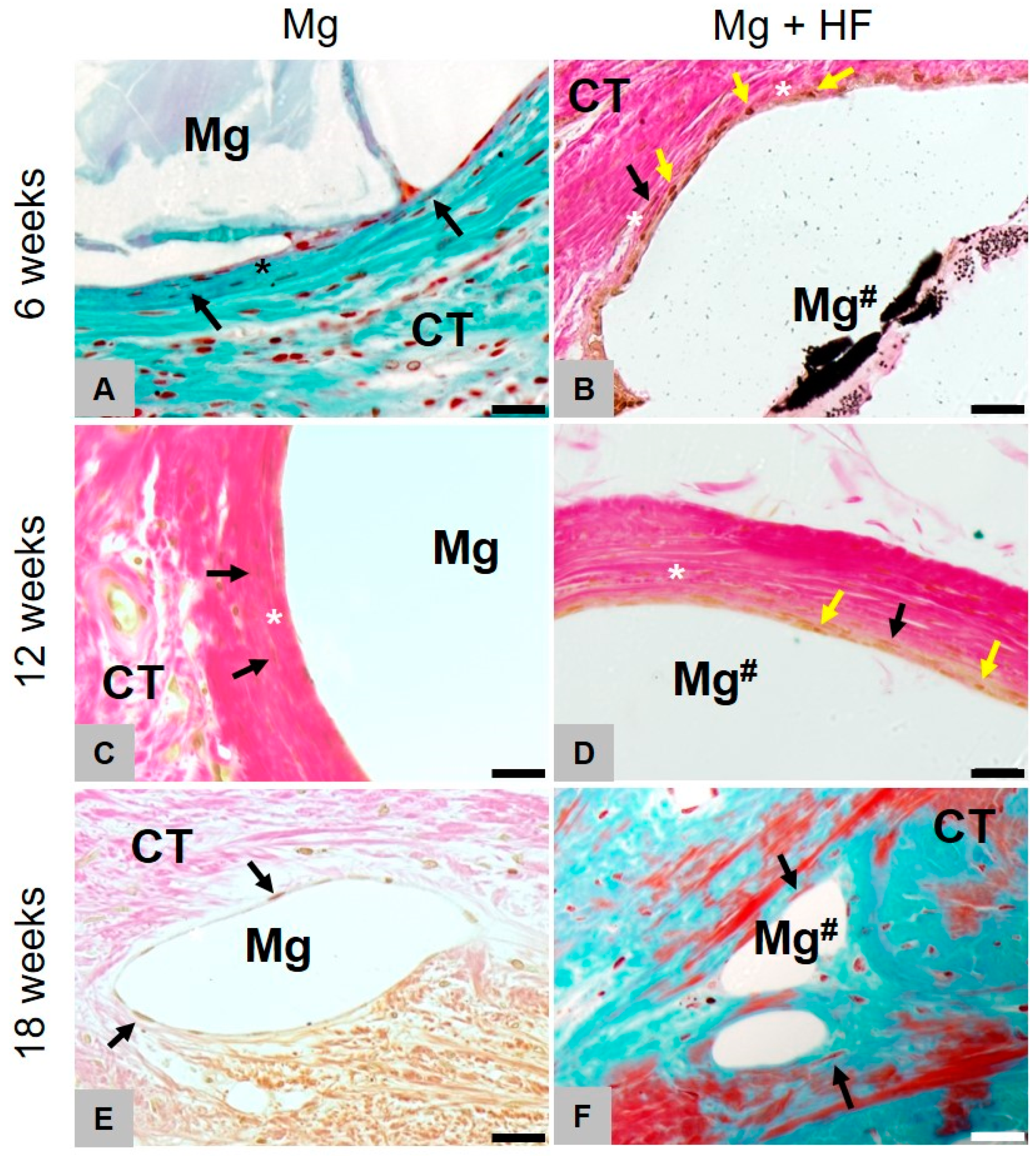
2.3. Histopathological Results
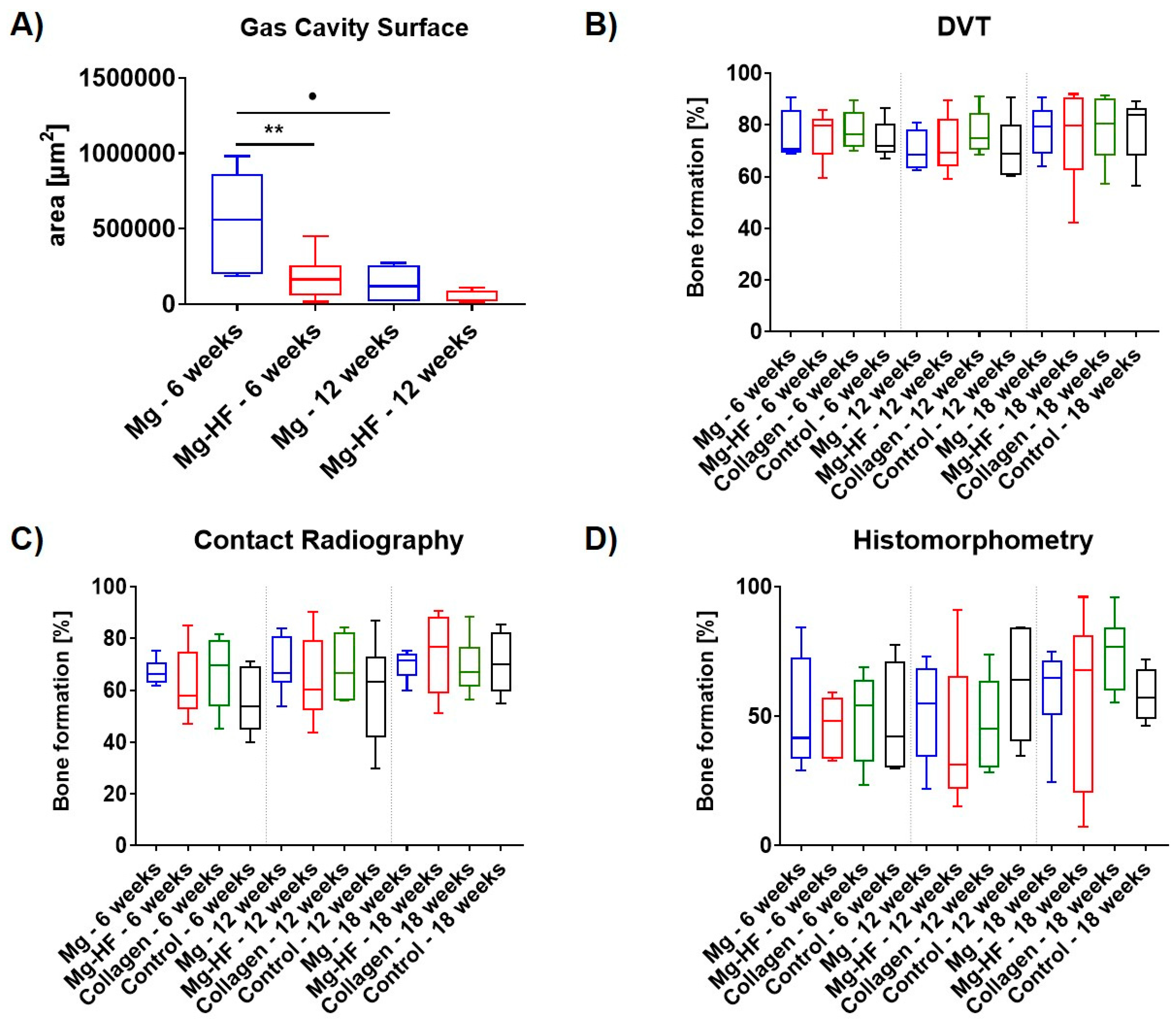
2.4. Digital Volume Tomography (DVT) Analysis
2.5. Contact Radiography
2.6. Histomorphometrical Analysis
2.7. Results of the Gas Cavity Measurements
3. Discussion
4. Materials and Methods
4.1. Biomaterial Preparation
4.2. Cytocompatibility Analysis
4.2.1. Reference Material
4.2.2. Cells and Cell Culture
4.2.3. Extract Analysis
Extraction
Assay Procedure
Bromodeoxyuridine/5-Bromo-2′-Deoxyuridine (BrdU)-Assay
Sodium 3,3′-[1(Phenylamino)carbonyl]-3,4-tetrazolium]-3is(4-methoxy-6-nitro) Benzene Sulfonic Acid Hydrate (XTT)-Assay
Lactate Dehydrogenase (LDH)-Assay
4.2.4. Live-Dead Staining
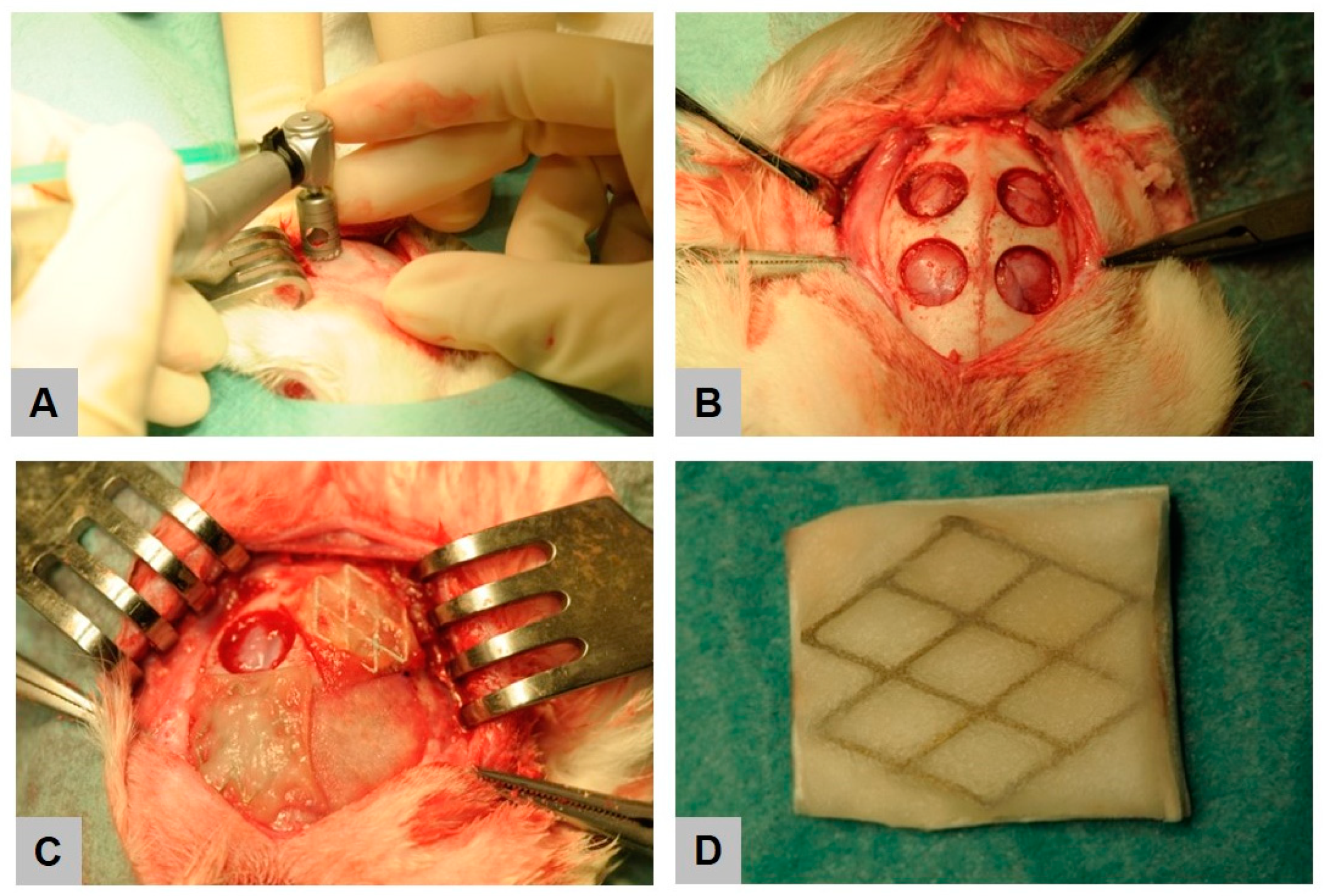
4.3. Experimental Animals and Surgical Procedure
4.4. Digital Volume Tomography (DVT) Analysis
4.5. Radiological Analysis
4.6. Histological Work Up
4.7. Histopathological Analysis
4.8. Histomorphometrical Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Basler, T.; Naenni, N.; Schneider, D.; Hämmerle, C.H.; Jung, R.E.; Thoma, D.S. Randomized controlled clinical study assessing two membranes for guided bone regeneration of peri-implant bone defects: 3-year results. Clin. Oral Implant. Res. 2018, 29, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Milinkovic, I.; Cordaro, L. Are there specific indications for the different alveolar bone augmentation procedures for implant placement? A systematic review. Int. J. Oral Maxillofac. Surg. 2014, 43, 606–625. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Jung, O.; Hanken, H.; Hartjen, P.; Al Dam, A.; Gröbe, A.; Heiland, M.; Gosau, M.; Rothamel, D.; Schlee, M. Was können regenerative Materialien in der Zahnmedizin leisten–und wo sind die Grenzen. Deutsceh Zahnärztliceh Zeitschrift. 2014, 69, 708–721. [Google Scholar]
- Linde, A.; Alberius, P.; Dahlin, C.; Bjurstam, K.; Sundin, Y. Osteopromotion: A soft-tissue exclusion principle using a membrane for bone healing and bone neogenesis. J. Periodontol. 1993, 64, 1116–1128. [Google Scholar] [CrossRef] [PubMed]
- Linde, A.; Thorén, C.; Dahlin, C.; Sandberg, E. Creation of new bone by an osteopromotive membrane technique: An experimental study in rats. J. Oral Maxillofac. Surg. 1993, 51, 892–897. [Google Scholar] [CrossRef]
- Karring, T.; Nyman, S.; Gottlow, J.; Laurell, L. Development of the biological concept of guided tissue regeneration—Animal and human studies. Periodontology 2000 1993, 1, 26–35. [Google Scholar] [CrossRef]
- Meinig, R.P. Clinical use of resorbable polymeric membranes in the treatment of bone defects. Orthop. Clin. N. Am. 2010, 41, 39–47. [Google Scholar] [CrossRef]
- Dimitriou, R.; Mataliotakis, G.I.; Calori, G.M.; Giannoudis, P.V. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence. BMC Med. 2012, 10, 81. [Google Scholar] [CrossRef]
- Gottlow, J. Guided Tissue Regeneration using bioresorbable and nonresorbable devices. J. Jpn. Soc. Periodontol. 1993, 35, 37. [Google Scholar] [CrossRef]
- Scantlebury, T.V. 1982-1992: A decade of technology development for guided tissue regeneration. J. Periodontol. 1993, 64, 1129–1137. [Google Scholar] [CrossRef]
- Ghanaati, S. Non-cross-linked porcine-based collagen I-III membranes do not require high vascularization rates for their integration within the implantation bed: A paradigm shift. Acta Biomater. 2012, 8, 3061–3072. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Hamdan, N.; Ikeda, Y.; Grynpas, M.; Ganss, B.; Glogauer, M. Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: A review. Biomater. Res. 2017, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Tolstunov, L.; Hamrick, J.F.E.; Broumand, V.; Shilo, D.; Rachmiel, A. Bone Augmentation Techniques for Horizontal and Vertical Alveolar Ridge Deficiency in Oral Implantology. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 163–191. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; El-Moawen, A.; Jung, O.; Hanken, H.; Hartjen, P.; Heiland, M.; Kansy, K.; Kloss, F.; Kolk, A. From bench to application: Current practices in tissue engineering and its realisation at maxillofacial units in Germany, Austria and Switzerland. J. Craniomaxillofac. Surg. 2014, 42, 1128–1132. [Google Scholar] [CrossRef]
- Lundgren, A.; Sennerby, L.; Lundgren, D. Guided jaw-bone regeneration using an experimental rabbit model. Int. J. Oral Maxillofac. Surg. 1998, 27, 135–140. [Google Scholar] [CrossRef]
- Wiltfang, J.; Merten, H.-A.; Peters, J.-H. Comparative study of guided bone regeneration using absorbable and permanent barrier membranes: A histologic report. Int. J. Oral Maxillofac. Implant. 1998, 13, 416–421. [Google Scholar]
- Liu, J.; Kerns, D.G. Mechanisms of guided bone regeneration: A review. Open Dent. J. 2014, 8, 56–65. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Korzinskas, T.; Jung, O.; Smeets, R.; Stojanovic, S.; Najman, S.; Glenske, K.; Hahn, M.; Wenisch, S.; Schnettler, R.; Barbeck, M. In Vivo Analysis of the Biocompatibility and Macrophage Response of a Non-Resorbable PTFE Membrane for Guided Bone Regeneration. Int. J. Mol. Sci. 2018, 19, 2952. [Google Scholar] [CrossRef]
- Sanctis, M.; Zucchelli, G.; Clauser, C. Bacterial colonization of barrier material and periodontal regeneration. J. Clin. Periodontol. 1996, 23, 1039–1046. [Google Scholar] [CrossRef]
- Tempro, P.J.; Nalbandian, J. Colonization of retrieved polytetrafluoroethylene membranes: Morphological and microbiological observations. J. Periodontol. 1993, 64, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Selvig, K.A.; Kersten, B.G.; Chamberlain, A.D.H.; Wikesjö, U.M.; Nilvúus, R.E. Regenerative surgery of intrabony periodontal defects using ePTFE barrier membranes: Scanning electron microscopic evaluation of retrieved membranes versus clinical healing. J. Periodontol. 1992, 63, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Akbay, E.; Aydogan, F. Reconstruction of isolated mandibular bone defects with non-vascularized corticocancellous bone autograft and graft viability. Auris Nasus Larynx 2014, 41, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Gu, X.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef]
- Witte, F.; Fischer, J.; Nellesen, J.; Crostack, H.A.; Kaese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef]
- Jung, O.; Smeets, R.; Hartjen, P.; Schnettler, R.; Feyerabend, F.; Klein, M.; Wegner, N.; Walther, F.; Stangier, D.; Henningsen, A.; et al. Improved In Vitro Test Procedure for Full Assessment of the Cytocompatibility of Degradable Magnesium Based on ISO 10993-5/-12. Int. J. Mol. Sci. 2019, 20, 255. [Google Scholar] [CrossRef]
- Hornberger, H.; Virtanen, S.; Boccaccini, A. Biomedical coatings on magnesium alloys—A review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef]
- Wang, J.; Tang, J.; Zhang, P.; Li, Y.; Wang, J.; Lai, Y.; Qin, L. Surface modification of magnesium alloys developed for bioabsorbable orthopedic implants: A general review. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Liu, X. Surface modification of biodegradable magnesium and its alloys for biomedical applications. Regen. Biomater. 2015, 2, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Thomann, M.; Krause, C.; Angrisani, N.; Bormann, D.; Hassel, T.; Windhagen, H.; Meyer-Lindenberg, A. Influence of a magnesium-fluoride coating of magnesium-based implants (MgCa0. 8) on degradation in a rabbit model. J. Biomed. Mater. Res. A 2010, 93, 1609–1619. [Google Scholar] [PubMed]
- Carboneras, M.; García-Alonso, M.; Escudero, M. Biodegradation kinetics of modified magnesium-based materials in cell culture medium. Corros. Sci. 2011, 53, 1433–1439. [Google Scholar] [CrossRef]
- Yan, T.; Tan, L.; Xiong, D.; Liu, X.; Zhang, B.; Yang, K. Fluoride treatment and in vitro corrosion behavior of an AZ31B magnesium alloy. Mater. Sci. Eng. C 2010, 30, 740–748. [Google Scholar] [CrossRef]
- Witte, F.; Fischer, J.; Nellesen, J.; Vogt, C.; Vogt, J.; Donath, T.; Beckmann, F. In vivo corrosion and corrosion protection of magnesium alloy LAE442. Acta Biomater. 2010, 6, 1792–1799. [Google Scholar] [CrossRef]
- Kang, M.H.; Jang, T.S.; Kim, S.W.; Park, H.S.; Song, J.; Kim, H.E.; Jung, K.H.; Jung, H.D. MgF2-coated porous magnesium/alumina scaffolds with improved strength, corrosion resistance, and biological performance for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 634–642. [Google Scholar] [CrossRef]
- Da Conceicao, T.; Scharnagl, N.; Blawert, C.; Dietzel, W.; Kainer, K. Surface modification of magnesium alloy AZ31 by hydrofluoric acid treatment and its effect on the corrosion behaviour. Thin Solid Films 2010, 518, 5209–5218. [Google Scholar] [CrossRef]
- Sieger, D.; Korzinskas, T.; Jung, O.; Stojanovic, S.; Wenisch, S.; Smeets, R.; Gosau, M.; Schnettler, R.; Najman, S.; Barbeck, M. The Addition of High Doses of Hyaluronic Acid to a Biphasic Bone Substitute Decreases the Proinflammatory Tissue Response. Int. J. Mol. Sci. 2019, 20, 1969. [Google Scholar] [CrossRef]
- Jung, O.; Smeets, R.; Porchetta, D.; Kopp, A.; Ptock, C.; Muller, U.; Heiland, M.; Schwade, M.; Behr, B.; Kroger, N.; et al. Optimized in vitro procedure for assessing the cytocompatibility of magnesium-based biomaterials. Acta Biomater. 2015, 23, 354–363. [Google Scholar] [CrossRef]
- Tawil, G.; Barbeck, M.; Unger, R.; Tawil, P.; Witte, F. Sinus Floor Elevation Using the Lateral Approach and Window Repositioning and a Xenogeneic Bone Substitute as a Grafting Material: A Histologic, Histomorphometric, and Radiographic Analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Barbeck, M.; Booms, P.; Unger, R.; Hoffmann, V.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Multinucleated giant cells in the implant bed of bone substitutes are foreign body giant cells-New insights into the material-mediated healing process. J. Biomed. Mater. Res. A 2017, 105, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Barbeck, M.; Unger, R.E.; Booms, P.; Dohle, E.; Sader, R.A.; Kirkpatrick, C.J.; Ghanaati, S. Monocyte preseeding leads to an increased implant bed vascularization of biphasic calcium phosphate bone substitutes via vessel maturation. J. Biomed. Mater. Res. A 2016, 104, 2928–2935. [Google Scholar] [CrossRef] [PubMed]
- Chaya, A.; Yoshizawa, S.; Verdelis, K.; Myers, N.; Costello, B.J.; Chou, D.T.; Pal, S.; Maiti, S.; Kumta, P.N.; Sfeir, C. In vivo study of magnesium plate and screw degradation and bone fracture healing. Acta Biomater 2015, 18, 262–269. [Google Scholar] [CrossRef]
- Chaya, A.; Yoshizawa, S.; Verdelis, K.; Noorani, S.; Costello, B.J.; Sfeir, C. Fracture healing using degradable magnesium fixation plates and screws. J. Oral Maxillofac. Surg. 2015, 73, 295–305. [Google Scholar] [CrossRef]
- Ding, W. Opportunities and challenges for the biodegradable magnesium alloys as next-generation biomaterials. Regen. Biomater. 2016, 3, 79–86. [Google Scholar] [CrossRef]
- Yu, W.; Zhao, H.; Ding, Z.; Zhang, Z.; Sun, B.; Shen, J.; Chen, S.; Zhang, B.; Yang, K.; Liu, M.; et al. In vitro and in vivo evaluation of MgF2 coated AZ31 magnesium alloy porous scaffolds for bone regeneration. Colloids Surf. B Biointerfaces 2017, 149, 330–340. [Google Scholar] [CrossRef]
- Mao, L.; Yuan, G.; Niu, J.; Zong, Y.; Ding, W. In vitro degradation behavior and biocompatibility of Mg-Nd-Zn-Zr alloy by hydrofluoric acid treatment. Mater Sci. Eng. C Mater Biol. Appl. 2013, 33, 242–250. [Google Scholar] [CrossRef]
- Zhao, N.; Workman, B.; Zhu, D. Endothelialization of novel magnesium-rare earth alloys with fluoride and collagen coating. Int. J. Mol. Sci. 2014, 15, 5263–5276. [Google Scholar] [CrossRef]
- Makkar, P.; Kang, H.J.; Padalhin, A.R.; Park, I.; Moon, B.G.; Lee, B.T. Development and properties of duplex MgF2/PCL coatings on biodegradable magnesium alloy for biomedical applications. PLoS ONE 2018, 13, e0193927. [Google Scholar] [CrossRef]
- Liu, W.; Yan, Z.; Ma, X.; Geng, T.; Wu, H.; Li, Z.J.M. Mg-MOF-74/MgF2 Composite Coating for Improving the Properties of Magnesium Alloy Implants: Hydrophilicity and Corrosion Resistance. Materials 2018, 11, 396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kong, N.; Niu, J.; Shi, Y.; Li, H.; Zhou, Y.; Yuan, G. Influence of fluoride treatment on surface properties, biodegradation and cytocompatibility of Mg–Nd–Zn–Zr alloy. J. Mater. Sci. Mater. Med. 2014, 25, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Seitz, J.M.; Eifler, R.; Weber, C.; Lenarz, T.H.; Maier, H.J.; Durisin, M. In vivo degradation effects of alloy MgNd2 in contact with mucous tissue. J. Biomed. Mater. Res. A 2015, 103, 2427–2440. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.M.; Eifler, R.; Seitz, J.M.; Maier, H.J.; Reifenrath, J.; Lenarz, T.; Durisin, M. Biocompatibility of MgF2-coated MgNd2 specimens in contact with mucosa of the nasal sinus—A long term study. Acta Biomater. 2015, 18, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Durisin, M.; Reifenrath, J.; Weber, C.M.; Eifler, R.; Maier, H.J.; Lenarz, T.; Seitz, J.M. Biodegradable nasal stents (MgF2 -coated Mg-2 wt %Nd alloy)-A long-term in vivo study. J. Biomed. Mater Res. B Appl. Biomater. 2017, 105, 350–365. [Google Scholar] [CrossRef] [PubMed]
- Behring, J.; Junker, R.; Walboomers, X.F.; Chessnut, B.; Jansen, J.A. Toward guided tissue and bone regeneration: Morphology, attachment, proliferation, and migration of cells cultured on collagen barrier membranes. A systematic review. Odontology 2008, 96, 1–11. [Google Scholar] [CrossRef]
- Bunyaratavej, P.; Wang, H.L. Collagen membranes: A review. J. Periodontol. 2001, 72, 215–229. [Google Scholar] [CrossRef]
- Guda, T.; Walker, J.A.; Singleton, B.M.; Hernandez, J.W.; Son, J.S.; Kim, S.G.; Oh, D.S.; Appleford, M.R.; Ong, J.L.; Wenke, J.C. Guided bone regeneration in long-bone defects with a structural hydroxyapatite graft and collagen membrane. Tissue Eng. Part A 2013, 19, 1879–1888. [Google Scholar] [CrossRef]
- Hoornaert, A.; d’Arros, C.; Heymann, M.F.; Layrolle, P. Biocompatibility, resorption and biofunctionality of a new synthetic biodegradable membrane for guided bone regeneration. Biomed. Mater. 2016, 11, 045012. [Google Scholar] [CrossRef]
- Byun, S.H.; Lim, H.K.; Kim, S.M.; Lee, S.M.; Kim, H.E.; Lee, J.H. The Bioresorption and Guided Bone Regeneration of Absorbable Hydroxyapatite-Coated Magnesium Mesh. J. Craniofac. Surg. 2017, 28, 518–523. [Google Scholar] [CrossRef]
- Fischerauer, S.F.; Kraus, T.; Wu, X.; Tangl, S.; Sorantin, E.; Hanzi, A.C.; Loffler, J.F.; Uggowitzer, P.J.; Weinberg, A.M. In vivo degradation performance of micro-arc-oxidized magnesium implants: A micro-CT study in rats. Acta Biomater. 2013, 9, 5411–5420. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 948–963. [Google Scholar] [CrossRef] [PubMed]
- Jung, O.; Smeets, R.; Kopp, A.; Porchetta, D.; Hiester, P.; Heiland, M.; Friedrich, R.E.; Precht, C.; Hanken, H.; Grobe, A.; et al. PEO-generated Surfaces Support Attachment and Growth of Cells In Vitro with No Additional Benefit for Micro-roughness in Sa (0.2–4 μm). In Vivo 2016, 30, 27–33. [Google Scholar] [PubMed]
- Sun, J.; Wang, J.; Jiang, H.; Chen, M.; Bi, Y.; Liu, D. In vivo comparative property study of the bioactivity of coated Mg-3Zn-0.8Zr alloy. Mater Sci. Eng. C Mater Biol. Appl. 2013, 33, 3263–3272. [Google Scholar] [CrossRef] [PubMed]
- Kastellorizios, M.; Tipnis, N.; Burgess, D.J. Foreign Body Reaction to Subcutaneous Implants. Adv. Exp. Med. Biol. 2015, 865, 93–108. [Google Scholar] [CrossRef]
- Gruionu, G.; Stone, A.L.; Schwartz, M.A.; Hoying, J.B.; Williams, S.K. Encapsulation of ePTFE in prevascularized collagen leads to peri-implant vascularization with reduced inflammation. J. Biomed. Mater Res. A 2010, 95, 811–818. [Google Scholar] [CrossRef]
- Klopfleisch, R.; Jung, F. The pathology of the foreign body reaction against biomaterials. J. Biomed. Mater. Res. A 2017, 105, 927–940. [Google Scholar] [CrossRef]
- Major, M.R.; Wong, V.W.; Nelson, E.R.; Longaker, M.T.; Gurtner, G.C. The foreign body response: At the interface of surgery and bioengineering. Plast. Reconstr. Surg. 2015, 135, 1489–1498. [Google Scholar] [CrossRef]
- DiEgidio, P.; Friedman, H.I.; Gourdie, R.G.; Riley, A.E.; Yost, M.J.; Goodwin, R.L. Biomedical implant capsule formation: Lessons learned and the road ahead. Ann. Plast. Surg. 2014, 73, 451–460. [Google Scholar] [CrossRef]
- Ghanaati, S.; Unger, R.E.; Webber, M.J.; Barbeck, M.; Orth, C.; Kirkpatrick, J.A.; Booms, P.; Motta, A.; Migliaresi, C.; Sader, R.A.; et al. Scaffold vascularization in vivo driven by primary human osteoblasts in concert with host inflammatory cells. Biomaterials 2011, 32, 8150–8160. [Google Scholar] [CrossRef]
- Klopfleisch, R. Macrophage reaction against biomaterials in the mouse model—Phenotypes, functions and markers. Acta Biomater. 2016, 43, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Abshagen, K.; Schrodi, I.; Gerber, T.; Vollmar, B. In vivo analysis of biocompatibility and vascularization of the synthetic bone grafting substitute NanoBone. J. Biomed. Mater. Res. A 2009, 91, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Perumal, G.; Ramasamy, B.; Doble, M.J.C.; Biointerfaces, S.B. Nanostructure coated AZ31 magnesium cylindrical mesh cage for potential long bone segmental defect repair-in vitro degradation and cytocompatibility studies. Colloids Surf. B Biointerfaces 2018, 172, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, W.; Yu, Q.; Wu, Y.; Wang, D.; Liang, J.; Zhou, F.J.L. New Method for the Corrosion Resistance of AZ31 Mg Alloy with a Porous Micro-Arc Oxidation Membrane as an Ionic Corrosion Inhibitor Container. Langmuir 2018, 35, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Zomorodian, A.; Garcia, M.P.; Moura e Silva, T.; Fernandes, J.C.; Fernandes, M.H.; Montemor, M.F. Corrosion resistance of a composite polymeric coating applied on biodegradable AZ31 magnesium alloy. Acta Biomater. 2013, 9, 8660–8670. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.S.; Zomorodian, A.; Kwiatkowski, L.; Lutze, R.; Balkowiec, A.; Colaço, B.; Pinheiro, V.; Fernandes, J.C.; Montemor, M.F.; Fernandes, M.H.J.B.M. In vivo assessment of a new multifunctional coating architecture for improved Mg alloy biocompatibility. Biomed. Mater. 2016, 11, 045007. [Google Scholar] [CrossRef]
- Neacsu, P.; Staras, A.I.; Voicu, S.I.; Ionascu, I.; Soare, T.; Uzun, S.; Cojocaru, V.D.; Pandele, A.M.; Croitoru, S.M.; Miculescu, F.; et al. Characterization and In Vitro and In Vivo Assessment of a Novel Cellulose Acetate-Coated Mg-Based Alloy for Orthopedic Applications. Materials 2017, 10, 686. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, B.; Jia, Z.; Lu, X.; Fang, L.; Wang, K.; Ren, F. Polydopamine mediated assembly of hydroxyapatite nanoparticles and bone morphogenetic protein-2 on magnesium alloys for enhanced corrosion resistance and bone regeneration. Biomed. Mater. 2017, 105, 2750–2761. [Google Scholar] [CrossRef]
- Li, G.; Zhang, L.; Wang, L.; Yuan, G.; Dai, K.; Pei, J.; Hao, Y. Dual modulation of bone formation and resorption with zoledronic acid-loaded biodegradable magnesium alloy implants improves osteoporotic fracture healing: An in vitro and in vivo study. Acta Biomater. 2018, 65, 486–500. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, J.; Chen, M.; Liu, D. Biological activity evaluation of magnesium fluoride coated Mg-Zn-Zr alloy in vivo. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 1068–1074. [Google Scholar] [CrossRef]
- Li, Z.; Shizhao, S.; Chen, M.; Fahlman, B.D.; Liu, D.; Bi, H. In vitro and in vivo corrosion, mechanical properties and biocompatibility evaluation of MgF2-coated Mg-Zn-Zr alloy as cancellous screws. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 1268–1280. [Google Scholar] [CrossRef] [PubMed]
- Donath, K.; Breuner, G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Sage-Schliff (sawing and grinding) technique. J. Oral Pathol. 1982, 11, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.; Vogel, M.; Delling, G. Undecalcified preparation of bone tissue: Report of technical experience and development of new methods. Virchows Arch A Pathol. Anat. Histopathol. 1991, 418, 1–7. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbeck, M.; Kühnel, L.; Witte, F.; Pissarek, J.; Precht, C.; Xiong, X.; Krastev, R.; Wegner, N.; Walther, F.; Jung, O. Degradation, Bone Regeneration and Tissue Response of an Innovative Volume Stable Magnesium-Supported GBR/GTR Barrier Membrane. Int. J. Mol. Sci. 2020, 21, 3098. https://doi.org/10.3390/ijms21093098
Barbeck M, Kühnel L, Witte F, Pissarek J, Precht C, Xiong X, Krastev R, Wegner N, Walther F, Jung O. Degradation, Bone Regeneration and Tissue Response of an Innovative Volume Stable Magnesium-Supported GBR/GTR Barrier Membrane. International Journal of Molecular Sciences. 2020; 21(9):3098. https://doi.org/10.3390/ijms21093098
Chicago/Turabian StyleBarbeck, Mike, Lennart Kühnel, Frank Witte, Jens Pissarek, Clarissa Precht, Xin Xiong, Rumen Krastev, Nils Wegner, Frank Walther, and Ole Jung. 2020. "Degradation, Bone Regeneration and Tissue Response of an Innovative Volume Stable Magnesium-Supported GBR/GTR Barrier Membrane" International Journal of Molecular Sciences 21, no. 9: 3098. https://doi.org/10.3390/ijms21093098
APA StyleBarbeck, M., Kühnel, L., Witte, F., Pissarek, J., Precht, C., Xiong, X., Krastev, R., Wegner, N., Walther, F., & Jung, O. (2020). Degradation, Bone Regeneration and Tissue Response of an Innovative Volume Stable Magnesium-Supported GBR/GTR Barrier Membrane. International Journal of Molecular Sciences, 21(9), 3098. https://doi.org/10.3390/ijms21093098








