Microbes and Cancer: Friends or Faux?
Abstract
1. Introduction
- (1)
- The relationship between infection and cancer;
- (2)
- The susceptibility of cancer patients to acquire infectious disease(s);
- (3)
- The role of the microbiota in cancer susceptibility;
- (4)
- The role of tumor microbioma in cancer therapy.
2. Infection and Cancer
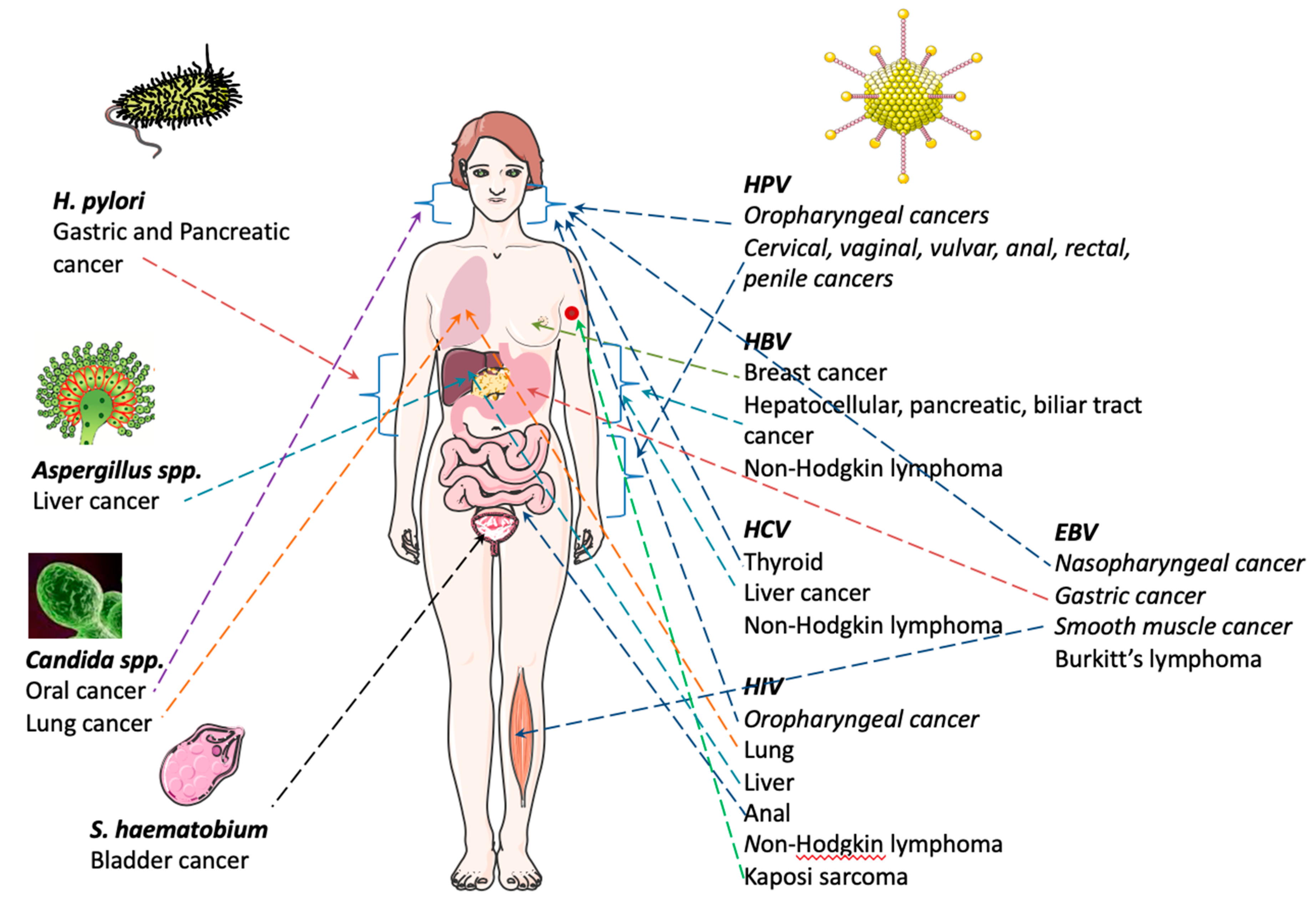
2.1. Viral Infections
2.1.1. Epstein–Barr Virus (EBV)
2.1.2. Human Papillomavirus (HPV)
2.1.3. Hepatitis Virus: B (HBV) and C Virus (HCV)
2.1.4. Human Immunodeficiency Virus (HIV)
3. Bacterial Infections
3.1. Helicobacter pylori (H. pylori)
3.2. Fungal Infections
3.3. Helminth Infections
3.4. Schistosoma haematobium (S. haematobium)
4. Susceptibility of Cancer Patients to Acquire Infectious Diseases
4.1. Bacterial Infections
4.2. Fungal Infections
5. Tissue Microbioma and Cancer Susceptibility
5.1. Breast Cancer
5.2. Digestive Tract Cancer
5.2.1. Gastric Cancer
5.2.2. Colorectal Cancer
5.2.3. Pancreatic Cancer
5.2.4. Oral Cancer
6. Role of Microbioma in Cancer Drug Response and Toxicity
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics for Hispanics/Latinos, 2012. CA Cancer J. Clin. 2012, 62, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Mariotto, A.B.; Yabroff, K.R.; Shao, Y.; Feuer, E.J.; Brown, M.L. Projections of the cost of cancer care in the United States: 2010–2020. J. Natl. Cancer Inst. 2011, 103, 117–128. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Noguchi, M.; Nicholson, A.G.; Geisinger, K.; Yatabe, Y.; Ishikawa, Y.; Wistuba, I.; Flieder, D.B.; Franklin, W.; et al. Diagnosis of lung cancer in small biopsies and cytology: Implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. Arch Pathol. Lab. Med. 2013, 137, 668–684. [Google Scholar] [CrossRef]
- Denisov, E.V.; Litviakov, N.V.; Zavyalova, M.V.; Perelmuter, V.M.; Vtorushin, S.V.; Tsyganov, M.M.; Gerashchenko, T.S.; Garbukov, E.Y.; Slonimskaya, E.M.; Cherdyntseva, N.V. Intratumoral morphological heterogeneity of breast cancer: Neoadjuvant chemotherapy efficiency and multidrug resistance gene expression. Sci. Rep. 2014, 4, 4709. [Google Scholar] [CrossRef]
- Yasunaga, J.I.; Matsuoka, M. Oncogenic spiral by infectious pathogens: Cooperation of multiple factors in cancer development. Cancer Sci. 2018, 109, 24–32. [Google Scholar] [CrossRef]
- Parkin, D.M. The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer 2006, 118, 3030–3044. [Google Scholar] [CrossRef]
- Chang, X.B. Molecular mechanism of ATP-dependent solute transport by multidrug resistance-associated protein 1. Methods Mol. Biol. 2010, 596, 223–249. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- de Martel, C.; Ferlay, J.; Franceschi, S.; Vignat, J.; Bray, F.; Forman, D.; Plummer, M. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012, 13, 607–615. [Google Scholar] [CrossRef]
- Luo, G.G.; Ou, J.H.J. Oncogenic viruses and cancer. Virol. Sin. 2015, 30, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Khan, G.; Hashim, M.J. Global burden of deaths from Epstein-Barr virus attributable malignancies 1990–2010. Infect. Agent Cancer 2014, 9, 38. [Google Scholar] [CrossRef]
- Babcock, G.J.; Decker, L.L.; Volk, M.; Thorley-Lawson, D.A. EBV persistence in memory B cells in vivo. Immunity 1998, 9, 395–404. [Google Scholar] [CrossRef]
- Molyneux, E.M.; Rochford, R.; Griffin, B.; Newton, R.; Jackson, G.; Menon, G.; Harrison, C.J.; Israels, T.; Bailey, S. Burkitt’s lymphoma. Lancet 2012, 379, 1234–1244. [Google Scholar] [CrossRef]
- Miles, R.R.; Arnold, S.; Cairo, M.S. Risk factors and treatment of childhood and adolescent Burkitt lymphoma/leukaemia. Br. J. Haematol. 2012, 156, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Petrich, A.M.; Sparano, J.A.; Parekh, S. Paradigms and Controversies in the Treatment of HIV-Related Burkitt Lymphoma. Adv. Hematol. 2012, 2012, 403648. [Google Scholar] [CrossRef][Green Version]
- Kaplan, L.D. HIV-associated lymphoma. Best Pract. Res. Clin. Haematol. 2012, 25, 101–117. [Google Scholar] [CrossRef]
- Brady, G.; MacArthur, G.J.; Farrell, P.J. Epstein-Barr virus and Burkitt lymphoma. J. Clin. Pathol. 2007, 60, 1397–1402. [Google Scholar] [CrossRef]
- Godot, C.; Patte, C.; Blanche, S.; Rohrlich, P.; Dollfus, C.; Tabone, M.D. Characteristics and prognosis of B-cell lymphoma in HIV-infected children in the HAART era. J. Pediatr. Hematol. Oncol. 2012, 34, e282–e288. [Google Scholar] [CrossRef]
- Carbone, A.; Gloghini, A.; Serraino, D.; Spina, M. HIV-associated Hodgkin lymphoma. Curr. Opin. HIV AIDS 2009, 4, 3–10. [Google Scholar] [CrossRef]
- Akar Ozkan, E.; Ozdemir, B.H.; Akdur, A.; Deniz, E.E.; Haberal, M. Burkitt lymphoma after transplant: An aggressive lymphoproliferative disease. Exp. Clin. Transpl. 2014, 12 (Suppl. 1), 136–138. [Google Scholar]
- Shah, K.M.; Young, L.S. Epstein-Barr virus and carcinogenesis: Beyond Burkitt’s lymphoma. Clin. Microbiol. Infect. 2009, 15, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Wee, J.; Nei, W.L.; Yeoh, K.W.; Yeo, R.M.; Loong, S.L.; Qian, C.N. Why are East Asians more susceptible to several infection-associated cancers (carcinomas of the nasopharynx, stomach, liver, adenocarcinoma of the lung, nasal NK/T-cell lymphomas)? Med. Hypotheses 2012, 79, 833–842. [Google Scholar] [CrossRef]
- Petersson, F. Nasopharyngeal carcinoma: A review. Semin. Diagn. Pathol. 2015, 32, 54–73. [Google Scholar] [CrossRef]
- Alabdulqader, N.A.; Yousef, M.M.; Bernacki, K.D.; Al-Abbadi, M.A. Epstein-Barr virus associated smooth muscle tumors. Synchronous liver and lung involvement. Saudi. Med. J. 2012, 33, 1010–1013. [Google Scholar]
- Luo, G.; Hao, N.B.; Hu, C.J.; Yong, X.; Lu, M.H.; Cheng, B.J.; Zhang, Y.; Yang, S.M. HBV infection increases the risk of pancreatic cancer: A meta-analysis. Cancer Causes Control 2013, 24, 529–537. [Google Scholar] [CrossRef]
- Hsing, A.W.; Zhang, M.; Rashid, A.; McGlynn, K.A.; Wang, B.S.; Niwa, S.; Ortiz-Conde, B.A.; Goedert, J.J.; Fraumeni, J.F., Jr.; O’Brien, T.R.; et al. Hepatitis B and C virus infection and the risk of biliary tract cancer: A population-based study in China. Int. J. Cancer 2008, 122, 1849–1853. [Google Scholar] [CrossRef]
- Su, F.H.; Chang, S.N.; Chen, P.C.; Sung, F.C.; Su, C.T.; Yeh, C.C. Association between chronic viral hepatitis infection and breast cancer risk: A nationwide population-based case-control study. BMC Cancer 2011, 11, 495. [Google Scholar] [CrossRef]
- Available online: http://www.who.int/cancer/prevention/en/ (accessed on 4 March 2019).
- Bosch, F.X.; Broker, T.R.; Forman, D.; Moscicki, A.B.; Gillison, M.L.; Doorbar, J.; Stern, P.L.; Stanley, M.; Arbyn, M.; Poljak, M.; et al. Comprehensive control of human papillomavirus infections and related diseases. Vaccine 2013, 31 (Suppl. 7), H1–H31. [Google Scholar] [CrossRef] [PubMed]
- Viens, L.J.; Henley, S.J.; Watson, M.; Markowitz, L.E.; Thomas, C.C.; Thompson, T.D.; Razzaghi, H.; Saraiya, M. Human Papillomavirus-Associated Cancers-United States, 2008–2012. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.cdc.gov/std/hpv/HPV-FS-July-2017.pdf (accessed on 4 March 2019).
- Asiaf, A.; Ahmad, S.T.; Mohammad, S.O.; Zargar, M.A. Review of the current knowledge on the epidemiology, pathogenesis, and prevention of human papillomavirus infection. Eur. J. Cancer Prev. 2014, 23, 206–224. [Google Scholar] [CrossRef] [PubMed]
- Marlow, L.A.; Waller, J.; Wardle, J. Public awareness that HPV is a risk factor for cervical cancer. Br. J. Cancer 2007, 97, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Anhang, R.; Wright, T.C., Jr.; Smock, L.; Goldie, S.J. Women’s desired information about human papillomavirus. Cancer 2004, 100, 315–320. [Google Scholar] [CrossRef] [PubMed]
- McCaffery, K.; Waller, J.; Nazroo, J.; Wardle, J. Social and psychological impact of HPV testing in cervical screening: A qualitative study. Sex Transm. Infect. 2006, 82, 169–174. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Cuzick, J.; Arbyn, M.; Sankaranarayanan, R.; Tsu, V.; Ronco, G.; Mayrand, M.H.; Dillner, J.; Meijer, C.J. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine 2008, 26 (Suppl. 10), K29–K41. [Google Scholar] [CrossRef]
- Stanley, M. Tumour virus vaccines: Hepatitis B virus and human papillomavirus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372. [Google Scholar] [CrossRef]
- Lieberman, P.M. Virology. Epstein-Barr virus turns 50. Science 2014, 343, 1323–1325. [Google Scholar] [CrossRef]
- Bosch, F.X.; Ribes, J.; Diaz, M.; Cleries, R. Primary liver cancer: Worldwide incidence and trends. Gastroenterology 2004, 127, S5–S16. [Google Scholar] [CrossRef] [PubMed]
- Minuk, G.Y.; Bautista, W.; Klein, J. Evidence of Hepatitis B Virus Infection in Cancer and Noncancer Stem Cells Associated with Human Hepatocellular Carcinoma. Can. J. Infect. Dis. Med. Microbiol. 2016, 2016, 8931591. [Google Scholar] [CrossRef] [PubMed]
- Lemon, S.M.; McGivern, D.R. Is hepatitis C virus carcinogenic? Gastroenterology 2012, 142, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, F.; Mele, A. Hepatitis viruses and non-Hodgkin lymphoma: Epidemiology, mechanisms of tumorigenesis, and therapeutic opportunities. Blood 2011, 117, 1792–1798. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Garcia-Buey, L.; Pajares, J.M.; Moreno-Otero, R. Prevalence of hepatitis C virus infection in B-cell non-Hodgkin’s lymphoma: Systematic review and meta-analysis. Gastroenterology 2003, 125, 1723–1732. [Google Scholar] [CrossRef]
- Matsuo, K.; Kusano, A.; Sugumar, A.; Nakamura, S.; Tajima, K.; Mueller, N.E. Effect of hepatitis C virus infection on the risk of non-Hodgkin’s lymphoma: A meta-analysis of epidemiological studies. Cancer Sci. 2004, 95, 745–752. [Google Scholar] [CrossRef]
- Wang, P.; Jing, Z.; Liu, C.; Xu, M.; Wang, P.; Wang, X.; Yin, Y.; Cui, Y.; Ren, D.; Rao, X. Hepatitis C virus infection and risk of thyroid cancer: A systematic review and meta-analysis. Arab. J. Gastroenterol. 2017, 18, 1–5. [Google Scholar] [CrossRef]
- Omland, L.H.; Farkas, D.K.; Jepsen, P.; Obel, N.; Pedersen, L. Hepatitis C virus infection and risk of cancer: A population-based cohort study. Clin. Epidemiol. 2010, 2, 179–186. [Google Scholar] [CrossRef]
- Gao, W.Y.; Johns, D.G.; Tanaka, M.; Mitsuya, H. Suppression of replication of multidrug-resistant HIV type 1 variants by combinations of thymidylate synthase inhibitors with zidovudine or stavudine. Mol. Pharmacol. 1999, 55, 535–540. [Google Scholar]
- Sharp, P.M.; Hahn, B.H. Origins of HIV and the AIDS pandemic. Cold Spring Harb. Perspect. Med. 2011, 1, a006841. [Google Scholar] [CrossRef]
- Faria, N.R.; Rambaut, A.; Suchard, M.A.; Baele, G.; Bedford, T.; Ward, M.J.; Tatem, A.J.; Sousa, J.D.; Arinaminpathy, N.; Pepin, J.; et al. HIV epidemiology. The early spread and epidemic ignition of HIV-1 in human populations. Science 2014, 346, 56–61. [Google Scholar] [CrossRef]
- Antman, K.; Chang, Y. Kaposi’s sarcoma. N. Engl. J. Med. 2000, 342, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Beatrous, S.V.; Grisoli, S.B.; Riahi, R.R.; Cohen, P.R. Cutaneous HIV-associated Kaposi sarcoma: A potential setting for management by clinical observation. Dermatol. Online J. 2017, 23. [Google Scholar]
- Goncalves, P.H.; Uldrick, T.S.; Yarchoan, R. HIV-associated Kaposi sarcoma and related diseases. AIDS 2017, 31, 1903–1916. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Hanson, D.L.; Sullivan, P.S.; Novak, R.M.; Moorman, A.C.; Tong, T.C.; Holmberg, S.D.; Brooks, J.T.; Adult and Adolescent Spectrum of Disease Project and HIV Outpatient Study Investigators. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann. Intern. Med. 2008, 148, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Biggar, R.J.; Curtis, R.E.; Cote, T.R.; Rabkin, C.S.; Melbye, M. Risk of Other Cancers Following Kaposis-Sarcoma-Relation to Acquired-Immunodeficiency-Syndrome. Am. J. Epidemiol. 1994, 139, 362–368. [Google Scholar] [CrossRef]
- Schneider, J.W.; Dittmer, D.P. Diagnosis and Treatment of Kaposi Sarcoma. Am. J. Clin. Dermatol. 2017, 18, 529–539. [Google Scholar] [CrossRef]
- Muhammad, J.S.; Zaidi, S.F.; Sugiyama, T. Epidemiological ins and outs of helicobacter pylori: A review. J. Pak. Med. Assoc. 2012, 62, 955–959. [Google Scholar]
- Camilo, V.; Sugiyama, T.; Touati, E. Pathogenesis of Helicobacter pylori infection. Helicobacter 2017, 22 (Suppl. 1). [Google Scholar] [CrossRef]
- Rugge, M.; Genta, R.M.; Di Mario, F.; El-Omar, E.M.; El-Serag, H.B.; Fassan, M.; Hunt, R.H.; Kuipers, E.J.; Malfertheiner, P.; Sugano, K.; et al. Gastric Cancer as Preventable Disease. Clin. Gastroenterol. Hepatol. 2017, 15, 1833–1843. [Google Scholar] [CrossRef]
- Venerito, M.; Vasapolli, R.; Rokkas, T.; Delchier, J.C.; Malfertheiner, P. Helicobacter pylori, gastric cancer and other gastrointestinal malignancies. Helicobacter 2017, 22 (Suppl. 1). [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, T.; Nakagawa, M.; Kiriyama, Y.; Toyoda, T.; Cao, X. Prevention of Gastric Cancer: Eradication of Helicobacter pylori and Beyond. Int. J. Mol. Sci. 2017, 18, 1699. [Google Scholar] [CrossRef] [PubMed]
- Maev, I.G.; Kazyulin, A.N. [New opportunities for the prevention of gastric cancer]. Ter Arkh 2017, 89, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Houghton, J.; Wang, T.C. Helicobacter pylori and gastric cancer: A new paradigm for inflammation-associated epithelial cancers. Gastroenterology 2005, 128, 1567–1578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Powell, S.E.; Betel, D.; Shah, M.A. The Gastric Microbiome and Its Influence on Gastric Carcinogenesis: Current Knowledge and Ongoing Research. Hematol. Oncol. Clin. N. Am. 2017, 31, 389–408. [Google Scholar] [CrossRef]
- Gigek, C.O.; Calcagno, D.Q.; Rasmussen, L.T.; Santos, L.C.; Leal, M.F.; Wisnieski, F.; Burbano, R.R.; Lourenco, L.G.; Lopes-Filho, G.J.; Smith, M.A.C. Genetic variants in gastric cancer: Risks and clinical implications. Exp. Mol. Pathol. 2017, 103, 101–111. [Google Scholar] [CrossRef]
- Sugano, K.; Tack, J.; Kuipers, E.J.; Graham, D.Y.; El-Omar, E.M.; Miura, S.; Haruma, K.; Asaka, M.; Uemura, N.; Malfertheiner, P.; et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015, 64, 1353–1367. [Google Scholar] [CrossRef]
- Asaka, M.; Kato, M.; Sakamoto, N. Roadmap to eliminate gastric cancer with Helicobacter pylori eradication and consecutive surveillance in Japan. J. Gastroenterol. 2014, 49, 1–8. [Google Scholar] [CrossRef]
- Driscoll, L.J.; Brown, H.E.; Harris, R.B.; Oren, E. Population Knowledge, Attitude, and Practice Regarding Helicobacter pylori Transmission and Outcomes: A Literature Review. Front. Public Health 2017, 5, 144. [Google Scholar] [CrossRef]
- Martins, H.M.; Almeida, I.; Marques, M.; Bernardo, F. Interaction of wild strains of Aspergilla with Aspergillus parasiticus ATCC15517 and aflatoxin production. Int. J. Mol. Sci. 2008, 9, 394–400. [Google Scholar] [CrossRef]
- Sohrabi, N.; Hassan, Z.M.; Khosravi, A.R.; Tebianian, M.; Mahdavi, M.; Tootian, Z.; Ebrahimi, S.M.; Yadegari, M.H.; Gheflati, Z. Invasive aspergillosis promotes tumor growth and severity in a tumor-bearing mouse model. Can. J. Microbiol. 2010, 56, 771–776. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ramirez-Garcia, A.; Gallot, N.; Abad, A.; Mendoza, L.; Rementeria, A.; Hernando, F.L. Molecular fractionation and characterization of a Candida albicans fraction that increases tumor cell adhesion to hepatic endothelium. Appl. Microbiol. Biotechnol. 2011, 92, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Garcia, A.; Arteta, B.; Abad-Diaz-de-Cerio, A.; Pellon, A.; Antoran, A.; Marquez, J.; Rementeria, A.; Hernando, F.L. Candida albicans increases tumor cell adhesion to endothelial cells in vitro: Intraspecific differences and importance of the mannose receptor. PLoS ONE 2013, 8, e53584. [Google Scholar] [CrossRef] [PubMed]
- Mohd Bakri, M.; Mohd Hussaini, H.; Rachel Holmes, A.; David Cannon, R.; Mary Rich, A. Revisiting the association between candidal infection and carcinoma, particularly oral squamous cell carcinoma. J. Oral. Microbiol. 2010, 2. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Garcia, A.; Rementeria, A.; Aguirre-Urizar, J.M.; Moragues, M.D.; Antoran, A.; Pellon, A.; Abad-Diaz-de-Cerio, A.; Hernando, F.L. Candida albicans and cancer: Can this yeast induce cancer development or progression? Crit. Rev. Microbiol. 2016, 42, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, A.S.; Suryavanshi, M.V.; Bhute, S.; Agunloye, A.M.; Isokpehi, R.D.; Anumudu, C.I.; Shouche, Y.S. The microbiome in urogenital schistosomiasis and induced bladder pathologies. PLoS Negl. Trop. Dis. 2017, 11, e0005826. [Google Scholar] [CrossRef]
- Chng, K.R.; Chan, S.H.; Ng, A.H.Q.; Li, C.; Jusakul, A.; Bertrand, D.; Wilm, A.; Choo, S.P.; Tan, D.M.Y.; Lim, K.H.; et al. Tissue Microbiome Profiling Identifies an Enrichment of Specific Enteric Bacteria in Opisthorchis viverrini Associated Cholangiocarcinoma. EBioMedicine 2016, 8, 195–202. [Google Scholar] [CrossRef]
- Itthitaetrakool, U.; Pinlaor, P.; Pinlaor, S.; Chomvarin, C.; Dangtakot, R.; Chaidee, A.; Wilailuckana, C.; Sangka, A.; Lulitanond, A.; Yongvanit, P. Chronic Opisthorchis viverrini Infection Changes the Liver Microbiome and Promotes Helicobacter Growth. PLoS ONE 2016, 11, e0165798. [Google Scholar] [CrossRef]
- Plieskatt, J.L.; Deenonpoe, R.; Mulvenna, J.P.; Krause, L.; Sripa, B.; Bethony, J.M.; Brindley, P.J. Infection with the carcinogenic liver fluke Opisthorchis viverrini modifies intestinal and biliary microbiome. FASEB J. 2013, 27, 4572–4584. [Google Scholar] [CrossRef]
- Sripa, B.; Deenonpoe, R.; Brindley, P.J. Co-infections with liver fluke and Helicobacter species: A paradigm change in pathogenesis of opisthorchiasis and cholangiocarcinoma? Parasitol. Int. 2017, 66, 383–389. [Google Scholar] [CrossRef]
- Botelho, M.C.; Alves, H.; Richter, J. Halting Schistosoma haematobium-associated bladder cancer. Int. J. Cancer Manag. 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Sanli, O.; Dobruch, J.; Knowles, M.A.; Burger, M.; Alemozaffar, M.; Nielsen, M.E.; Lotan, Y. Bladder cancer. Nat. Rev. Dis. Primers 2017, 3, 17022. [Google Scholar] [CrossRef]
- La Vecchia, C.; Negri, E.; D’Avanzo, B.; Savoldelli, R.; Franceschi, S. Genital and urinary tract diseases and bladder cancer. Cancer Res. 1991, 51, 629–631. [Google Scholar] [PubMed]
- Afonso, J.; Longatto-Filho, A.; Baltazar, F.; Sousa, N.; Costa, F.E.; Morais, A.; Amaro, T.; Lopes, C.; Santos, L.L. CD147 overexpression allows an accurate discrimination of bladder cancer patients’ prognosis. Eur. J. Surg. Oncol. 2011, 37, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Gryseels, B.; Polman, K.; Clerinx, J.; Kestens, L. Human schistosomiasis. Lancet 2006, 368, 1106–1118. [Google Scholar] [CrossRef]
- Mostafa, M.H.; Sheweita, S.A.; O’Connor, P.J. Relationship between schistosomiasis and bladder cancer. Clin. Microbiol. Rev. 1999, 12, 97–111. [Google Scholar] [CrossRef]
- Grimes, J.E.; Croll, D.; Harrison, W.E.; Utzinger, J.; Freeman, M.C.; Templeton, M.R. The roles of water, sanitation and hygiene in reducing schistosomiasis: A review. Parasit Vectors 2015, 8, 156. [Google Scholar] [CrossRef]
- Grimes, J.E.; Croll, D.; Harrison, W.E.; Utzinger, J.; Freeman, M.C.; Templeton, M.R. The relationship between water, sanitation and schistosomiasis: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2014, 8, e3296. [Google Scholar] [CrossRef]
- Okwuosa, V.N.; Osuala, F.O. Toxicity of washing soaps to Schistosoma mansoni cercariae and effects of sublethal concentrations on infectivity in mice. Appl. Parasitol. 1993, 34, 69–75. [Google Scholar]
- Birrie, H.; Balcha, F.; Erko, B.; Bezuneh, A.; Gemeda, N. Investigation into the cercariacidal and miracidiacidal properties of Endod (Phytolacca dodecandra) berries (type 44). East Afr. Med. J. 1998, 75, 311–314. [Google Scholar]
- Botelho, M.C.; Machado, J.C.; da Costa, J.M. Schistosoma haematobium and bladder cancer: What lies beneath? Virulence 2010, 1, 84–87. [Google Scholar] [CrossRef][Green Version]
- Botelho, M.C.; Soares, R.; Vale, N.; Ribeiro, R.; Camilo, V.; Almeida, R.; Medeiros, R.; Gomes, P.; Machado, J.C.; Correia da Costa, J.M. Schistosoma haematobium: Identification of new estrogenic molecules with estradiol antagonistic activity and ability to inactivate estrogen receptor in mammalian cells. Exp. Parasitol. 2010, 126, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Rolston, K.V. Infections in Cancer Patients with Solid Tumors: A Review. Infect. Dis. Ther. 2017, 6, 69–83. [Google Scholar] [CrossRef] [PubMed]
- John, C.; Ruckdeschel, M.; John Greene, M.D.K.; Eric Sommers, M.D.; Karen, K.; Fields, M.D. Respiratory infections in patients with cancer. In Holland-Frei Cancer Medicine, 6th ed.; BC Decker: Hamilton, ON, Canada, 2003. [Google Scholar]
- Rolston, K.V.; Dholakia, N.; Rodriguez, S.; Rubenstein, E.B. Nature and outcome of febrile episodes in patients with pancreatic and hepatobiliary cancer. Support Care Cancer 1995, 3, 414–417. [Google Scholar] [CrossRef]
- Nesher, L.; Rolston, K.V. The current spectrum of infection in cancer patients with chemotherapy related neutropenia. Infection 2014, 42, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Cancer Facts & Figures 2019. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html (accessed on 23 February 2019).
- Denning, D.W.; Bromley, M.J. Infectious Disease. How to bolster the antifungal pipeline. Science 2015, 347, 1414–1416. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Lass-Florl, C. The changing face of epidemiology of invasive fungal disease in Europe. Mycoses 2009, 52, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive mycoses in North America. Crit. Rev. Microbiol. 2010, 36, 1–53. [Google Scholar] [CrossRef]
- Pappas, P.G.; Alexander, B.D.; Andes, D.R.; Hadley, S.; Kauffman, C.A.; Freifeld, A.; Anaissie, E.J.; Brumble, L.M.; Herwaldt, L.; Ito, J.; et al. Invasive fungal infections among organ transplant recipients: Results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 2010, 50, 1101–1111. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Almirante, B.; Rodriguez, D.; Park, B.J.; Cuenca-Estrella, M.; Planes, A.M.; Almela, M.; Mensa, J.; Sanchez, F.; Ayats, J.; Gimenez, M.; et al. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: Results from population-based surveillance, barcelona, Spain, from 2002 to 2003. J. Clin. Microbiol. 2005, 43, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Lalla, R.V.; Latortue, M.C.; Hong, C.H.; Ariyawardana, A.; D’Amato-Palumbo, S.; Fischer, D.J.; Martof, A.; Nicolatou-Galitis, O.; Patton, L.L.; Elting, L.S.; et al. A systematic review of oral fungal infections in patients receiving cancer therapy. Support Care Cancer 2010, 18, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Bergamasco, M.D.; Garnica, M.; Colombo, A.L.; Nucci, M. Epidemiology of candidemia in patients with hematologic malignancies and solid tumours in Brazil. Mycoses 2013, 56, 256–263. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Marr, K.A.; Park, B.J.; Alexander, B.D.; Anaissie, E.J.; Walsh, T.J.; Ito, J.; Andes, D.R.; Baddley, J.W.; Brown, J.M.; et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: Overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin. Infect. Dis. 2010, 50, 1091–1100. [Google Scholar] [CrossRef]
- Steinbach, W.J.; Marr, K.A.; Anaissie, E.J.; Azie, N.; Quan, S.P.; Meier-Kriesche, H.U.; Apewokin, S.; Horn, D.L. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J. Infect. 2012, 65, 453–464. [Google Scholar] [CrossRef]
- Marr, K.A.; Carter, R.A.; Crippa, F.; Wald, A.; Corey, L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 2002, 34, 909–917. [Google Scholar] [CrossRef]
- Anaissie, E. Opportunistic mycoses in the immunocompromised host: Experience at a cancer center and review. Clin. Infect. Dis. 1992, 14 (Suppl. 1), S43–S53. [Google Scholar] [CrossRef]
- Latge, J.P. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 1999, 12, 310–350. [Google Scholar] [CrossRef]
- Pannuti, C.; Gingrich, R.; Pfaller, M.A.; Kao, C.; Wenzel, R.P. Nosocomial pneumonia in patients having bone marrow transplant. Attributable mortality and risk factors. Cancer 1992, 69, 2653–2662. [Google Scholar] [CrossRef]
- Lass-Florl, C.; Griff, K.; Mayr, A.; Petzer, A.; Gastl, G.; Bonatti, H.; Freund, M.; Kropshofer, G.; Dierich, M.P.; Nachbaur, D. Epidemiology and outcome of infections due to Aspergillus terreus: 10-year single centre experience. Br. J. Haematol. 2005, 131, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Auberger, J.; Lass-Florl, C.; Ulmer, H.; Nogler-Semenitz, E.; Clausen, J.; Gunsilius, E.; Einsele, H.; Gastl, G.; Nachbaur, D. Significant alterations in the epidemiology and treatment outcome of invasive fungal infections in patients with hematological malignancies. Int. J. Hematol. 2008, 88, 508–515. [Google Scholar] [CrossRef] [PubMed]
- von Eiff, M.; Roos, N.; Schulten, R.; Hesse, M.; Zuhlsdorf, M.; van de Loo, J. Pulmonary aspergillosis: Early diagnosis improves survival. Respiration 1995, 62, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Anaissie, E. Fusarium infections in immunocompromised patients. Clin. Microbiol. Rev. 2007, 20, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P. Mucormycosis. In Goldman’s Cecil Medicine, 24th ed.; Lee Goldman, M.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 2, pp. 1994–1997. [Google Scholar]
- Pagano, L.; Caira, M.; Valentini, C.G.; Posteraro, B.; Fianchi, L. Current therapeutic approaches to fungal infections in immunocompromised hematological patients. Blood Rev. 2010, 24, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Garrett, W.S. Cancer and the microbiota. Science 2015, 348, 80–86. [Google Scholar] [CrossRef]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef]
- Burgess, S.L.; Buonomo, E.; Carey, M.; Cowardin, C.; Naylor, C.; Noor, Z.; Wills-Karp, M.; Petri, W.A., Jr. Bone marrow dendritic cells from mice with an altered microbiota provide interleukin 17A-dependent protection against Entamoeba histolytica colitis. MBio 2014, 5, e01817. [Google Scholar] [CrossRef]
- Tai, E.; Richardson, L.C.; Townsend, J.; Howard, E.; Mcdonald, L.C. Clostridium Difficile Infection among Children with Cancer. Pediatr. Infect. Dis. J. 2011, 30, 610–612. [Google Scholar] [CrossRef]
- Rajagopala, S.V.; Yooseph, S.; Harkins, D.M.; Moncera, K.J.; Zabokrtsky, K.B.; Torralba, M.G.; Tovchigrechko, A.; Highlander, S.K.; Pieper, R.; Sender, L.; et al. Gastrointestinal microbial populations can distinguish pediatric and adolescent Acute Lymphoblastic Leukemia (ALL) at the time of disease diagnosis. BMC Genom. 2016, 17, 635. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xue, J.; Zhou, X.; You, M.; Du, Q.; Yang, X.; He, J.; Zou, J.; Cheng, L.; Li, M.; et al. Oral microbiota distinguishes acute lymphoblastic leukemia pediatric hosts from healthy populations. PLoS ONE 2014, 9, e102116. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Jobin, C.; Balkwill, F. Chemotherapy, immunity and microbiota—a new triumvirate? Nat. Med. 2014, 20, 126–127. [Google Scholar] [CrossRef]
- Urbaniak, C.; Gloor, G.B.; Brackstone, M.; Scott, L.; Tangney, M.; Reid, G. The Microbiota of Breast Tissue and Its Association with Breast Cancer. Appl. Environ. Microbiol. 2016, 82, 5039–5048. [Google Scholar] [CrossRef] [PubMed]
- Hieken, T.J.; Chen, J.; Hoskin, T.L.; Walther-Antonio, M.; Johnson, S.; Ramaker, S.; Xiao, J.; Radisky, D.C.; Knutson, K.L.; Kalari, K.R.; et al. The Microbiome of Aseptically Collected Human Breast Tissue in Benign and Malignant Disease. Sci. Rep. 2016, 6, 30751. [Google Scholar] [CrossRef]
- Kwa, M.; Plottel, C.S.; Blaser, M.J.; Adams, S. The Intestinal Microbiome and Estrogen Receptor-Positive Female Breast Cancer. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimaraes, V.; Sokol, H.; Dore, J.; Corthier, G.; Furet, J.P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef]
- Hullar, M.A.; Burnett-Hartman, A.N.; Lampe, J.W. Gut microbes, diet, and cancer. Cancer Treat. Res. 2014, 159, 377–399. [Google Scholar] [CrossRef]
- Modi, S.R.; Collins, J.J.; Relman, D.A. Antibiotics and the gut microbiota. J. Clin. Invest. 2014, 124, 4212–4218. [Google Scholar] [CrossRef]
- Jakobsson, H.E.; Jernberg, C.; Andersson, A.F.; Sjolund-Karlsson, M.; Jansson, J.K.; Engstrand, L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS ONE 2010, 5, e9836. [Google Scholar] [CrossRef]
- Dethlefsen, L.; Huse, S.; Sogin, M.L.; Relman, D.A. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008, 6, e280. [Google Scholar] [CrossRef] [PubMed]
- Kilkkinen, A.; Rissanen, H.; Klaukka, T.; Pukkala, E.; Heliovaara, M.; Huovinen, P.; Mannisto, S.; Aromaa, A.; Knekt, P. Antibiotic use predicts an increased risk of cancer. Int. J. Cancer 2008, 123, 2152–2155. [Google Scholar] [CrossRef] [PubMed]
- Goedert, J.J.; Jones, G.; Hua, X.; Xu, X.; Yu, G.; Flores, R.; Falk, R.T.; Gail, M.H.; Shi, J.; Ravel, J.; et al. Investigation of the association between the fecal microbiota and breast cancer in postmenopausal women: A population-based case-control pilot study. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Pereira-Marques, J.; Pinto-Ribeiro, I.; Costa, J.L.; Carneiro, F.; Machado, J.C.; Figueiredo, C. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut 2018, 67, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Aviles-Jimenez, F.; Vazquez-Jimenez, F.; Medrano-Guzman, R.; Mantilla, A.; Torres, J. Stomach microbiota composition varies between patients with non-atrophic gastritis and patients with intestinal type of gastric cancer. Sci. Rep. 2014, 4, 4202. [Google Scholar] [CrossRef] [PubMed]
- Bik, E.M.; Eckburg, P.B.; Gill, S.R.; Nelson, K.E.; Purdom, E.A.; Francois, F.; Perez-Perez, G.; Blaser, M.J.; Relman, D.A. Molecular analysis of the bacterial microbiota in the human stomach. Proc. Natl. Acad. Sci. USA 2006, 103, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Delgado, S.; Cabrera-Rubio, R.; Mira, A.; Suarez, A.; Mayo, B. Microbiological survey of the human gastric ecosystem using culturing and pyrosequencing methods. Microb. Ecol. 2013, 65, 763–772. [Google Scholar] [CrossRef]
- Andersson, A.F.; Lindberg, M.; Jakobsson, H.; Backhed, F.; Nyren, P.; Engstrand, L. Comparative analysis of human gut microbiota by barcoded pyrosequencing. PLoS ONE 2008, 3, e2836. [Google Scholar] [CrossRef]
- Rajilic-Stojanovic, M.; de Vos, W.M. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol. Rev. 2014, 38, 996–1047. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.P.; LaMont, J.T. Clostridium difficile—more difficult than ever. N. Engl. J. Med. 2008, 359, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Dicksved, J.; Lindberg, M.; Rosenquist, M.; Enroth, H.; Jansson, J.K.; Engstrand, L. Molecular characterization of the stomach microbiota in patients with gastric cancer and in controls. J. Med. Microbiol. 2009, 58, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Tjalsma, H.; Boleij, A.; Marchesi, J.R.; Dutilh, B.E. A bacterial driver-passenger model for colorectal cancer: Beyond the usual suspects. Nat. Rev. Microbiol. 2012, 10, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Xu, S.; Yin, W.; Zhang, Y.; Lv, Q.; Yang, Y.; He, J. Foes or Friends? Bacteria Enriched in the Tumor Microenvironment of Colorectal Cancer. Cancers 2020, 12, 372. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef]
- Arthur, J.C.; Gharaibeh, R.Z.; Muhlbauer, M.; Perez-Chanona, E.; Uronis, J.M.; McCafferty, J.; Fodor, A.A.; Jobin, C. Microbial genomic analysis reveals the essential role of inflammation in bacteria-induced colorectal cancer. Nat. Commun. 2014, 5, 4724. [Google Scholar] [CrossRef]
- Castellarin, M.; Warren, R.L.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012, 22, 299–306. [Google Scholar] [CrossRef]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013, 14, 207–215. [Google Scholar] [CrossRef]
- Mira-Pascual, L.; Cabrera-Rubio, R.; Ocon, S.; Costales, P.; Parra, A.; Suarez, A.; Moris, F.; Rodrigo, L.; Mira, A.; Collado, M.C. Microbial mucosal colonic shifts associated with the development of colorectal cancer reveal the presence of different bacterial and archaeal biomarkers. J. Gastroenterol. 2015, 50, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.; Barnich, N.; Nguyen, H.T.T. Microbiota, Inflammation and Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 1310. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.E.; Moore, L.H. Intestinal floras of populations that have a high risk of colon cancer. Appl. Environ. Microbiol. 1995, 61, 3202–3207. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, F.; Ling, Z.; Tong, X.; Xiang, C. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS ONE 2012, 7, e39743. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Guo, B.; Gao, R.; Zhu, Q.; Qin, H. Microbiota disbiosis is associated with colorectal cancer. Front. Microbiol. 2015, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Zambirinis, C.P.; Pushalkar, S.; Saxena, D.; Miller, G. Pancreatic cancer, inflammation, and microbiome. Cancer J. 2014, 20, 195–202. [Google Scholar] [CrossRef]
- Takayama, S.; Takahashi, H.; Matsuo, Y.; Okada, Y.; Manabe, T. Effects of Helicobacter pylori infection on human pancreatic cancer cell line. Hepatogastroenterology 2007, 54, 2387–2391. [Google Scholar]
- Schulte, A.; Pandeya, N.; Fawcett, J.; Fritschi, L.; Risch, H.A.; Webb, P.M.; Whiteman, D.C.; Neale, R.E. Association between Helicobacter pylori and pancreatic cancer risk: A meta-analysis. Cancer Causes Control 2015, 26, 1027–1035. [Google Scholar] [CrossRef]
- Farrell, J.J.; Zhang, L.; Zhou, H.; Chia, D.; Elashoff, D.; Akin, D.; Paster, B.J.; Joshipura, K.; Wong, D.T. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 2012, 61, 582–588. [Google Scholar] [CrossRef]
- Archibugi, L.; Signoretti, M.; Capurso, G. The Microbiome and Pancreatic Cancer: An Evidence-based Association? Proceedings from the 9th Probiotics, Prebiotics and New Foods, Nutraceuticals and Botanicals for Nutrition & Human and Microbiota Health Meeting, held in Rome, Italy from September 10 to 12, 2017. J. Clin. Gastroenterol. 2018, 52 (Suppl. 1), S82–S85. [Google Scholar] [CrossRef]
- Torres, P.J.; Fletcher, E.M.; Gibbons, S.M.; Bouvet, M.; Doran, K.S.; Kelley, S.T. Characterization of the salivary microbiome in patients with pancreatic cancer. PeerJ 2015, 3, e1373. [Google Scholar] [CrossRef]
- Ren, Z.; Jiang, J.; Xie, H.; Li, A.; Lu, H.; Xu, S.; Zhou, L.; Zhang, H.; Cui, G.; Chen, X.; et al. Gut microbial profile analysis by MiSeq sequencing of pancreatic carcinoma patients in China. Oncotarget 2017, 8, 95176–95191. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.H.; Satagopan, J.; Xu, Y.; Ling, L.; Leong, S.; Orlow, I.; Saldia, A.; Li, P.; Nunes, P.; Madonia, V.; et al. The oral microbiota in patients with pancreatic cancer, patients with IPMNs, and controls: A pilot study. Cancer Causes Control 2017, 28, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Leal-Lopes, C.; Velloso, F.J.; Campopiano, J.C.; Sogayar, M.C.; Correa, R.G. Roles of Commensal Microbiota in Pancreas Homeostasis and Pancreatic Pathologies. J. Diabetes Res. 2015, 2015, 284680. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, K.; Nosho, K.; Sukawa, Y.; Matsunaga, Y.; Ito, M.; Kurihara, H.; Kanno, S.; Igarashi, H.; Naito, T.; Adachi, Y.; et al. Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget 2015, 6, 7209–7220. [Google Scholar] [CrossRef] [PubMed]
- Bultman, S.J. Emerging roles of the microbiome in cancer. Carcinogenesis 2014, 35, 249–255. [Google Scholar] [CrossRef]
- Memba, R.; Duggan, S.N.; Ni Chonchubhair, H.M.; Griffin, O.M.; Bashir, Y.; O’Connor, D.B.; Murphy, A.; McMahon, J.; Volcov, Y.; Ryan, B.M.; et al. The potential role of gut microbiota in pancreatic disease: A systematic review. Pancreatol. Off. J. Int. Assoc. Pancreatol. 2017, 17, 867–874. [Google Scholar] [CrossRef]
- McAllister, F.; Khan, M.A.W.; Helmink, B.; Wargo, J.A. The Tumor Microbiome in Pancreatic Cancer: Bacteria and Beyond. Cancer Cell 2019, 36, 577–579. [Google Scholar] [CrossRef]
- Lax, A.J.; Thomas, W. How bacteria could cause cancer: One step at a time. Trends Microbiol. 2002, 10, 293–299. [Google Scholar] [CrossRef]
- Mager, D.L.; Haffajee, A.D.; Devlin, P.M.; Norris, C.M.; Posner, M.R.; Goodson, J.M. The salivary microbiota as a diagnostic indicator of oral cancer: A descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects. J. Transl. Med. 2005, 3, 27. [Google Scholar] [CrossRef]
- Mager, D.L. Bacteria and cancer: Cause, coincidence or cure? A review. J. Transl. Med. 2006, 4, 14. [Google Scholar] [CrossRef]
- Schmidt, B.L.; Kuczynski, J.; Bhattacharya, A.; Huey, B.; Corby, P.M.; Queiroz, E.L.; Nightingale, K.; Kerr, A.R.; DeLacure, M.D.; Veeramachaneni, R.; et al. Changes in abundance of oral microbiota associated with oral cancer. PLoS ONE 2014, 9, e98741. [Google Scholar] [CrossRef]
- Healy, C.M.; Moran, G.P. The microbiome and oral cancer: More questions than answers. Oral Oncol. 2019, 89, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Bloching, M.; Reich, W.; Schubert, J.; Grummt, T.; Sandner, A. The influence of oral hygiene on salivary quality in the Ames Test, as a marker for genotoxic effects. Oral Oncol. 2007, 43, 933–939. [Google Scholar] [CrossRef]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillere, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Bronckaers, A.; Balzarini, J.; Liekens, S. The cytostatic activity of pyrimidine nucleosides is strongly modulated by Mycoplasma hyorhinis infection: Implications for cancer therapy. Biochem. Pharmacol. 2008, 76, 188–197. [Google Scholar] [CrossRef]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef]
- Paulos, C.M.; Wrzesinski, C.; Kaiser, A.; Hinrichs, C.S.; Chieppa, M.; Cassard, L.; Palmer, D.C.; Boni, A.; Muranski, P.; Yu, Z.; et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J. Clin. Invest. 2007, 117, 2197–2204. [Google Scholar] [CrossRef]
- Vetizou, M.; Pitt, J.M.; Daillere, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Trinchieri, G. Microbiota: A key orchestrator of cancer therapy. Nat. Rev. Cancer 2017, 17, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Xiao, H.; Li, Y.; Zhou, L.; Zhao, S.; Luo, D.; Zheng, Q.; Dong, J.; Zhao, Y.; Zhang, X.; et al. Faecal microbiota transplantation protects against radiation-induced toxicity. EMBO Mol. Med. 2017, 9, 448–461. [Google Scholar] [CrossRef]
- Rollins, K.E.; Javanmard-Emamghissi, H.; Acheson, A.G.; Lobo, D.N. The Role of Oral Antibiotic Preparation in Elective Colorectal Surgery: A Meta-analysis. Ann. Surg. 2019, 270, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Villeger, R.; Lopes, A.; Carrier, G.; Veziant, J.; Billard, E.; Barnich, N.; Gagniere, J.; Vazeille, E.; Bonnet, M. Intestinal Microbiota: A Novel Target to Improve Anti-Tumor Treatment? Int. J. Mol. Sci. 2019, 20, 4584. [Google Scholar] [CrossRef]
- Roberts, A.B.; Wallace, B.D.; Venkatesh, M.K.; Mani, S.; Redinbo, M.R. Molecular insights into microbial beta-glucuronidase inhibition to abrogate CPT-11 toxicity. Mol. Pharmacol. 2013, 84, 208–217. [Google Scholar] [CrossRef]
- Nakayama, H.; Kinouchi, T.; Kataoka, K.; Akimoto, S.; Matsuda, Y.; Ohnishi, Y. Intestinal anaerobic bacteria hydrolyse sorivudine, producing the high blood concentration of 5-(E)-(2-bromovinyl)uracil that increases the level and toxicity of 5-fluorouracil. Pharmacogenetics 1997, 7, 35–43. [Google Scholar] [CrossRef]
- Ferreira, M.R.; Muls, A.; Dearnaley, D.P.; Andreyev, H.J. Microbiota and radiation-induced bowel toxicity: Lessons from inflammatory bowel disease for the radiation oncologist. Lancet Oncol. 2014, 15, e139–e147. [Google Scholar] [CrossRef]

| Cancer Types | No. of New Cases (% of All Sites) | No. of Deaths (% of All Sites) |
|---|---|---|
| Bladder | 549,393 (3) | 199,922 (2,1) |
| Breast | 2,088,849 (11,6) | 626,679 (6,6) |
| Cervical | 569,847 (3,2) | 311,365 (3,3) |
| Colon | 1,096,601 (6,1) | 551,269 (5,8) |
| Gastric | 1,033,701 (5,7) | 782,685 (8,2) |
| Kaposi | 41,799 (0,2) | 19,902 (0,2) |
| Liver | 841,080 (4,7) | 781,631 (8,2) |
| Lung | 2,093,876 (11,6) | 1,761,007 (18,4) |
| Nasopharyngeal | 129,079 (0,7) | 72,987 (0,8) |
| Non-Hodgkin lymphoma | 509,590 (2,8) | 248,724 (2,6) |
| Pancreatic | 458,918 (2,5) | 432,242 (4,5) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo, M.M.; Pina-Vaz, C.; Baltazar, F. Microbes and Cancer: Friends or Faux? Int. J. Mol. Sci. 2020, 21, 3115. https://doi.org/10.3390/ijms21093115
Azevedo MM, Pina-Vaz C, Baltazar F. Microbes and Cancer: Friends or Faux? International Journal of Molecular Sciences. 2020; 21(9):3115. https://doi.org/10.3390/ijms21093115
Chicago/Turabian StyleAzevedo, Maria Manuel, Cidália Pina-Vaz, and Fátima Baltazar. 2020. "Microbes and Cancer: Friends or Faux?" International Journal of Molecular Sciences 21, no. 9: 3115. https://doi.org/10.3390/ijms21093115
APA StyleAzevedo, M. M., Pina-Vaz, C., & Baltazar, F. (2020). Microbes and Cancer: Friends or Faux? International Journal of Molecular Sciences, 21(9), 3115. https://doi.org/10.3390/ijms21093115






