Binder-Free α-MnO2 Nanowires on Carbon Cloth as Cathode Material for Zinc-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrode and Battery Fabrication
2.3. Characterization
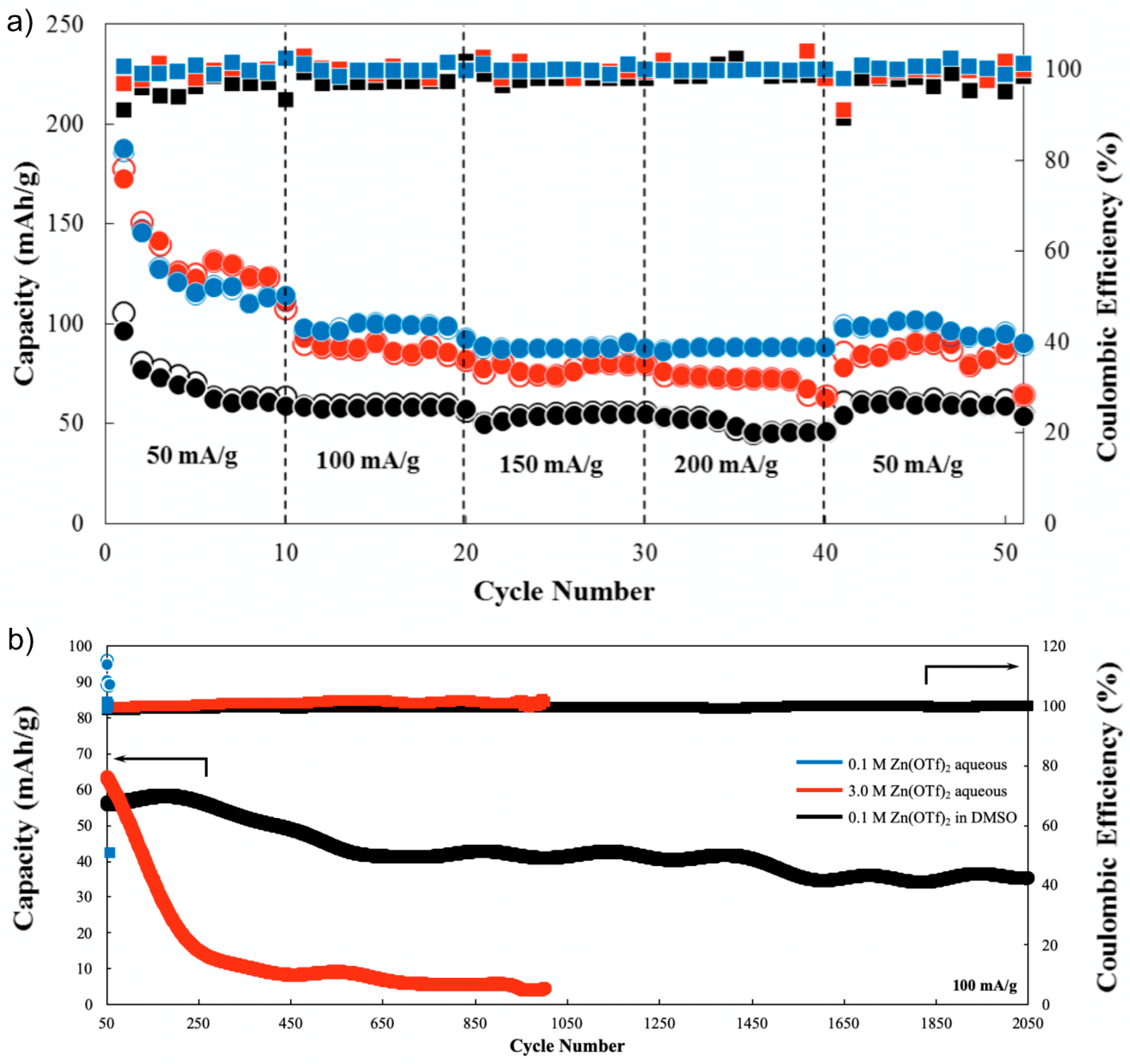
- Galvanostatic charge-discharge tests were conducted via Battery Testing System (NEWARE, BTS-4000 series, Neware Technology Ltd., Shenzhen, China) at 30 °C within the voltage range of 0.4–1.9 V. The equivalent C-rate per current density was indicated: 50, 100, 150, and 200 mA g−1 correspond to 0.16 C, 0.32 C, 0.49 C, and 0.65 C, respectively.
- EIS tests were conducted using VersaSTAT 3F (AMETEK, Berwyn, PA, USA) at an amplitude potential of 10 mV around the open circuit voltage (OCV) within the frequency range of 0.01–100,000 Hz.
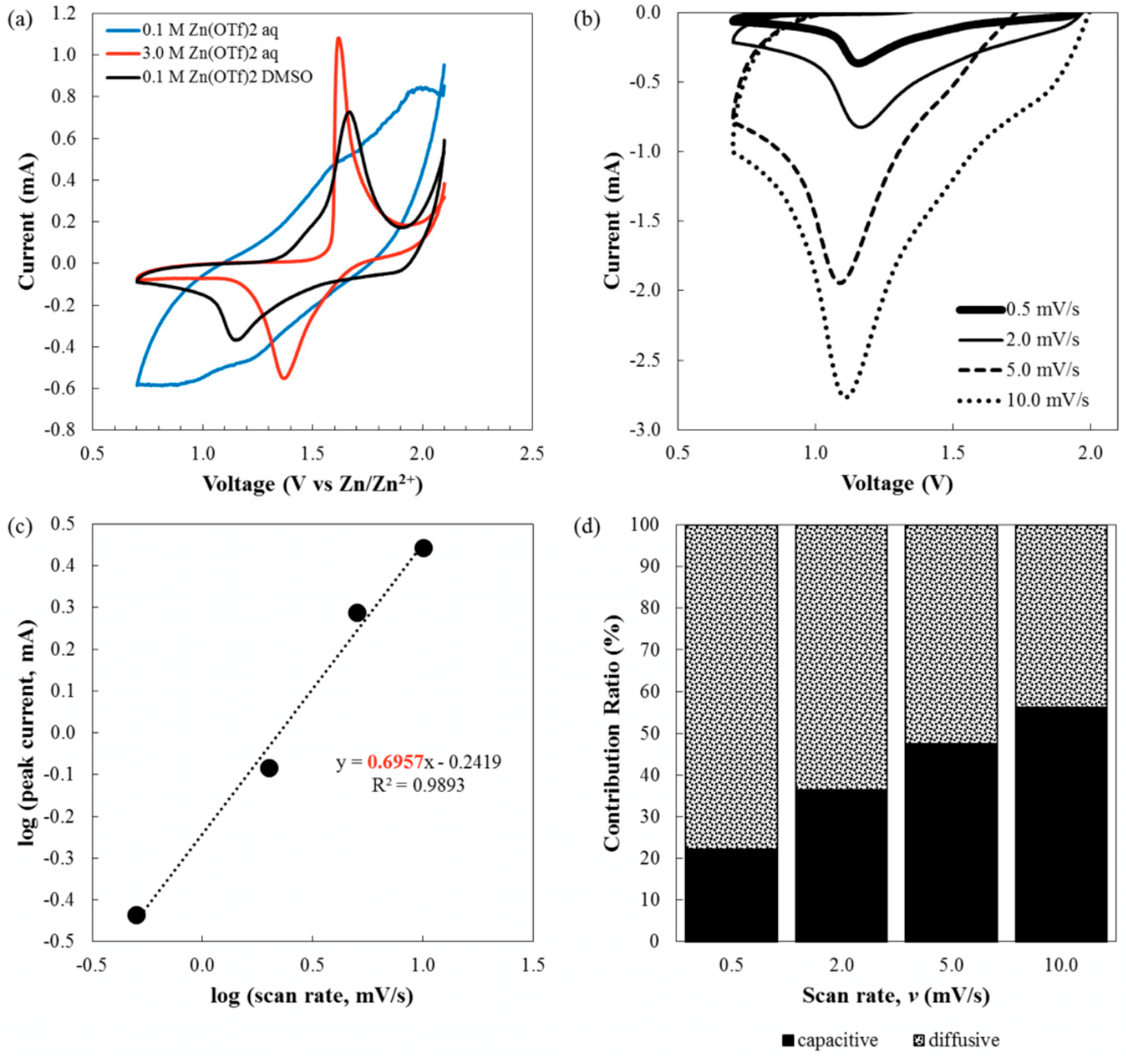
- CV tests were conducted using VersaSTAT 3F (AMETEK, Berwyn, PA, USA) within the voltage range of 0.7–2.1 V vs. Zn/Zn2+ with scan rates of 0.5, 2.0, 5.0, and 10.0 mV/s. The tests were carried out using the CR2032 cell with a two-electrode configuration. In this configuration, the positive electrode of the cell was used as the working electrode. The negative electrode of the cell was used as both counter and reference electrode.
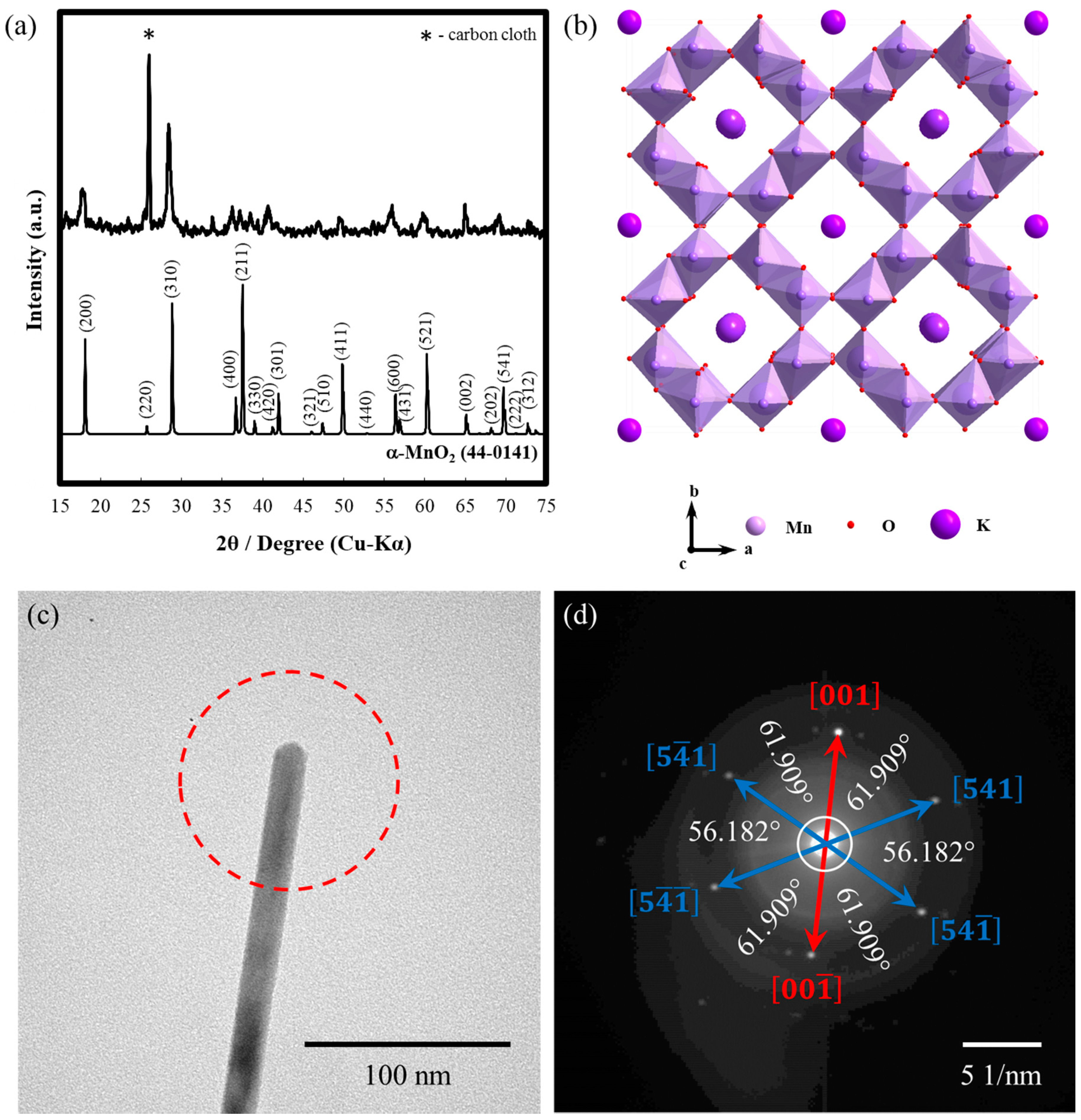
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ZIB | Zinc-ion battery |
| DMSO | Dimethyl sulfoxide |
| LIB | Lithium-ion battery |
| rGO | Reduced graphene oxide |
| CNT | Carbon nanotube |
| NMP | N-methyl-2-pyrrolidone |
| DES | Deep eutectic solvent |
| DI water | Deionized water |
| CV | Cyclic voltammetry |
| EIS | Electrochemical Impedance Spectroscopy |
| OCV | Open circuit voltage |
| SEM | Scanning electron microscope |
| TEM | Transmission electron microscope |
| XRD | X-ray diffraction |
| EDS | Energy dispersive X-Ray spectroscopy |
| SAED | Selected area electron diffraction |
References
- Wu, X.; Song, K.; Zhang, X.; Hu, N.; Li, L.; Li, W.; Zhang, L.; Zhang, H. Safety Issues in Lithium Ion Batteries: Materials and Cell Design. Front. Energy Res. 2019, 7. [Google Scholar] [CrossRef]
- Ouyang, D.; Chen, M.; Huang, Q.; Weng, J.; Wang, Z.; Wang, J. A Review on the Thermal Hazards of the Lithium-Ion Battery and the Corresponding Countermeasures. Appl. Sci. 2019, 9, 2483. [Google Scholar] [CrossRef]
- Ould Ely, T.; Kamzabek, D.; Chakraborty, D. Batteries Safety: Recent Progress and Current Challenges. Front. Energy Res. 2019, 7. [Google Scholar] [CrossRef]
- Su, B.; Zhang, J.; Fujita, M.; Zhou, W.; Sit, P.H.-L.; Yu, D.Y.W. Na2SeO3: A Na-Ion Battery Positive Electrode Material with High Capacity. J. Electrochem. Soc. 2019, 166, A5075–A5080. [Google Scholar] [CrossRef]
- Zhang, R.; Tutusaus, O.; Mohtadi, R.; Ling, C. Magnesium-Sodium Hybrid Battery With High Voltage, Capacity and Cyclability. Front. Chem. 2018, 6. [Google Scholar] [CrossRef]
- Leisegang, T.; Meutzner, F.; Zschornak, M.; Münchgesang, W.; Schmid, R.; Nestler, T.; Eremin, R.A.; Kabanov, A.A.; Blatov, V.A.; Meyer, D.C. The Aluminum-Ion Battery: A Sustainable and Seminal Concept? Front. Chem. 2019, 7. [Google Scholar] [CrossRef]
- Mokhtar, M.; Talib, M.Z.M.; Majlan, E.H.; Tasirin, S.M.; Ramli, W.M.F.W.; Daud, W.R.W.; Sahari, J. Recent developments in materials for aluminum–air batteries: A review. J. Ind. Eng. Chem. 2015, 32, 1–20. [Google Scholar] [CrossRef]
- Ling, C.; Zhang, R. Manganese Dioxide As Rechargeable Magnesium Battery Cathode. Front. Energy Res. 2017, 5. [Google Scholar] [CrossRef]
- Shrestha, N.; Raja, K.S.; Utgikar, V. Mg-RE Alloy Anode Materials for Mg-Air Battery Application. J. Electrochem. Soc. 2019, 166, A3139–A3153. [Google Scholar] [CrossRef]
- Tian, H.; Gao, T.; Li, X.; Wang, X.; Luo, C.; Fan, X.; Yang, C.; Suo, L.; Ma, Z.; Han, W.; et al. High power rechargeable magnesium/iodine battery chemistry. Nat. Commun. 2017, 8, 14083. [Google Scholar] [CrossRef]
- Lao-atiman, W.; Julaphatachote, T.; Boonmongkolras, P.; Kheawhom, S. Printed Transparent Thin Film Zn-MnO2 Battery. J. Electrochem. Soc. 2017, 164, A859–A863. [Google Scholar] [CrossRef]
- De Juan-Corpuz, L.M.; Corpuz, R.D.; Somwangthanaroj, A.; Nguyen, M.T.; Yonezawa, T.; Ma, J.; Kheawhom, S. Binder-Free Centimeter-Long V2O5 Nanofibers on Carbon Cloth as Cathode Material for Zinc-Ion Batteries. Energies 2019, 13, 31. [Google Scholar] [CrossRef]
- Hosseini, S.; Abbasi, A.; Uginet, L.-O.; Haustraete, N.; Praserthdam, S.; Yonezawa, T.; Kheawhom, S. The Influence of Dimethyl Sulfoxide as Electrolyte Additive on Anodic Dissolution of Alkaline Zinc-Air Flow Battery. Sci. Rep. 2019, 9, 14958. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Tan, H.; Rui, X.; Yu, Y. Electrode Materials for Rechargeable Zinc-Ion and Zinc-Air Batteries: Current Status and Future Perspectives. Electrochem. Energy Rev. 2019, 2, 395–427. [Google Scholar] [CrossRef]
- Konarov, A.; Voronina, N.; Jo, J.H.; Bakenov, Z.; Sun, Y.-K.; Myung, S.-T. Present and Future Perspective on Electrode Materials for Rechargeable Zinc-Ion Batteries. ACS Energy Lett. 2018, 3, 2620–2640. [Google Scholar] [CrossRef]
- Abbasi, A.; Hosseini, S.; Somwangthanaroj, A.; Mohamad, A.A.; Kheawhom, S. Poly(2,6-Dimethyl-1,4-Phenylene Oxide)-Based Hydroxide Exchange Separator Membranes for Zinc–Air Battery. Int. J. Mol. Sci. 2019, 20, 3678. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Sun, X.; Xiao, M.; Cheng, J.; Shen, Y. Equivalent Circuit Model Construction and Dynamic Flow Optimization Based on Zinc–Nickel Single-Flow Battery. Energies 2019, 12, 582. [Google Scholar] [CrossRef]
- Hosseini, S.; Han, S.J.; Arponwichanop, A.; Yonezawa, T.; Kheawhom, S. Ethanol as an electrolyte additive for alkaline zinc-air flow batteries. Sci. Rep. 2018, 8, 11273. [Google Scholar] [CrossRef]
- Poolnapol, L.; Kao-ian, W.; Somwangthanaroj, A.; Mahlendorf, F.; Nguyen, M.T.; Yonezawa, T.; Kheawhom, S. Silver Decorated Reduced Graphene Oxide as Electrocatalyst for Zinc–Air Batteries. Energies 2020, 13, 462. [Google Scholar] [CrossRef]
- Xie, C.; Liu, Y.; Lu, W.; Zhang, H.; Li, X. Highly stable zinc–iodine single flow batteries with super high energy density for stationary energy storage. Energy Environ. Sci. 2019, 12, 1834–1839. [Google Scholar] [CrossRef]
- Selverston, S.; Savinell, R.F.; Wainright, J.S. Zinc-Iron Flow Batteries with Common Electrolyte. J. Electrochem. Soc. 2017, 164, A1069–A1075. [Google Scholar] [CrossRef]
- Liu, J.; Xu, C.; Chen, Z.; Ni, S.; Shen, Z.X. Progress in aqueous rechargeable batteries. Green Energy Environ. 2018, 3, 20–41. [Google Scholar] [CrossRef]
- Ao, H.; Zhao, Y.; Zhou, J.; Cai, W.; Zhang, X.; Zhu, Y.; Qian, Y. Rechargeable aqueous hybrid ion batteries: Developments and prospects. J. Mater. Chem. A 2019, 7, 18708–18734. [Google Scholar] [CrossRef]
- Van Genderen, E.; Wildnauer, M.; Santero, N.; Sidi, N. A global life cycle assessment for primary zinc production. Int. J. Life Cycle Assess. 2016, 21, 1580–1593. [Google Scholar] [CrossRef]
- Li, H.; Ma, L.; Han, C.; Wang, Z.; Liu, Z.; Tang, Z.; Zhi, C. Advanced rechargeable zinc-based batteries: Recent progress and future perspectives. Nano Energy 2019, 62, 550–587. [Google Scholar] [CrossRef]
- Wang, B.; Han, Y.; Wang, X.; Bahlawane, N.; Pan, H.; Yan, M.; Jiang, Y. Prussian Blue Analogs for Rechargeable Batteries. iScience 2018, 3, 110–133. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Zhou, X.; Liu, Z. Towards High-Voltage Aqueous Metal-Ion Batteries Beyond 1.5 V: The Zinc/Zinc Hexacyanoferrate System. Adv. Energy Mater. 2015, 5, 1400930. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Zhou, X.; Liu, Z. Morphology-Dependent Electrochemical Performance of Zinc Hexacyanoferrate Cathode for Zinc-Ion Battery. Sci. Rep. 2015, 5, 18263. [Google Scholar] [CrossRef]
- Tang, B.; Zhou, J.; Fang, G.; Guo, S.; Guo, X.; Shan, L.; Tang, Y.; Liang, S. Structural Modification of V2O5 as High-Performance Aqueous Zinc-Ion Battery Cathode. J. Electrochem. Soc. 2019, 166, A480–A486. [Google Scholar] [CrossRef]
- Zhou, J.; Shan, L.; Wu, Z.; Guo, X.; Fang, G.; Liang, S. Investigation of V2O5 as a low-cost rechargeable aqueous zinc ion battery cathode. Chem. Commun. 2018, 54, 4457–4460. [Google Scholar] [CrossRef]
- Xie, C.; Li, T.; Deng, C.; Song, Y.; Zhang, H.; Li, X. A highly reversible neutral zinc/manganese battery for stationary energy storage. Energy Environ. Sci. 2020, 13, 135–143. [Google Scholar] [CrossRef]
- Julien, M.C.; Mauger, A. Nanostructured MnO2 as Electrode Materials for Energy Storage. Nanomaterials 2017, 7, 396. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Lao-atiman, W.; Han, S.J.; Arpornwichanop, A.; Yonezawa, T.; Kheawhom, S. Discharge Performance of Zinc-Air Flow Batteries Under the Effects of Sodium Dodecyl Sulfate and Pluronic F-127. Sci. Rep. 2018, 8, 14909. [Google Scholar] [CrossRef] [PubMed]
- Valipour, A.; Hamnabard, N.; Meshkati, S.M.H.; Pakan, M.; Ahn, Y.-H. Effectiveness of phase- and morphology-controlled MnO2 nanomaterials derived from flower-like δ-MnO2 as alternative cathode catalyst in microbial fuel cells. Dalton Trans. 2019, 48, 5429–5443. [Google Scholar] [CrossRef] [PubMed]
- Lao-atiman, W.; Olaru, S.; Arpornwichanop, A.; Kheawhom, S. Discharge performance and dynamic behavior of refuellable zinc-air battery. Sci. Data 2019, 6, 168. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, Q.; Abraham, A.; West, P.J.; Housel, L.M.; Singh, G.; Sadique, N.; Quilty, C.D.; Wu, D.; Takeuchi, E.S.; et al. Silver-Containing α-MnO2 Nanorods: Electrochemistry in Rechargeable Aqueous Zn-MnO2 Batteries. J. Electrochem. Soc. 2019, 166, A3575–A3584. [Google Scholar] [CrossRef]
- Singh, S.; Sahoo, R.K.; Shinde, N.M.; Yun, J.M.; Mane, R.S.; Kim, K.H. Synthesis of Bi2O3-MnO2 Nanocomposite Electrode for Wide-Potential Window High Performance Supercapacitor. Energies 2019, 12, 3320. [Google Scholar] [CrossRef]
- Wu, D.; Xie, X.; Zhang, Y.; Zhang, D.; Du, W.; Zhang, X.; Wang, B. MnO2/Carbon Composites for Supercapacitor: Synthesis and Electrochemical Performance. Front. Mater. 2020, 7. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.-G.; Liu, H.; Wei, C.; Kang, F. Zinc ion stabilized MnO2 nanospheres for high capacity and long lifespan aqueous zinc-ion batteries. J. Mater. Chem. A 2019, 7, 13727–13735. [Google Scholar] [CrossRef]
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 16039. [Google Scholar] [CrossRef]
- Khamsanga, S.; Pornprasertsuk, R.; Yonezawa, T.; Mohamad, A.A.; Kheawhom, S. δ-MnO2 nanoflower/graphite cathode for rechargeable aqueous zinc ion batteries. Sci. Rep. 2019, 9, 8441. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, J.; Huang, Q.; Zheng, Z.; Hiralal, P.; Zheng, F.; Ozgit, D.; Su, S.; Chen, S.; Tan, P.-H.; et al. Flexible high energy density zinc-ion batteries enabled by binder-free MnO2/reduced graphene oxide electrode. NPJ Flex. Electron. 2018, 2, 21. [Google Scholar] [CrossRef]
- Xu, D.; Li, B.; Wei, C.; He, Y.-B.; Du, H.; Chu, X.; Qin, X.; Yang, Q.-H.; Kang, F. Preparation and Characterization of MnO2/acid-treated CNT Nanocomposites for Energy Storage with Zinc Ions. Electrochim. Acta 2014, 133, 254–261. [Google Scholar] [CrossRef]
- Jiao, T.; Yang, Q.; Wu, S.; Wang, Z.; Chen, D.; Shen, D.; Liu, B.; Cheng, J.; Li, H.; Ma, L.; et al. Binder-free hierarchical VS2 electrodes for high-performance aqueous Zn ion batteries towards commercial level mass loading. J. Mater. Chem. A 2019, 7, 16330–16338. [Google Scholar] [CrossRef]
- Yin, B.; Zhang, S.; Ke, K.; Xiong, T.; Wang, Y.; Lim, B.K.D.; Lee, W.S.V.; Wang, Z.; Xue, J. Binder-free V2O5/CNT paper electrode for high rate performance zinc ion battery. Nanoscale 2019, 11, 19723–19728. [Google Scholar] [CrossRef]
- Corpuz, R.D.; De Juan, L.M.Z.; Praserthdam, S.; Pornprasertsuk, R.; Yonezawa, T.; Nguyen, M.T.; Kheawhom, S. Annealing induced a well-ordered single crystal δ-MnO2 and its electrochemical performance in zinc-ion battery. Sci. Rep. 2019, 9, 15107. [Google Scholar] [CrossRef]
- Demir-Cakan, R.; Palacin, M.R.; Croguennec, L. Rechargeable aqueous electrolyte batteries: From univalent to multivalent cation chemistry. J. Mater. Chem. A 2019, 7, 20519–20539. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, K.; Chen, S.; Liu, Y.; Tao, Y.; Zhang, X.; Ding, Y.; Dai, S. Proton Inserted Manganese Dioxides as a Reversible Cathode for Aqueous Zn-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 319–327. [Google Scholar] [CrossRef]
- Zhang, N.; Dong, Y.; Wang, Y.; Wang, Y.; Li, J.; Xu, J.; Liu, Y.; Jiao, L.; Cheng, F. Ultrafast Rechargeable Zinc Battery Based on High-Voltage Graphite Cathode and Stable Nonaqueous Electrolyte. ACS Appl. Mater. Interfaces 2019, 11, 32978–32986. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef]
- Zhu, G.; Angell, M.; Pan, C.-J.; Lin, M.-C.; Chen, H.; Huang, C.-J.; Lin, J.; Achazi, A.J.; Kaghazchi, P.; Hwang, B.-J.; et al. Rechargeable aluminum batteries: Effects of cations in ionic liquid electrolytes. RSC Adv. 2019, 9, 11322–11330. [Google Scholar] [CrossRef]
- Kao-ian, W.; Pornprasertsuk, R.; Thamyongkit, P.; Maiyalagan, T.; Kheawhom, S. Rechargeable Zinc-Ion Battery Based on Choline Chloride-Urea Deep Eutectic Solvent. J. Electrochem. Soc. 2019, 166, A1063–A1069. [Google Scholar] [CrossRef]
- Chen, B.-R.; Sun, W.; Kitchaev, D.A.; Mangum, J.S.; Thampy, V.; Garten, L.M.; Ginley, D.S.; Gorman, B.P.; Stone, K.H.; Ceder, G.; et al. Understanding crystallization pathways leading to manganese oxide polymorph formation. Nat. Commun. 2018, 9, 2553. [Google Scholar] [CrossRef]
- Qu, Q.; Zhang, P.; Wang, B.; Chen, Y.; Tian, S.; Wu, Y.; Holze, R. Electrochemical Performance of MnO2 Nanorods in Neutral Aqueous Electrolytes as a Cathode for Asymmetric Supercapacitors. J. Phys. Chem. C 2009, 113, 14020–14027. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive Contributions to Electrochemical Energy Storage in TiO2 (Anatase) Nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, J.; Hu, Z.; Li, J.; Li, J.; Zhang, Y.; Wang, C.; Cui, G. Long-life and deeply rechargeable aqueous Zn anodes enabled by a multifunctional brightener-inspired interphase. Energy Environ. Sci. 2019, 12, 1938–1949. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Y. Recent Progress on Zinc-Ion Rechargeable Batteries. Nano-Micro Lett. 2019, 11, 90. [Google Scholar] [CrossRef]
- Zhang, N.; Cheng, F.; Liu, J.; Wang, L.; Long, X.; Liu, X.; Li, F.; Chen, J. Rechargeable aqueous zinc-manganese dioxide batteries with high energy and power densities. Nat. Commun. 2017, 8, 405. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corpuz, R.D.; De Juan-Corpuz, L.M.; Nguyen, M.T.; Yonezawa, T.; Wu, H.-L.; Somwangthanaroj, A.; Kheawhom, S. Binder-Free α-MnO2 Nanowires on Carbon Cloth as Cathode Material for Zinc-Ion Batteries. Int. J. Mol. Sci. 2020, 21, 3113. https://doi.org/10.3390/ijms21093113
Corpuz RD, De Juan-Corpuz LM, Nguyen MT, Yonezawa T, Wu H-L, Somwangthanaroj A, Kheawhom S. Binder-Free α-MnO2 Nanowires on Carbon Cloth as Cathode Material for Zinc-Ion Batteries. International Journal of Molecular Sciences. 2020; 21(9):3113. https://doi.org/10.3390/ijms21093113
Chicago/Turabian StyleCorpuz, Ryan Dula, Lyn Marie De Juan-Corpuz, Mai Thanh Nguyen, Tetsu Yonezawa, Heng-Liang Wu, Anongnat Somwangthanaroj, and Soorathep Kheawhom. 2020. "Binder-Free α-MnO2 Nanowires on Carbon Cloth as Cathode Material for Zinc-Ion Batteries" International Journal of Molecular Sciences 21, no. 9: 3113. https://doi.org/10.3390/ijms21093113
APA StyleCorpuz, R. D., De Juan-Corpuz, L. M., Nguyen, M. T., Yonezawa, T., Wu, H.-L., Somwangthanaroj, A., & Kheawhom, S. (2020). Binder-Free α-MnO2 Nanowires on Carbon Cloth as Cathode Material for Zinc-Ion Batteries. International Journal of Molecular Sciences, 21(9), 3113. https://doi.org/10.3390/ijms21093113







