Abstract
Benzodiazepines (BZDs) are widely used in patients of all ages. Unlike adults, neonatal animals treated with BZDs exhibit a variety of behavioral deficits later in life; however, the mechanisms underlying these deficits are poorly understood. This study aims to examine whether administration of clonazepam (CZP; 1 mg/kg/day) in 7–11-day-old rats affects Gama aminobutyric acid (GABA)ergic receptors in both the short and long terms. Using RT-PCR and quantitative autoradiography, we examined the expression of the selected GABAA receptor subunits (α1, α2, α4, γ2, and δ) and the GABAB B2 subunit, and GABAA, benzodiazepine, and GABAB receptor binding 48 h, 1 week, and 2 months after treatment discontinuation. Within one week after CZP cessation, the expression of the α2 subunit was upregulated, whereas that of the δ subunit was downregulated in both the hippocampus and cortex. In the hippocampus, the α4 subunit was downregulated after the 2-month interval. Changes in receptor binding were highly dependent on the receptor type, the interval after treatment cessation, and the brain structure. GABAA receptor binding was increased in almost all of the brain structures after the 48-h interval. BZD-binding was decreased in many brain structures involved in the neuronal networks associated with emotional behavior, anxiety, and cognitive functions after the 2-month interval. Binding of the GABAB receptors changed depending on the interval and brain structure. Overall, the described changes may affect both synaptic development and functioning and may potentially cause behavioral impairment.
1. Introduction
Gama aminobutyric acid (GABA) is the main inhibitory neurotransmitter in the adult central nervous system. The role of the GABAergic system in the developing brain appears to differ substantially from that in the adult central nervous system (CNS). During early development, GABA exerts an important neurotrophic function and is implicated in many neurodevelopmental processes (for review, see Reference [1]).
The effects of GABA are mediated by two major types of receptors—the ionotropic GABAA and the metabotropic GABAB receptors. In the adult brain, GABA acts primarily through hyperpolarizing GABAA receptors, ligand-gated Cl- channels. Each receptor comprises three to five different subunits α, β, γ, and δ in heteropentameric structure. Specific GABAA receptor subtypes, defined by the subunit composition, differ by their functional properties, pharmacological sensitivity, location (synaptic vs. extrasynaptic), and distribution in the brain (for review, see Reference [2]). In contrast, metabotropic GABAB receptors are composed of two subunits, GABAB1 and GABAB2, and they are found both pre- and postsynaptically [3]. The subunit composition, distribution, and properties of GABA receptors at early development differ markedly from those expressed in the adult brain [4].
During early development, GABA is essential for a wide spectrum of developmental phenomena, including cell proliferation and migration, synaptic formation and plasticity, neuronal maturation, and network formation [5]. For this reason, neurodevelopmental disturbances of the GABAergic system have been associated with neuropsychiatric disorders and behavioral dysfunctions. The finding that GABA has critical functions in brain development, in particular during the late embryonic and neonatal period, raises questions regarding the safety and possible undesirable effects of GABAergic drugs that have, for decades, been commonly used as anesthetics, sedatives, and anticonvulsants in patients of all age groups.
Benzodiazepines (BZDs) exert their effects via modulation of the GABAA receptor properties. They have multiple clinical uses, including therapy of anxiety, insomnia, muscle spasm, alcohol withdrawal, and seizures in patients of all age groups—including neonates, children, and pregnant women [6]; thus, the possible undesirable effects of BZDs on the developing brain are of great concern. Studies conducted using laboratory animals have shown that pre- and/or postnatal BZD exposure has short- and long-lasting consequences on brain chemistry and behavior and that it leads to increased apoptosis and suppresses neurogenesis and synaptogenesis (for review, see References [7,8,9,10]). Also, in humans, prenatal exposure to BZDs is associated with increased risk of behavioral problems later in life [11].
Previous studies have demonstrated that the effects of BZDs are stable during development and that these drugs exert strong anticonvulsant and anxiolytic effects even in neonatal animals [12,13]. Neonatal rats also develop side effects similar to those reported in adults, such as sedation [14]. However, unlike in adults, some of the effects of early BZD exposure can outlast the drug itself. Neonatal animals treated for a short period of time with clonazepam (CZP) were shown to exhibit a variety of behavioral deficits during adolescence and adulthood, including deficits in learning, emotions, and social behavior [15,16], and similar alterations were reported also after perinatal exposure to other benzodiazepines [17,18,19,20]. Infantile rats have also been shown to develop signs of BZD withdrawal after very short exposure [12,14,21]. Irrespective of the obvious clinical importance of BZDs, studies regarding the long-term effects of early benzodiazepine exposure are sparse and the mechanisms underlying the associated changes are still poorly described.
In our previous study [22], we described changes in the glutamatergic receptors after short-term administration of CZP to perinatal rats. The expressions of NMDA (N-methyl D aspartate) and AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) glutamate receptor subunit mRNAs and NMDA receptor binding were assessed in three intervals after therapy cessation—48 h, 1 week, and 2 months. Using the remaining tissue, the present study aimed to characterize the changes of the GABAA and GABAB receptors and analyzed the expressions of α1, α2, α4 γ2, and δ GABAA receptor subunit mRNA; GABAB2 receptor subunit mRNA; and BZD, GABAA, and GABAB receptor binding.
2. Results
2.1. The Effect of CZP Administration on GABAA and GABAB R2 Receptor (Rp) Subunit mRNA Expression
Early exposure to CZP resulted in moderate changes only in GABAA Rp subunit mRNA expression. Significant differences between the control and CZP-treated animals were found only in short intervals after CZP cessation. In the cortex, early CZP exposure caused a significant decrease of total mRNA for all of the evaluated GABAA Rp subunits 48 h after treatment cessation (t = 2.526 df = 92; p = 0.0132). In the hippocampus, total mRNA was not affected at any interval. Interestingly, the expression of GABAA Rp δ subunit mRNA was downregulated in the cortex as well as in the hippocampus two days after the end of therapy (Figure 1A and Table A1). The receptors containing the δ subunit were insensitive to classical BZDs, suggesting that changes in δ subunit expression are not due to a direct interaction between the receptors and CZP. The role of the δ subunit-containing receptors in the regulation of neuronal excitability, synaptic plasticity, and network synchrony [23,24] predetermines their important function in the maturation of brain circuitry. Therefore, even transient alterations of these receptors during the critical developmental period may result in enduring changes in brain structure.
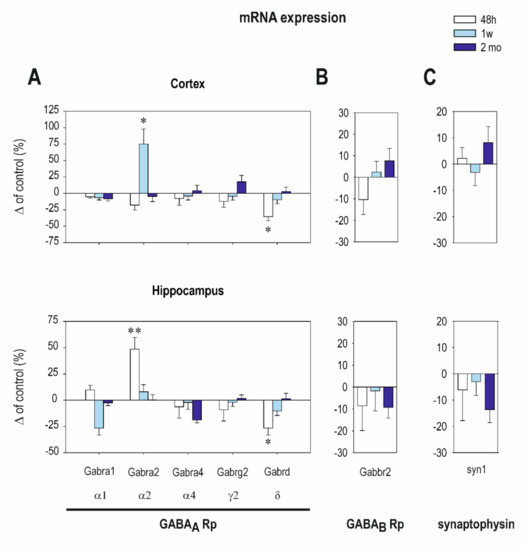
Figure 1.
The transcription levels of the Gama aminobutyric acid A (GABAA) receptor subunits (A) and the GABAB receptor subunit (B) in the cortex (on the top) and in the hippocampus (on the bottom) at three intervals after clonazepam (CZP) cessation (48 h, 1 week, and 2 months—legend at the top). (C) The graphs on the right demonstrate the transcription levels of synaptophysin at the same intervals. The mRNA levels were determined using quantitative RT-PCR, and values were converted to a percentage of the control values considered as the baseline (zero) levels. Each experimental group consisted of 10 animals. Data were analyzed using GraphPad Prism 7 (GraphPad Software, United States) software. Using the D’Agostino–Pearson normality test, all data sets were first analyzed to determine whether the values were derived from a Gaussian distribution. Outliers were identified with the ROUT test (Q = 1%). The differences between age-matched controls and CZP-treated animals were analyzed using ordinary one-way ANOVA with post hoc Sidak’s multiple comparison test, and a p-value < 0.05 was required for significance. Data are expressed as the means ± SEM and plotted as a % of the controls. Asterisks denote significant differences from the controls (* p < 0.05; ** p < 0.01).
In addition to the changes of δ subunit expression, early CZP exposure significantly upregulated α2 subunit mRNA expression in the hippocampus 48 h after the end of therapy as well as in the cortex 1 week later (Figure 1A and Table A1). The δ and α2 subunits exhibit opposing developmental profiles; whereas δ subunit mRNA appears gradually during postnatal development with a dramatic increase in expression in the second week after birth, receptors containing the α2 subunit disappear from many areas as maturation progresses and are replaced with the α1 subunit containing assembly [25]. Therefore, the CZP-induced shifts in δ and α2 subunit expressions may be due to developmental delay. Such alterations in GABAA receptor development can affect the formation of brain circuitry and can participate in enduring alterations of the brain’s structure and functions.
Administration of CZP did not affect the expression of GABAB R2 mRNA receptor subunit at any interval used in this study (Figure 1B and Table A1). The expression of synaptophysin mRNA tended to be reduced by approximately 15% in the hippocampus 2 months after the end of treatment (Figure 1C and Table A1), but difference between the treated animals and controls was not significant.
2.2. Effect of CZP Administration on [3H] Muscimol Binding and [3H] Flunitrazepam Binding
Our results revealed striking differences in the effects of early CZP exposure on [3H] muscimol and [3H] flunitrazepam binding. Muscimol is regarded as a universal nonselective GABAA-site agonist with exceptionally high sensitivity to the δ subunit-containing GABAA receptors [26,27]. On the other hand, [3H] flunitrazepam binds only to a specific, benzodiazepine-sensitive subpopulation of the GABAA receptors. In our study, early CZP exposure resulted in only a transient and short-lasting increase of [3H] muscimol binding (Figure 2 and Table A2). In almost all of the measured brain areas, binding was significantly elevated 48 h after treatment cessation and returned to control levels thereafter. Interestingly, a marked increase of [3H] muscimol binding was not accompanied by a parallel increase of [3H] flunitrazepam binding (Figure 3 and Table A3). In contrast, binding to the BZD receptors tended to be lower in the CZP-exposed animals 48 h after the end of treatment in most of the cortical, amygdalar, and thalamic areas; a significant decrease was, however, found only in the ventromedial thalamus. The discrepancy between [3H] muscimol and [3H] flunitrazepam binding is surprising and is not easy to explain. It may be related to a reduction of the coupling between the GABA site and the BZD site, which has been documented before [28], or by different receptor selectivity of both ligands.
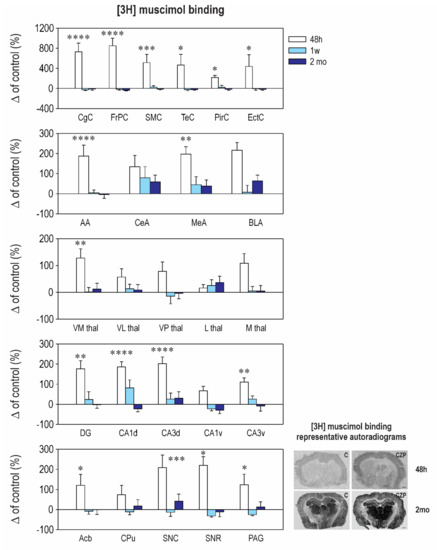
Figure 2.
Relative changes in [3H] muscimol binding to GABAA receptors (means ± SEM): The binding in the control animals is considered as the baseline (zero) level. From the top to the bottom: cortical structures, amygdalar structures, thalamic structures, and hippocampal structures. On the bottom: Ncl. accumbens (Acb), caudate putamen (CPu), substantia nigra pars compacta (SNC), substantia nigra pars reticulate (SNR), and periaqueductal gray (PAG). [3H] Muscimol binding was assessed at three different intervals (48 h, 1 week, and 2 months—legend at the top) after CZP cessation. Abbreviations: CgC, cingulate cortex; FrPc, frontoparietal cortex; SMC, sensorimotor cortex; TeC, temporal cortex; PirC, piriform cortex; EctC, entorhinal cortex; AA, anterior amygdala; CeA, central amygdala; MeA, medial amygdala; BLA, basolateral amygdala; VN thal, ventromedial thalamus; VL thal, ventrolateral thalamus; VP thal, ventroposterior thalamus; L thal, lateral thalamus; M thal, medial thalamus; DG-, dentate gyrus of the hippocampus; CA1, CA1 subfield of the hippocampus; CA3, CA3 subfield of the hippocampus; d, dorsal; v, ventral. Each experimental group consisted of 5 animals. Asterisks denote significant differences from the controls (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001). Scale bar = 1 mm. For other details, see Figure 1.
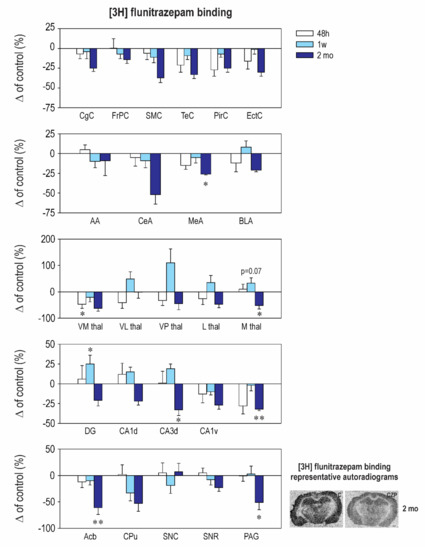
Figure 3.
Relative changes in [3H] flunitrazepam binding to Benzodiazepine (BZD) receptors (means ± SEM). Panels on the right: Representative autoradiograms showing decreased binding to BZD Rp labeled with [3H] flunitrazepam. Sections were taken at the level of the hippocampus 2 months after the end of therapy. Details as in Figure 1 and Figure 2. Asterisks denote significant differences from the controls (* p < 0.05; ** p < 0.01). Scale bar = 1 mm.
[3H] muscimol binding quickly returned back to control levels, and no differences between the control and CZP animals were observed at later points (Figure 2 and Table A2). By contrast, alterations in [3H] flunitrazepam binding caused by early CZP exposure were delayed and became evident 2 months after the end of therapy. In most of the cortical, amygdalar, and hippocampal areas measured in our study, [3H] flunitrazepam binding tended to be lower by 20–50% at this interval, but differences between the control and CZP-treated animals reached a significant level only in the medial amygdala, the nucleus (ncl.) accumbens, the dorsal hippocampus, and the periaqeductal grey (Figure 3 and Table A3). All of these structures are part of the neuronal networks associated with emotional behavior, anxiety, and cognitive functions, i.e., with the behavioral domains that are permanently affected by administration of BZDs during critical developmental periods. Our findings support the hypothesis that, in the immature brain, drug-induced changes can be delayed and can become detectable later in life (for review, see Reference [29]).
Another discrepancy occurred between the downregulated expression of the δ subunit seen 48 h after treatment cessation and an increase in [3H] muscimol binding at the same interval. As mentioned above, muscimol exhibits high sensitivity to the δ subunit-containing GABAA receptors [27]; thus, one can expect a decrease of binding due to reduction of the number of receptors. In our study, we assessed only mRNA expression and not the protein levels, and changes in messages do not necessarily result in changes in protein levels or vice versa. The functional significance of δ subunit mRNA expression must therefore be interpreted with caution.
Panels on the right: Representative autoradiograms illustrating the distribution of binding to GABAA receptors labeled with [3H] muscimol in the brain sections at the level of the striatum 48 h (on the top) and 2 months (on the bottom) after treatment withdrawal of rats treated with vehicle (C—left panel) and clonazepam (CZP—right panels). An increase in binding is obvious in most of the brain structures 48 h after therapy discontinuation; high binding appears as darker areas.
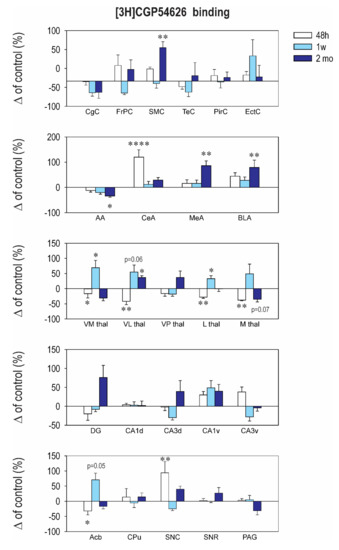
2.3. Effect of CZP Administration on [3H] CGP54626 Binding
The pattern of changes of [3H] CGP54626 binding, used to assess the GABAB receptors, induced by early CZP exposure was highly dependent on brain structure and the interval after CZP cessation (Figure 4 and Table A4). Long-term, early CZP exposure resulted in an increase of [3H] CGP54626 in the sensorimotor cortex, the ventroposterior thalamus, and the medial and basolateral amygdala, whereas in the anterior amygdala, binding was reduced compared to the control. Two days after the end of administration, a significant increase in binding was observed in the central amygdala and the substantia nigra pars compacta, whereas in the ncl. accumbens and the ventrolateral and lateral thalamus, binding was decreased. One week later, an increase in binding occurred in several areas of the thalamus (ventromedial, lateral, and ventrolateral) (Figure 4 and Table A4). The GABAB receptors were critically involved in the regulation of synaptic activity. Although no physical contact or complex formation was reported, there is functional crosstalk between the GABAB receptors and the ionotropic glutamate receptors, with both the NMDA and AMPA receptors involved in GABAB receptor endocytosis, trafficking, degradation, and phosphorylation. As reported before, early CZP exposure leads to both short- and long-term changes of the NMDA and AMPA receptors, which can be partially responsible for the changes observed in the GABAB receptors in our study.
Taken together, the results demonstrate that early CZP exposure leads to both transient and permanent changes in the GABAA and GABAB receptors and, as reported previously, also in the glutamatergic receptors [22]. Alterations of both the GABA and glutamate receptors occur shortly after the end of CZP administration, i.e., during a very sensitive developmental period of brain growth spurt [30,31]. We hypothesize that these changes can underlie disturbances in brain network development and can result in the loss of neurons or synapses [10,32], thus contributing to long-term enduring changes in the structure or number of receptors, which consequently results in behavioral deficits.
3. Discussion
Our data demonstrate that exposure to CZP during the early stages of postnatal development affects the BZD/GABAA and GABAB receptors, both in the short and long terms after drug cessation. In adults exposed to CZP during the neonatal period, alterations were seen in BZDs and GABAB binding. The exact pattern of change was dependent on the individual receptor and/or the receptor subunit, brain structure, and interval after CZP discontinuation.
Interestingly, CZP administration affected the expression of individual GABAA Rp subunit mRNAs only moderately and only within the first week after CZP cessation (Figure 1 and Table A1). In addition, the pattern of these changes differed considerably from those described in adults exposed chronically to BZDs. It has to be emphasized, however, that studies regarding the effects of BZD exposure on GABAA receptor subunit composition performed in adult animals have brought about inconsistent results. The pattern of change seems to be highly dependent on the BZD used; the duration of exposure; the brain structure; and, most significantly, the time of the analysis (during treatment vs. during withdrawal) (for review, see Reference [33]). In our study, early CZP exposure resulted in overexpression of the α2 subunit within one week after treatment cessation, whereas in adult animals, the expression of α2 was not affected by chronic BZD treatment [34,35,36]. Moreover, the effects of long-term BZD administration on the expression of α1 subunit mRNA were significantly different in neonates that from those in adult rats. Depending on the timing of the analysis, α1 subunit mRNA was either upregulated or downregulated during treatment and/or the withdrawal period in adults [37,38,39], whereas in our study, the expression of the α1 subunit was not affected in any posttreatment interval or in any brain structure analyzed. Although GABA is already in abundance in the prenatal brain (for review, see Reference [40]), its function and receptor composition change during development and each receptor subunit exhibits a unique developmental profile and an age-dependent distribution [25,41,42,43,44]. The expression of receptors containing the αl subunit increases markedly throughout most of the brain during postnatal development, whereas receptors containing the α2 subunit disappear from many areas shortly after the onset of αl subunit expression [25]. This switch coincides with synaptogenesis, suggesting that the emergence of α1 subunit-containing receptors parallels the formation of neuronal circuits [44]. The upregulation of the α2 subunit seen shortly after early CZP exposure may reflect a developmental delay in the α2/α1 subunit switch and may affect the development of brain circuitry, as it occurs during a sensitive developmental period.
Significant downregulation of the δ subunit expression was seen in both the cortex and the hippocampus 48 h after the end of CZP exposure (Figure 1 and Table A1). Activation of receptors containing the δ subunit generates a persistent tonic current that profoundly affects neuronal excitability [45,46]. During early development, tonic inhibition is important in regulating the excitation/inhibition balance during hippocampal maturation [47]. In a study with transgenic mice that lacked δ GABAA Rp, Korpi and collaborators demonstrated the role of this subunit in dentate gyrus neurogenesis, suggesting that even transient alteration in δ subunit expression can affect hippocampal maturation by several mechanisms [48].
Taking these data together, we hypothesize that even limited changes in GABAA receptor composition together with altered composition of the NMDA and AMPA receptors [22] can significantly impair synaptogenesis, formation and maturation of brain circuitry, as well as synaptic plasticity later in life. This hypothesis is supported by a previously published study [32] that showed disrupted synaptic development after early exposure to antiepileptic drugs.
Interestingly, CZP exposure resulted in a marked increase of [3H] muscimol 48 h after treatment withdrawal (Figure 2 and Table A2). At the same time point, [3H] flunitrazepam binding was not affected (Figure 3 and Table A3). Thus far, there is very little experimental evidence to demonstrate that long-term BZD administration results in changes of BZD Rp binding, and the results published to date are conflicting (for review, see Reference [49]). Most studies conducted in adults chronically exposed to BZDs found no change in BZD Rp number or a decrease in receptor density after a very high dose of benzodiazepines [50]. An increase of both [3H] muscimol and [3H] flunitrazepam binding after BZD exposure was demonstrated in vitro in cell cultures [51]. However, the mechanisms responsible for the discrepancy seen in our study have to be further studied.
Whereas [3H] flunitrazepam binding was not affected in the withdrawal period, two months after treatment cessation, a decrease of BZD receptor density was observed in many cortical areas, including in both the dorsal and ventral hippocampus, the ncl. accumbens, the caudate putamen, and the periaqueductal grey (Figure 3 and Table A3). These structures are part of the neuronal networks associated with emotional behavior, anxiety, and cognitive functions (for review, see References [52,53,54,55,56]), which are impaired in animals exposed to BZDs early in life [15,16,17,18,19,20,57]. Whether the decrease of [3H] flunitrazepam binding is associated with a decreased number of neurons or synapses remains to be further studied.
Early exposure to CZP also affected [3H] CGP54626 binding to metabotropic GABAB receptors, and the changes were structure and interval dependent (Figure 4 and Table A4). GABAB receptors are located presynaptically as well as postsynaptically and play an important role in controlling synaptic activity. Released GABA can feed back into the GABAB autoreceptors located on the GABAergic terminals, can activate the GABAB receptors on a neighboring gutamatergic terminal, and can inhibit neurotransmitter release. GABA can also signal postsynaptic GABAB receptors with the potential to modulate various properties of postsynaptic transmission (for review, see Reference [58]). GABAB receptors appear to exhibit neurotrophic properties during development [59]. Data from in vivo studies are inconsistent, but the complete silencing of GABAB receptors in mice causes many behavioral alterations, such as epilepsy and hyperalgesia [60,61]. In utero knockdown of GABAB receptors alters cortical development in rats [62]. Early CZP expression affected GABAB receptor density in a structure- and interval-dependent manner. We presume that the changes seen in GABAB receptor density within the withdrawal period, i.e., during the crucial period of postnatal brain development, can participate in an impairment of the normal development of neural networks, with functional consequences occurring later in life. GABAB receptor activity was found to modulate synaptic plasticity in the adult nervous system as well as LTP and many behavioral functions (for review, see Reference [63]), and GABAB receptors have been implicated in a wide variety of neuropsychiatric disorders (for review, see Reference [64]). Therefore, the chronic changes in the GABAB receptors observed in in our study—in several amygdalar nuclei, in the sensorimotor cortex, and in the ventrolateral thalamus—can directly participate in the functional deficits described before.
To the best of our knowledge, this and our previous study [22] are the first reports showing the long-term effects of relatively short-lasting BZD exposure during the neonatal period on the GABAA, GABAB, and glutamate receptors. However, there are several limitations in our studies that have to be noted. First, not all changes found in mRNA expression have to correlate with changes in protein levels. Therefore, any interpretation of our data that suggests changes in receptor composition has to be taken with caution. Second, although our study primarily aimed to map principal receptor changes at three intervals after treatment cessation, the mechanisms underlying the observed changes remain to be clarified. Previously published data suggest, however, several possible mechanisms that can play a role in the observed alterations. Early exposure to BZDs was found to increase apoptosis and to supress neurogenesis in many brain areas [65,66,67], and pro-apoptotic drugs have been found to disrupt synaptic development [32]. All of these mechanisms are likely involved in the receptor alterations seen in both our studies mapping the effect of early CZP on the GABA and glutamate [22] receptors. We also expect that the interplay between the GABAergic and glutamatergic receptors participates substantially in long-term receptor changes because alterations of one receptor type can trigger compensatory changes in other receptor types. Third, our study does not provide any information concerning receptor changes during BZD exposure. All of the described changes were observed either during the withdrawal period or much later in adulthood.
Nevertheless, our results support the hypothesis that, in the immature brain, drug-induced changes may be incorporated as a permanent developmental alteration of the system (for review, see Reference [29]). In our study, animals were exposed to CZP and CZP withdrawal during a highly vulnerable period of development, covering the period of growth spurt and increased synaptic plasticity [30,31,64], in order to create a synaptic network and to process properly environmental stimuli. Even transient disruption of the balance between the two major neurotransmitter systems that are critically involved in synaptogenesis and in formation and maturation of neural networks may be responsible for the pathological consequences seen in our previous studies and studies by others. This should be taken into consideration in the development of new and safe drugs for pediatric patients.
4. Materials and Methods
The experiments were performed using male Wistar albino rats (n = 90). The day of birth was counted as zero (P0). Rats were housed in a controlled environment (temperature 22 ± 1 °C, humidity 50–60%, lights on 600–1800 h) with free access to food and water. Animals were weaned at P28. All procedures involving animals and their care were conducted according to the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines in compliance with national (Act No 246/1992 Coll.) and international laws and policies (EEC Council Directive 86/609, OJ L 358, 1, December 12, 1987; Guide for the Care and Use of Laboratory Animals, U.S. National Research Council, 1996). The Ethical Committee of the Czech Academy of Sciences approved the experimental protocol (Approval No. 128/2013, approval date: 23 September 2013).
4.1. Pharmacological Treatment
Clonazepam (CZP) was suspended in physiological saline with addition of Tween 80 (1 mg/5 mL of saline with one drop of Tween 80) and injected intraperitoneally in a dose of 1 mg/kg/day for 5 consecutive days starting at postnatal day 7 (P7) until P11. The selection of the used dose and the duration of administration were done according to our previous studies that demonstrated long-lasting behavioral alterations [15,16] in animals treated in the same way. Control animals received solvent instead. After injection, pups were immediately returned to their dams. Separation from mothers during drug administration never exceeded 20 min. During the drug administration, pups were kept on an electric heating pad connected to a digital thermometer at 34 ± 1 °C to compensate for the immature thermoregulation at this age [68].
Body weight was checked daily during drug administration until the end of the experiment. The difference in body weight between two consecutive days was used to assess weight gain.
4.2. Quantitative Real-Time RT-PCR
Hippocampi and sensorimotor cortices obtained from 10 animals per treatment and interval groups were immediately dissected and homogenized in RNAzol RT (Molecular Research Center). Total RNA was extracted by Direct-zol™ RNA MiniPrep (Zymo research, Irvine, CA, USA) according to the manufacturer’s instructions. Total RNA (1 µg) was converted to cDNA using the one-step SuperScript® VILO cDNA Synthesis Kit and Master Mix (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Samples of cDNA (1 μL) were amplified in 20 μL of PCR reaction mixture containing 5× HOT FIREPol® Probe qPCR Mix Plus (Baria, Prague, Czech Repubic) plus TaqMan probes (Life technologies, Carlsbad, CA, USA; Table 1). All qPCR reactions were performed in duplicate in a LightCycler® 480 Instrument (Roche Life Science, Indianapolis, IN, USA) using the following temperature profile: initial denaturation at 95 °C for 15 min, followed by 60 cycles consisting of denaturation at 95 °C for 18 s and annealing/elongation at 60 °C for 60 s. The mean of the crossing point (Cp) obtained from qPCR was normalized to the level of the housekeeping gene Ppia (Cyclophilin A) and then used for analysis of relative gene expression by the ΔΔCT method [69] as described in Kubová et al. [22]. Mean values of the controls were normalized to zero, and values of the CZP-treated animals were plotted as percent difference from the controls (i.e., zero). For statistics, all of the values for the controls were counted as percent distribution around the mean and compared with the treatment groups. The reproducibility of the assays was evaluated using calculation of the coefficient of variation according to following formula: % CV = σ/µ (σ = SD and µ = mean value). The chosen criterion was %CV < 10.

Table 1.
List of TaqMan probes used.
4.3. Receptor Binding
Rats (5 animals per treatment and interval group) were sacrificed under light ether anesthesia by decapitation, and the brains were rapidly frozen in pulverized dry ice and stored at –70 °C until processing. Serial sections (1 of 5) through the entire brain were thaw-mounted on gelatin-coated slides and stored at –70 °C until the day of incubation. Serial and parallel sections were produced from each brain for subsequent autoradiography procedures. The brains of the CZP-treated and age-matched control rats were always obtained and examined simultaneously.
Quantitative autoradiography: The experiments were performed as described previously [70]. Brain sections were removed from the freezer, dried in a stream of cool air, and immediately washed to eliminate endogenous ligands. Then, sections were incubated in a solution with the specific ligand labeled with tritium ([3H]) in the presence or absence of a non-labeled specific ligand. Specific conditions for individual ligands are summarized in Table 2. The specific binding was established from the difference of values between both experimental conditions. Incubation was concluded with two consecutive washes in buffer solution and finally with cold distilled water for 2 s. Sections were quickly dried in a mild steam of cold air. Thereafter, they were arranged in X-ray cassettes with 3H standards (Amersham) and exposed to [3H]-sensitive film (Kodak MR) at 22 °C. Each film allowed the simultaneous exposition of 21 slides plus one standard, i.e., each film included sections from 7 animals. Each film contained sections from CZP animals and age-matched controls. All slides were processed in one autoradiography assay in order to avoid variability of the experimental conditions.

Table 2.
Conditions for the autoradiography experiments.
The film was developed at 18–20 °C using the Kodak D19 developer and fast fixer solutions. In every animal, the optical density was assessed as a mean of 10 measurements that were done in at least three parallel sections for each measured structure. The mean value was calculated and used for statistical evaluation. Optical densities were determined using JAVA Jandel image analysis software. We used tritium standards previously calibrated to brain homogenates with known protein concentrations to allow a transformation of gray values into total binding per milligram of protein as follows: (a) The optical density readings of the standards were used to construct a standard curve to determine tissue radioactivity values for the accompanying tissue sections (dpm/mm2). The optical density readings of the standards were used to construct a standard curve to determine tissue radioactivity values for the accompanying tissue sections (dpm/mm2). (b) Then, these values (dpm/mm2) were converted to fmol/mg of protein based on the specific activity of each [3H] ligand and tissue thickness (20 μm).
4.4. Statistics
Sample size was determined in advance based on previous experience and following the principles of the three Rs (replacement, reduction, and refinement; https://www.nc3rs.org.uk/the-3rs). At the beginning of the experiment, individual animals were randomly allocated to a particular treatment group.
All efforts were made to minimize the number of animals used and their suffering. Before the beginning of the experiment, a simple randomization was used to assign each rat to a particular treatment group. Data analysis was done blind to the treatment. The ages and time points for each group consisted of five to seven animals for the binding study and 10 animals per group used for real-time PCR. Data were analyzed using GraphPad Prism 7 (GraphPad Software, United States) software. Using the D’Agostino–Pearson normality test, all data sets were first analyzed to determine whether the values were derived from a Gaussian distribution. Outliers were identified with the ROUT (Robust regression and Outlier removal) test (Q = 1%). The differences between the age-matched controls and the CZP-treated animals were analyzed using ordinary one-way ANOVA with post hoc Sidak’s multiple comparison test, and a p-value < 0.05 was required for significance. Data are expressed as the means ± SEM and plotted as % of controls.
5. Conclusions
Our results support the hypothesis that, in the immature brain, drug-induced changes may be incorporated as a permanent developmental alteration of the system (for review, see Reference [29]). In our study, animals were exposed to CZP and CZP withdrawal during a highly vulnerable period of development, covering the period of growth spurt and increased synaptic plasticity [30,31,64], in order to create a synaptic network and to process properly environmental stimuli. Even transient disruption of the balance between the two major neurotransmitter systems—glutamatergic and GABAergic—that are critically involved in the synaptogenesis and in formation and maturation of neural networks may be responsible for the pathological consequences seen in our previous studies and studies by others. This should be taken into consideration in the development of new and safe drugs for pediatric patients.
Author Contributions
H.K., P.M., L.R., and Z.B. conceived and designed the experiments. H.K., L.R., Z.B., S.M., P.M., and D.P. selected and validated the used methodology, performed the experiments, and analyzed the data. H.K., Z.B., and P.M. wrote the original draft of the manuscript. Z.B., L.R., and P.M. reviewed and edited the original draft. All authors read and agreed to the published version of the manuscript.
Funding
This research was supported by the European Regional Development Fund—Projects “PharmaBrain” No. CZ.CZ.02.1.01/0.0/0.0/16_025/0007444 (H.K., P.M., and Z.B.) and 19-11931S of the Czech Science Foundation (H.K.); by support for long-term conceptual development of research organization RVO: 67985823; and by the Institutional Project of the Ministry of Education, Youth, and Sport of the Czech Republic (SVV-260434/2019) (S.M. and D.P.).
Acknowledgments
The authors gratefully acknowledge the expert technical help of Blanka Čejková and Irinka Necheva.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| DOAJ | Directory of open access journals |
| TLA | Three-letter acronym |
| LD | Linear dichroism |
Appendix
Table A1 demonstrates the relative mRNA abundance measured by qPCR of GABAA, synaptophysin, and GABAB receptor subunits in the cortex and hippocampus of control and clonazepam (CZP)-treated rats. Each treatment and interval group consisted of 10 animals. The differences between the control and CZP-treated animals were analyzed using ordinary one-way ANOVA with post hoc Sidak’s multiple comparison test, and a p-value < 0.05 was required for significance. Data presented as mean ± SEM. ⬇, significant decrease of binding compared to age-matched controls; ⬆, significant increase of binding compared to age-matched controls.

Table A1.
The relative mRNA abundance of synaptophysine and GABAA and GABAB receptor subuntis.
Table A1.
The relative mRNA abundance of synaptophysine and GABAA and GABAB receptor subuntis.
| Cortex | Interval 48 h Mean ± SEM Control CZP | Interval 1 Week Mean ± SEM Control CZP | Interval 2 Months Mean ± SEM Control CZP | |||
|---|---|---|---|---|---|---|
| Gabra1 (α1) | 16.3 ± 0.7 | 15.3 ± 0.6 | 24.2 ± 1.7 | 22.7 ± 0.9 | 27.3 ± 2.4 | 26.6 ± 1.6 |
| Gabra2 (α2) | 11.7 ± 1.3 | 10.7 ± 1.1 | 6.6 ± 1.2 | ⬆ 11.1 ± 1.1 t = 2.705, df= 13, p = 0.018 | 14.5 ± 1.8 | 13.8 ± 1.3 |
| Gabra4 (α4) | 2.3 ± 0.3 | 2.2 ± 0.2 | 2.8 ± 0.3 | 2.7 ± 0.3 | 3.2 ± 0.3 | 3.3 ± 0.2 |
| Gabrg2 (γ2) | 3.4 ± 0.4 | 3.08 ± 0.32 | 3.5 ± 0.4 | 3.4 ± 0.3 | 5.5 ± 0.506 | 6.2 ± 0.3 |
| Gabrd (δ) | 0.14 ± 0.01 | ⬇ 0.06 ± 0.0 t = 2.196, df = 17, p = 0.0423 | 0.14 ± 0.02 | 0.13 ± 0.01 | 0.24 ± 0.02 | 0.25 ± 0.01 |
| Synaptophysin | 3.03 ± 0.27 | 3.400 ± 0.1 | 3.8 ± 0.4 | 3.8 ± 0.4 | 3.7 ± 0.27 | 4.0 ± 0.1 |
| Gabbr2 | 2.1 ± 0.1 | 1.8 ± 0.1 | 1.9 ± 0.2 | 2.02 ± 0.2 | 2.1 ± 0.2 | 2.3 ± 0.1 |
| Hippocampus | ||||||
| Gabra1 (α1) | 7.8 ± 0.9 | 8.6 ± 0.9 | 10.0 ± 1.1 | 9.6 ± 0.6 | 12.2 ± 1.0 | 11.2 ± 0.9 |
| Gabra2 (α2) | 17.2 ± 1.3 | ⬆ 26.1 ± 2.6 t = 2.956, df = 15, p = 0.0098 | 29.7 ± 4.0 | 27.4 ± 3.9 | 36.3 ± 4.7 | 37.8 ± 5.7 |
| Gabra4 (α4) | 2.1 ± 0.5 | 1.7 ± 0.4 | 3.2 ± 0.8 | 3.2 ± 0.8 | 9.2 ± 1.42 | 4.267 ± 1.1 |
| Gabrg2 (γ2) | 9.9 ± 1.1 | 7.8 ± 0.6 | 13.5 ± 1.6 | 12.6 ± 1.6 | 19.0 ± 2.2 | 19.50 ± 2.3 |
| Gabrd (δ) | 0.1 ± 0.02 | ⬇ 0.064 ± 0.01 t = 2.129, df = 16, p = 0.0435 | 0.2 ± 0.03 | 0.13 ± 0.03 | 0.3 ± 0.06 | 0.28 ± 0.06 |
| Synaptophysin | 45.0 ± 9.7 | 31.4 ± 5.1 | 69.0 ± 15.2 | 65.0 ± 16.9 | 103.2 ± 3.0 | 86.8 ± 6.2 |
| Gabbr2 | 3.3 ± 0.2 | 3.04 ± 0.4 | 3.9 ± 0.2 | 3.9 ± 0.4 | 4.7 ± 0.1 | 4.2 ± 0.2 |

Table A2.
[3H] muscimol binding to GABAA receptors
Table A2.
[3H] muscimol binding to GABAA receptors
| Interval 48 h Mean ± SEM | Interval 1 week Mean ± SEM | Interval 2 months Mean ± SEM | ||||
|---|---|---|---|---|---|---|
| Control | CZP | Control | CZP | Control | CZP | |
| Cingulate Cx. | 50.1 ± 8.6 | ⬆ 416.0 ± 86.8 t = 6.921, df = 35, p < 0.0001 | 120.0 ± 35.3 | 88.3 ± 18.8 | 67.8 ± 14.8 | 60.0 ± 14.1 |
| Frontoparital Cx. | 55.8 ± 10.4 | ⬆ 529.0 ± 84.3 t = 8.006, df = 35, p < 0.0001 | 152.0 ± 49.6 | 127.3 ± 32.9 | 93.8 ± 15.7 | 59.8 ± 12.3 |
| Sensorimotor Cx. | 52.8 ± 12.0 | ⬆ 324.0 ± 88.0 t = 4.289, df = 35, p = 0.0004 | 151.1 ± 49.0 | 189.0 ± 42.4 | 72.1 ± 14.6 | 62.4 ± 9.9 |
| Temporal Cx. | 50.0 ± 10.5 | ⬆ 283.2 ± 105.0 t = 2.901, df = 35, p = 0.0191 | 211.7 ± 75.3 | 165.9 ± 48.7 | 76.5 ± 23.7 | 59.4 ± 11.3 |
| Piriform Cx. | 34.2 ± 5.5 | ⬆ 109.0 ± 14.2 t = 3.036, df = 35, p = 0.0135 | 66.4 ± 18.5 | 81.8 ± 26.5 | 52.3 ± 12 | 42.2 ± 8.3 |
| Enthorinal Cx. | 34.5 ± 2.8 | ⬆ 104 ± 7.4 t = 3.036, df = 35, p = 0.0134 | 51.7 ± 19.6 | 37 ± 6.1 | 52.3 ± 16.1 | 43.2 ± 10.0 |
| Anterior AMG | 28.8 ± 4.6 | ⬆ 82.8 ± 15.8 t = 4.756, df = 36, p < 0.0001 | 33.2 ± 5.3 | 34.5 ± 5.0 | 43.6 ± 9.1 | 42.1 ± 8.1 |
| Central AMG | 25.2 ± 2.5 | 79.0 ± 19.0 | 32.7 ± 6.6 | 58.5 ± 18.2 | 27.0 ± 8.4 | 43.0 ± 8.8 |
| Medial AMG | 34.0 ± 3.1 | ⬆ 101.0 ± 12.0 t = 3.688, df = 35, p = 0.0023 | 49.2 ± 10.3 | 71.3 ± 19.0 | 35.5 ± 5.8 | 49.1 ± 10.8 |
| Basolateral AMG | 40.1 ± 11.0 | 126.8 ± 15.0 | 110.3 ± 37.6 | 119.6 ± 37.6 | 36.3 ± 7.5 | 59.4 ± 10 |
| N. Accumbens | 25.7 ± 3.9 | ⬆ 56.8 ± 13.0 t = 2.791, df = 35, p = 0.0252 | 22.0 ± 2.7 | 20.2 ± 3.3 | 30.1 ± 12.0 | 30.0 ± 7.0 |
| CPU | 31.0 ± 5.3 | 53.8 ± 14.0 | 20.4 ± 2.5 | 18.2 ± 3.0 | 29.3 ± 11.0 | 34.7 ± 9.0 |
| Ventromedial TH | 28.0 ± 4.1 | ⬆ 63.8 ± 9.0 t = 3.09, df = 35, p = 0.0117 | 37.4 ± 8.8 | 37.8 ± 6.0 | 44.5 ± 8.5 | 49.7 ± 9.6 |
| Ventrolateral TH | 24.1 ± 4.2 | 37.8 ± 7.0 | 27.0 ± 5.7 | 30.6 ± 4.5 | 46.3 ± 6.6 | 50.5 ± 9.4 |
| Ventralpost. TH | 34.7 ± 4.7 | 61.6 ± 12.0 | 137.4 ± 56.1 | 118.3 ± 39.0 | 63.6 ± 4.2 | 61.4 ± 13 |
| Lateral TH | 32.4 ± 5.9 | 37.6 ± 4.0 | 27.8 ± 4.9 | 34.8 ± 6.2 | 43.5 ± 10.6 | 59.0 ± 10.0 |
| Medial TH | 19.7 ± 2.8 | 41.0 ± 7.1 | 27.5 ± 4.8 | 29.0 ± 4.7 | 46.1 ± 7.9 | 48.4 ± 9.4 |
| Dentate Gyrus | 31.8 ± 2.8 | ⬆ 106.0 ± 15.9 t = 3.671, df = 35, p = 0.0024 | 47.1 ± 7.0 | 58.2 ± 18 | 49.6 ± 11.0 | 48.7 ± 8.7 |
| CA1 Dorsal | 29.4 ± 2.5 | ⬆ 84.1 ± 7.7 t = 5.424, df = 35, p < 0.0001 | 23.8 ± 2.6 | 43.3 ± 9.3 | 68.3 ± 2.9 | 52.7 ± 9.7 |
| CA2 Dorsal | 29.2 ± 5.1 | ⬆ 79.0 ± 14.0 t = 3.941, df = 35, p = 0.0011 | 26.2 ± 5.2 | 35.8 ± 6.8 | 36.1 ± 10.6 | 39.1 ± 9.3 |
| CA3 Dorsal | 31.2 ± 4.3 | ⬆ 94.5 ± 10.0 t = 5.175, df = 35, p < 0.0001 | 31.0 ± 5.9 | 39.0 ± 9.3 | 30.0 ± 9.0 | 39.0 ± 9.9 |
| CA1 Ventral | 44.1 ± 12.3 | 73.6 ± 9.6 | 40.5 ± 15.5 | 31.7 ± 4.0 | 55.8 ± 10.7 | 39.7 ± 9.7 |
| CA2 Ventral | 34.8 ± 8.4 | ⬆ 83.6 ± 10.0 t = 4.228, df = 35, p = 0.0005 | 24.8 ± 5.5 | 32.8 ± 3.2 | 56.8 ± 6.7 | 43.1 ± 11.2 |
| CA3 Ventral | 35.5 ± 7.3 | ⬆ 74.8 ± 7.6 t = 3.175, df = 35, p = 0.0093 | 25.5 ± 6.1 | 32.2 ± 4.1 | 45.1 ± 12.6 | 41.5 ± 11.9 |
| SN Compacta | 26.8 ± 4.0 | ⬆ 83.0 ± 16.5 t = 4.591, df = 35, p = 0.0002 | 35.7 ± 6.1 | 30.8 ± 7.2 | 24.3 ± 3.3 | 34.5 ± 8.5 |
| SN Reticulata | 31.5 ± 4.9 | ⬆ 101.0 ± 13.0 t = 2.883, df = 35, p = 0.0199 | 88.2 ± 23.5 | 60.6 ± 15.4 | 64.0 ± 20.3 | 56.1 ± 15.4 |
| PAG | 27.7 ± 3.3 | ⬆ 61.6 ± 14.0 t = 2.764, df = 35, p = 0.0269 | 41.2 ± 6.7 | 30.8 ± 4.9 | 35.6 ± 10.7 | 40.4 ± 8.7 |
Table A3 demonstrates [3H] flunitrazepam binding to BZD receptors expressed in fmol/mg of protein in the brain areas (abbreviations as in Table A1) of control and clonazapam-treated rats. All details as in Table A1 and Table A2.

Table A3.
[3H] flunitrazepam binding to BZD receptors.
Table A3.
[3H] flunitrazepam binding to BZD receptors.
| Interval 48 h Mean ± SEM | Interval 1 Week Mean ± SEM | Interval 2 Months Mean ± SEM | ||||
|---|---|---|---|---|---|---|
| Structure | Control | CZP | Control | CZP | Control | CZP |
| Cingulate Cx. | 388.0 ± 25.5 | 361.0 ± 17.2 | 387.4 ± 15.5 | 373.0 ± 36.0 | 370.2 ± 35.1 | 276.0 ± 15.0 |
| Frontoparital Cx. | 380.8 ± 23.9 | 382.4 ± 45.2 | 396.6 ± 26.9 | 368.9 ± 22.0 | 306.0 ± 34.0 | 263.1 ± 15.0 |
| Sensorimotor Cx. | 363.9 ± 48.2 | 342.1 ± 28.0 | 410.4 ± 37.6 | 364.1 ± 30.6 | 314.8 ± 25.2 | 198.4 ±19.0 |
| Temporal Cx. | 389.8 ± 55.2 | 270.3 ± 25.5 | 389.7 ± 19.0 | 355.5 ± 19.8 | 374.3 ± 49.6 | 250.7 ± 18.0 |
| Piriform Cx. | 336.0 ± 42.9 | 245.7 ± 25.5 | 281.0 ± 24.6 | 260.4 ± 11.0 | 282.3 ± 18.1 | 212.7 ± 14.0 |
| Enthorinal Cx. | 367.6 ± 54.0 | 271.3 ± 7.8 | 305.9 ± 22.0 | 301.6 ± 19.7 | 318.8 ± 52.2 | 223.0 ± 15.8 |
| Anterior AMG | 215.9 ± 21.8 | 226.1 ± 12.0 | 233.6 ± 7.8 | 211.1 ± 19.3 | 126.8 ± 44.4 | 115.0 ± 24.5 |
| Central AMG | 200.9 ± 40.0 | 190.9 ± 21.0 | 210.5 ± 7.2 | 210.6 ± 20.0 | 160.7 ± 39.0 | 77.2 ± 20.0 |
| Medial AMG | 317.3 ± 32.8 | 269.3 ± 15.9 | 295.0 ± 33.4 | 280.4 ± 19.0 | 304.0 ± 30.3 | ⬇ 224.7 ± 4.2 t = 2.572, df = 37, p = 0.0422 |
| Basolateral AMG | 343.6 ± 38.0 | 301.1 ± 37.1 | 318.0 ± 30.3 | 343.0 ± 24.0 | 319.3 ± 43.9 | 252.9 ± 5.5 |
| N. Accumbens | 223.3 ± 29.4 | 197.6 ± 24.0 | 216.7 ± 22.4 | 196.0 ± 17.4 | 233.8 ± 33.1 | ⬇ 91.3 ± 3.0 t = 3.637, df = 37, p = 0.0025 |
| CPU | 145.1 ± 30.1 | 148.7 ± 25.6 | 121.6 ± 26.1 | 81.1 ± 17.9 | 109.3 ± 21.4 | 51.1 ± 16.1 |
| Ventromedial TH | 230.1 ± 5.3 | ⬇ 122.9 ± 37.2 t = 2.859, df = 37, p = 0.0207 | 222.4 ± 17.2 | 175.1 ± 37.2 | 92 ± 37.0 | 33.9 ± 9.0 |
| Ventrolateral TH | 146.4 ± 28.6 | 86.1 ± 31.6 | 102.4 ± 27.4 | 153.0 ± 28.0 | 50.3 ± 19.1 | 49.8 ± 11.5 |
| Ventralpost. TH | 116.8 ± 26.7 | 78.2 ± 21.8 | 57.2 ± 12.9 | 120.5 ± 30.4 | 24.0 ± 11.4 | 13.1 ± 5.4 |
| Lateral TH | 157.0 ± 31.6 | 115.9 ± 33.9 | 106.6 ± 30.3 | 143.8 ± 28.5 | 74.0 ± 19.6 | 39.0 ± 9.7 |
| Medial TH | 193.3 ± 37.1 | 212.1 ± 36.5 | 158.1 ± 34.0 | 235.7 ± 19.7 | 178.0 ± 29.8 | 85.0 ± 23.4 |
| Dentate Gyrus | 313.4 ± 26.8 | 333.3 ± 53.6 | 311.1 ± 19.0 | 390.1 ± 33.6 | 337.8 ± 56.7 | 267.3 ± 23.2 |
| CA1 Dorsal | 261.5 ± 32.9 | 292.4 ± 35.7 | 288.9 ± 24.2 | 331.6 ± 17 | 249.7 ± 34.8 | 195.4 ± 16.4 |
| CA2 Dorsal | 228.9 ± 33.0 | 231.0 ± 41.4 | 230.6 ± 25.3 | 272.9 ± 15.1 | 206.8 ± 29.2 | 120.1 ± 23.2 |
| CA3 Dorsal | 294.8 ± 25.4 | 297.0 ± 45.5 | 261.6 ± 26.5 | 311.3 ± 14.9 | 267.8 ± 20.9 | ⬇ 179.0 ± 18.3 t = 3.795, df = 37, p = 0.0016 |
| CA1 Ventral | 373.1 ± 63.0 | 251.5 ± 10.9 | 289.1 ± 12.2 | 261.6 ± 12.9 | 280.0 ± 31.8 | 205.0 ± 14.8 |
| CA2 Ventral | 316.9 ± 60.8 | 248.9 ± 19.4 | 294.0 ± 20.5 | 280.9 ± 12.9 | 304.2 ± 41.4 | 218.0 ± 15.1 |
| CA3 Ventral | 356.9 ± 72.9 | 256.3 ± 35.0 | 294.4 ± 24.6 | 289.8 ± 19.8 | 320.8 ± 31.2 | 218.6 ± 7.3 |
| SN Compacta | 168.3 ± 49.4 | 177.3 ± 31.8 | 206.7 ± 11.9 | 168.1 ± 31.0 | 132.3 ± 49.2 | 141.0 ± 20.7 |
| SN Reticulata | 228.0 ± 60.4 | 238.4 ± 21.6 | 252.0 ± 21.6 | 232.1 ± 26.3 | 249.8 ± 27.6 | 192.0 ± 16.7 |
| PAG | 223.8 ± 35.9 | 220.9 ± 22.8 | 227.0 ± 22.7 | 233.3 ± 33.8 | 197.0 ± 22.0 | ⬇ 97.0 ± 28.1 t = 2.522, df = 37, p = 0.0492 |
Table A4 demonstrates [3H] CGP54626 binding to GABAB receptors in fmol/mg of protein in the brain areas (abbreviations as in Table A1) of control and CZP-treated rats. All details as in Table A1 and Table A2.

Table A4.
[3H] CGP54626 binding to GABAB receptors.
Table A4.
[3H] CGP54626 binding to GABAB receptors.
| Structure | Control 48 h | CZP 48 h | Control 1 week | CZP 1 week | Control 50 days | CZP 50 days |
|---|---|---|---|---|---|---|
| Cingulate Cx. | 274.1 ± 25.1 | 271.0 ± 19.0 | 311.1 ± 18.9 | 239.3 ± 20.9 | 404.7 ± 75.1 | 314.9 ± 49.3 |
| Frontoparital Cx. | 266.4 ± 9.3 | 349.8 ± 55.0 | 377.1 ± 39.6 | 287.6 ± 11.6 | 206.0 ± 26.3 | 253.6 ± 39.3 |
| Sensorimotor Cx. | 227.4 ± 15.0 | 283.0 ± 10.0 | 242.6 ± 14.7 | 230.6 ± 21.4 | 141.5 ± 16.5 | ⬆ 235.0 ± 17.7 t = 2.416, df = 37, p = 0.0017 |
| Temporal Cx. | 292.3 ± 27.4 | 260.1 ± 15.5 | 327.9 ± 38.6 | 256.0 ± 25.2 | 195.0 ± 26.9 | 215.7 ± 50.5 |
| Piriform Cx. | 210.1 ± 21.2 | 233.1 ± 25.6 | 237.1 ± 28.3 | 233.3 ± 27.5 | 269.0 ± 48.7 | 287.7 ± 30.6 |
| Enthorinal Cx. | 233.5 ± 24.7 | 262.3 ± 15.7 | 276.6 ± 24.0 | 414.9 ± 88.4 | 427.5 ± 87.3 | 462.6 ± 97.1 |
| Anterior AMG | 260.6 ± 20.0 | 228.7 ± 16.9 | 315.7 ± 31.2 | 253.9 ± 21.6 | 229.3 ± 49.3 | ⬇152.3 ± 10.7 t = 2.72, df = 37, p = 0.0293 |
| Central AMG | 231.1 ± 12.8 | ⬆ 507.7 ± 66.0 t = 5.706, df = 37, p < 0.0001 | 198.4 ± 19.4 | 221.5 ± 23.9 | 210.7 ± 37.8 | 271.9 ± 21.3 |
| Medial AMG | 439.9 ± 47.3 | 511.4 ± 63.3 | 298.1 ± 23.2 | 347.3 ± 40.0 | 365.5 ± 92.5 | ⬆ 678.6 ± 70.0 t = 3.679, df = 39, p = 0.0023 |
| Basolateral AMG | 251.9 ± 14.9 | 362.1 ± 34.0 | 218.7 ± 23.5 | 279.9 ± 27.0 | 268.0 ± 48.3 | ⬆ 479.1 ± 77 t = 3.422, df = 36, p = 0.0047 |
| N. Accumbens | 1028.0 ± 76.0 | ⬇ 697.6 ± 134.0 t = 2.857, df = 36, p = 0.021 | 384.6 ± 33.4 | 657.0 ± 85.0 | 369.5 ± 78.6 | 306.7 ± 29.6 |
| CPU | 673.1 ± 65.7 | 767.1 ± 191.0 | 454.3 ± 52.1 | 428.4 ± 68.0 | 174.8 ± 17.8 | 200.3 ± 22.8 |
| Ventromedial TH | 1151.0 ± 56.5 | 892.5 ± 68.7 | 734.3 ± 113 | ⬆ 1238.0 ± 175.0 t = 2.88, df = 36, p = 0.0199 | 601.0 ± 131 | 415.1 ± 51.4 |
| Ventrolateral TH | 1085.0 ± 66.5 | ⬇ 632.0 ± 120.0 t = 2.961, df = 36, p = 0.0161 | 735.6 ± 79.0 | ⬆ 1139.0 ± 166.0 t = 2.961, df = 36, p = 0.0293 | 606.5 ± 94.2 | 380.7 ± 37 |
| Ventralpost. TH | 1898.0 ± 321.0 | 1592.0 ± 178.0 | 1409.0 ± 77.8 | 1161.0 ± 99.0 | 249.7 ± 36.9 | ⬆ 342.4 ± 52.6 t = 3.738, df = 35, p = 0.0020 |
| Lateral TH | 2187.0 ± 125.0 | ⬇ 1566 ± 83.0 t = 3.608, df = 36, p = 0.0028 | 1532.0 ± 162.2 | ⬆ 2033.0 ± 156.0 t = 3.006, df = 39, p = 0.0143 | 718.7 ± 66.2 | 718.3 ± 63.8 |
| Medial TH | 1585.0 ± 119.0 | 1081 ± 16.5 | 1558.0 ± 190.3 | 2318.0 ± 501.0 | 1076 ± 179.6 | 694.0 ± 95 |
| Dentate Gyrus | 552.3 ± 66.9 | 441.9 ± 96.0 | 272.3 ± 17.3 | 250.8 ± 15.6 | 200.8 ± 24.5 | 353.1 ± 64 |
| CA1 Dorsal | 257.0 ± 18.8 | 267.0 ± 9.8 | 205.6 ± 19.6 | 211.8 ± 19.3 | 154.2 ± 10.3 | 138.7 ± 6.1 |
| CA2 Dorsal | 546.9 ± 61 | 611.6 ± 123.0 | 256.3 ± 13.0 | 281.8 ± 27.5 | 391.3 ± 87.2 | 324.9 ± 56.5 |
| CA3 Dorsal | 1087.0 ± 53.1 | 1060.0 ± 93.0 | 500.4 ± 76.0 | 351.8 ± 29.0 | 361.2 ± 34.5 | 500.9 ± 104 |
| CA1 Ventral | 257.7 ± 21.9 | 335.1 ± 22.0 | 352.1 ± 46.2 | 523.0 ± 69.0 | 503.8 ± 83.8 | 708.4 ± 92 |
| CA2 Ventral | 264.3 ± 35.5 | 312.3 ± 21.0 | 381.3 ± 40.0 | 442.0 ± 53.9 | 385.0 ± 68.2 | 292.7 ± 49.6 |
| CA3 Ventral | 693.0 ± 72.0 | 954.1 ± 88.0 | 714.3 ± 102.0 | 511.0 ± 78.0 | 772.7 ± 35.2 | 742.9 ± 71 |
| SN Compacta | 276.1 ± 15.0 | ⬆ 536.7 ± 102.0 t = 4.026, df = 36, p = 0.0008 | 352.4 ± 31.4 | 265.8 ± 22.0 | 113.5 ± 4.4 | 158.7 ± 11 |
| SN Reticulata | 264.4 ± 20.8 | 269.1 ± 17.3 | 290.0 ± 22.0 | 288.8 ± 12.0 | 124.5 ± 6.5 | 158.6 ± 22 |
| PAG | 967.6 ± 60.0 | 999.7 ± 54.0 | 564.1 ± 69.2 | 594.0 ± 76.0 | 154.0 ± 9.5 | 107.0 ± 22 |
References
- Wu, C.; Sun, D. GABA receptors in brain development, function, and injury. Metab. Brain Dis. 2015, 30, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Fritschy, J.M.; Panzanelli, P. GABAA receptors and plasticity of inhibitory neurotransmission in the central nervous system. Eur. J. Neurosci. 2014, 39, 1845–1865. [Google Scholar] [CrossRef] [PubMed]
- Terunuma, M. Diversity of structure and function of GABAB receptors: A complexity of GABAB-mediated signaling. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2018, 94, 390–411. [Google Scholar] [CrossRef] [PubMed]
- Fritschy, J.M. Significance of GABA(A) receptor heterogeneity: Clues from developing neurons. Adv. Pharmacol. 2015, 73, 13–39. [Google Scholar]
- Wang, D.D.; Kriegstein, A.R. Defining the role of GABA in cortical development. J. Physiol. 2009, 1873–1879. [Google Scholar] [CrossRef]
- Rennie, J.M.; Boylan, G.B. Neonatal seizures and their treatment. Curr. Opin. Neurol. 2003, 16, 177–181. [Google Scholar] [CrossRef]
- Gai, N.; Grimm, V.E. The effect of prenatal exposure to diazepam on aspects of postnatal development and behavior in rats. Psychopharmacology (Berl) 1982, 78, 225–229. [Google Scholar] [CrossRef]
- Tucker, J.C. Benzodiazepines and the developing rat:a critical review. Neurosci. Biobehav. Rev. 1985, 9, 101–111. [Google Scholar] [CrossRef]
- Kellogg, C.K. Benzodiazepines: Influence on the developing brain. Prog. Brain Res. 1988, 73, 207–228. [Google Scholar]
- Ikonomidoou, C.; Turski, L. Antiepileptic drugs and brain development. Epilepsy Res. 2010, 88, 11–22. [Google Scholar] [CrossRef]
- Sundbakk, L.M.; Wood, M.; Gran, J.M.; Nordeng, H. Impact of prenatal exposure to benzodiazepines and z-hypnotics on behavioral problems at 5 years of age: A study from the Norwegian Mother and Child Cohort Study. PLoS ONE 2019, 14, e0217830. [Google Scholar] [CrossRef] [PubMed]
- Kubová, H.; Mareš, P. Time course of the anticonvulsant action of clonazepam in the developing rats. Arch. Int. Pharmacodyn. 1989, 298, 15–24. [Google Scholar] [PubMed]
- Kubová, H.; Mareš, P.; Vorlíček, J. Stable anticonvulsant action of benzodiazepines during development in rats. J. Pharm. Pharmacol. 1993, 45, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Mikulecká, A.; Mareš, P.; Kubová, H. Rebound increase in seizure susceptibility but not isolation-induced calls after single administration of clonazepam and Ro 19-8022 in infant rats. Epilepsy Behav. 2011, 20, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Mikulecká, A.; Šubrt, M.; Stuchlík, A.; Kubová, H. Consequences of early postnatal benzodiazepines exposure in rats. I. Cognitive-like behavior. Front. Behav. Neurosci. 2014, 8, 101, eCollection 2014. [Google Scholar] [CrossRef]
- Mikulecká, A.; Šubrt, M.; Pařízková, M.; Mareš, P.; Kubová, H. Consequences of early postnatal benzodiazepines exposure in rats. II. Social behavior. Front. Behav. Neurosci. 2014, 8, 169, eCollection 2014. [Google Scholar] [CrossRef]
- File, S.E. Behavioral changes persisting in to adulthood after neonatal benzodiazepine administration in the rat. Neurobehav. Toxicol. Teratol. 1986, 8, 453–461. [Google Scholar]
- File, S.E. Effects of neonatal administration of diazepam and lorazepam on performance of adolescent rats in tests of anxiety, aggression, learning and convulsions. Neurobehav. Toxicol. Teratol. 1986, 8, 301–306. [Google Scholar]
- File, S.E. The effects of neonatal administration of clonazepam on passive avoidance and on social, aggressive and exploratory behavior of adolescent male rats. Neurobehav. Toxicol. Teratol. 1986, 8, 447–452. [Google Scholar]
- File, S.E. Diazepam and caffeine administration during the first week of life: Changes in neonatal and adolescent behavior. Neurotoxicol. Teratol. 1987, 9, 9–16. [Google Scholar] [CrossRef]
- Kubová, H.; Mareš, P. Partial agonist of benzodiazepine receptors Ro 19-8022 elicits withdrawal symptoms after short-term administration in immature rats. Physiol. Res. 2012, 61, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Kubová, H.; Bendová, Z.; Moravcová, S.; Pačesová, D.; Rocha, L.; Mareš, P. Neonatal clonazepam administration induced long-lasting changes in glutamate receptors. Front. Mol. Neurosci. 2018, 11, 382. [Google Scholar] [CrossRef] [PubMed]
- Whissell, P.D.; Rosenzweig, S.; Lecker, I.; Wang, D.S.; Wojtowicz, J.M.; Orser, B.A. γ-aminobutyric acid type A receptors that contain the δ subunit promote memory and neurogenesis in the dentate gyrus. Ann. Neurol. 2013, 74, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Whissell, P.D.; Lecker, I.; Wang, D.S.; Yu, J.; Orser, B.A. Altered expression of δGABAA receptors in health and disease. Neuropharmacology 2015, 88, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Laurie, D.J.; Wisden, W.; Seeburg, P.H. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J. Neurosci. 1992, 12, 4151–4172. [Google Scholar] [CrossRef]
- Martini, C.; Rigacci, T.; Lucacchini, A. [3H]muscimol binding site on purified benzodiazepine receptor. J. Neurochem. 1983, 41, 1183–1185. [Google Scholar] [CrossRef]
- Benkherouf, A.Y.; Taina, K.R.; Meera, P.; Aalto, A.J.; Li, X.G.; Soini, S.L.; Wallner, M.; Uusi-Oukari, M.J. Extrasynaptic δ-GABAA receptors are high-affinity muscimol receptors. J. Neurochem. 2019, 149, 41–53. [Google Scholar] [CrossRef]
- Brett, R.R.; Pratt, J.A. Changes in benzodiazepine-GABA receptor coupling in an accumbens-habenula circuit after chronic diazepam treatment. Br. J. Pharmacol. 1995, 116, 2375–2384. [Google Scholar] [CrossRef]
- Andersen, S.L.; Navalta, C.P. Altering the course of neurodevelopment: A framework for understanding the enduring effects of psychotropic drugs. Int. J. Dev. Neurosci. 2004, 22, 423–440. [Google Scholar] [CrossRef]
- Dobbing, J.; Smart, J.L. Vulnerability of developing brain and behaviour. Br. Med. Bull. 1974, 30, 164–168. [Google Scholar] [CrossRef]
- Lohmann, C.; Kessels, H.W. The developmental stages of synaptic plasticity. J. Physiol. 2014, 592, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Forcelli, P.A.; Janssen, M.J.; Vicini, S.; Gale, K. Neonatal exposure to antiepileptic drugs disrupts striatal synaptic development. Ann. Neurol. 2012, 72, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Uusi-Oukari, M.; Korpi, E.R. Regulation of GABA(A) receptor subunit expression by pharmacological agents. Pharmacol. Rev. 2010, 62, 97–135. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Rosenberg, H.C.; Chiu, T.H.; Zhao, T.J. Subunit- and brain region-specific reduction of GABAA receptor subunit mRNAs during chronic treatment of rats with diazepam. J. Mol. Neurosci. 1994, 5, 105–120. [Google Scholar] [CrossRef]
- Holt, R.A.; Bateson, A.N.; Martin, I.L. Chronic treatment with diazepam or abecarnil differently affects the expression of GABAA receptor subunit mRNAs in the rat cortex. Neuropharmacology 1996, 35, 1457–1463. [Google Scholar] [CrossRef]
- Tietz, E.I.; Huang, X.; Chen, S.; Ferencak, W.F., 3rd. Temporal and regional regulation of alpha1, beta2 and beta3, but not alpha2, alpha4, alpha5, alpha6, beta1 or gamma2 GABA(A) receptor subunit messenger RNAs following one-week oral flurazepam administration. Neuroscience 1999, 91, 327–341. [Google Scholar] [CrossRef]
- Tietz, E.I.; Huang, X.; Weng, X.; Rosenberg, H.C.; Chiu, T.H. Expression of alpha 1, alpha 5, and gamma 2 GABAA receptor subunit mRNAs measured in situ in rat hippocampus and cortex following chronic flurazepam administration. J. Mol. Neurosci. 1993, 4, 277–292. [Google Scholar] [CrossRef]
- Chen, S.; Huang, X.; Zeng, X.J.; Sieghart, W.; Tietz, E.I. Benzodiazepine-mediated regulation of alpha1, alpha2, beta1-3 and gamma2 GABA(A) receptor subunit proteins in the rat brain hippocampus and cortex. Neuroscience 1999, 93, 33–44. [Google Scholar] [CrossRef]
- Owens, D.F.; Kriegstein, A.R. Is there more to GABA than synaptic inhibition? Nat. Rev. Neurosci. 2002, 3, 715–727. [Google Scholar] [CrossRef]
- Sato, T.N.; Neale, J.H. Type I and type II gamma-aminobutyric acid/benzodiazepine receptors: Purification and analysis of novel receptor complex from neonatal cortex. J. Neurochem. 1989, 52, 1114–1122. [Google Scholar] [CrossRef]
- Zhang, J.H.; Sato, M.; Tohyama, M. Different postnatal development profiles of neurons containing distinct GABAA receptor beta subunit mRNAs (beta 1, beta 2, and beta 3) in the rat forebrain. J. Comp. Neurol. 1991, 308, 586–613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Sato, M.; Tohyama, M. Different postnatal ontogenic profiles of neurons containing beta (beta 1, beta 2 and beta 3) subunit mRNAs of GABAA receptor in the rat thalamus. Brain Res. Dev. Brain Res. 1991, 58, 289–292. [Google Scholar] [CrossRef]
- Zhang, J.H.; Sato, M.; Araki, T.; Tohyama, M. Postnatal ontogenesis of neurons containing GABAA alpha 1 subunit mRNA in the rat forebrain. Brain Res. Mol. Brain Res. 1992, 16, 193–203. [Google Scholar] [CrossRef]
- Hornung, J.P.; Fritschy, J.M. Developmental profile of GABAA-receptors in the marmoset monkey: Expression of distinct subtypes in pre- and postnatal brain. J. Comp. Neurol. 1996, 367, 413–430. [Google Scholar] [CrossRef]
- Stell, B.M.; Brickley, S.G.; Tang, C.Y.; Farrant, M.; Mody, I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc. Natl. Acad. Sci. USA 2003, 100, 14439–14444. [Google Scholar] [CrossRef]
- Glykys, J.; Peng, Z.; Chandra, D.; Homanics, G.E.; Houser, C.R.; Mody, I. A new naturally occurring GABA(A) receptor subunit partnership with high sensitivity to ethanol. Nat. Neurosci. 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Holter, N.I.; Zylla, M.M.; Zuber, N.; Bruehl, C.; Draguhn, A. Tonic GABAergic control of mouse dentate granule cells during postnatal development. Eur. J. Neurosci. 2010, 32, 1300–1309. [Google Scholar] [CrossRef]
- Korpi, E.R.; Mihalek, R.M.; Sinkkonen, S.T.; Hauer, B.; Hevers, W.; Homanics, G.E.; Sieghart, W.; Lüddens, H. Altered receptor subtypes in the forebrain of GABA(A) receptor delta subunit-deficient mice: Recruitment of gamma 2 subunits. Neuroscience 2002, 109, 733–743. [Google Scholar] [CrossRef]
- Allison, C.; Pratt, J.A. Neuroadaptive processes in GABAergic and glutamatergic systems in benzodiazepine dependence. Pharmacol. Ther. 2003, 98, 171–195. [Google Scholar] [CrossRef]
- Tietz, E.I.; Rosenberg, H.C.; Chiu, T.H. Autoradiographic localization of benzodiazepine receptor downregulation. J. Pharmacol. Exp. Ther. 1986, 236, 284–292. [Google Scholar]
- Percic, D.; Svob Strac, D.; Jazvincak Jemberek, M.; Vlainic, J. Allosteric uncoupling and up-regulation of benzodiazepine and GABA recognition sites following chronic diazepam treatment of HEK 293 cells stably transfected with α1β2γ2S subunits of GABAA receptors. Naunyn. Schmiedebergs Arch. Pharmacol. 2007, 375, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Fyhn, M.; Molden, S.; Witter, M.P.; Moser, E.I.; Moser, M.B. Spatial representation in the entorhinal cortex. Science 2004, 305, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- White, N.M. Some highlights of research on the effects of caudate nucleus lesions over the past 200 years. Behav. Brain Res. 2009, 199, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Panksepp, J. The basic emotional circuits of mammalian brains: Do animals have affective lives? Neurosci. Biobehav. Rev. 2011, 35, 1791–1804. [Google Scholar] [CrossRef]
- Benarroch, E.E. Periaqueductal gray: An interface for behavioral control. Neurology 2012, 78, 210–217. [Google Scholar] [CrossRef]
- Fanselow, M.S.; Dong, H.W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 2010, 65, 7–19. [Google Scholar] [CrossRef]
- Schroeder, H.; Humbert, A.C.; Desor, D.; Nehlig, A. Long-term consequences of neonatal exposure to diazepam on cerebral glucose utilization, learning, memory and anxiety. Brain Res. 1997, 766, 142–152. [Google Scholar] [CrossRef]
- Chalifoux, J.R.; Carter, A.G. GABAB receptor modulation of synaptic function. Curr. Opin. Neurobiol. 2011, 21, 339–344. [Google Scholar] [CrossRef]
- Gaiarsa, J.L.; Porcher, C. Emerging neurotrophic role of GABAB receptors in neuronal circuit development. Front. Cell Neurosci. 2013, 7, 206. [Google Scholar] [CrossRef]
- Prosser, H.M.; Gill, C.H.; Hirst, W.D.; Grau, E.; Robbins, M.; Calver, A.; Soffin, E.M.; Farmer, C.E.; Lanneau, C.; Gray, J.; et al. Epileptogenesis and enhanced prepulse inhibition in GABA(B1)-deficient mice. Mol. Cell Neurosci. 2001, 17, 1059–1070. [Google Scholar] [CrossRef]
- Haller, C.; Casanova, E.; Müller, M.; Vacher, C.M.; Vigot, R.; Doll, T.; Barbieri, S.; Gassmann, M.; Bettler, B. Floxed allele for conditional inactivation of the GABAB(1) gene. Genesis 2004, 40, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Bony, G.; Szczurkowska, J.; Tamagno, I.; Shelly, M.; Contestabile, A.; Cancedda, L. Non-hyperpolarizing GABAB receptor activation regulates neuronal migration and neurite growth and specification by cAMP/LKB1. Nat. Commun. 2013, 4, 1800. [Google Scholar] [CrossRef] [PubMed]
- Heaney, C.F.; Kinney, J.W. Role of GABA(B) receptors in learning and memory and neurological disorders. Neurosci. Biobehav. Rev. 2016, 63, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 106–107, 1–16. [Google Scholar] [CrossRef]
- Benarroch, E.E. GABAB receptors: Structure, functions, and clinical implications. Neurology 2012, 78, 578–584. [Google Scholar] [CrossRef]
- Bittigau, P.; Sifringer, M.; Genz, K.; Reith, E.; Pospischil, D.; Govindarajalu, S.; Dzietko, M.; Pesditschek, S.; Mai, I.; Dikranian, K.; et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc. Natl. Acad. Sci. USA 2002, 99, 15089–15094. [Google Scholar] [CrossRef]
- Stefovska, V.G.; Uckeremann, O.; Czuczwar, M.; Smitka, M.; Czuczwar, P.; Kis, J.; Kaindl, A.M.; Turski, L.; Turski, W.A.; Ikonomidou, C. Sedative and anticonvulsant drugs suppress postnatal neurogenesis. Ann. Neurol. 2008, 64, 434–445. [Google Scholar] [CrossRef]
- Conklin, P.; Heggeness, F.W. Maturation of temperature homeostasis in the rat. Am. J. Physiol. 1971, 220, 333–336. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.; Alonso-Vanegas, M.; Martínez-Juárez, I.E.; Orozco-Suárez, S.; Escalante-Santiago, D.; Feria-Romero, I.A.; Zavala-Tecuapetla, C.; Cisneros-Franco, J.M.; Buentello-García, R.M.; Cienfuegos, J. GABAergic alterations in neocortex of patients with pharmacoresistant temporal lobe epilepsy can explain the comorbidity of anxiety and depression: The potential impact of clinical factors. Front. Cell Neurosci. 2015, 8, 442. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).