An Ultra-Sensitive Electrochemical Sensor for the Detection of Carcinogen Oxidative Stress 4-Nitroquinoline N-Oxide in Biologic Matrices Based on Hierarchical Spinel Structured NiCo2O4 and NiCo2S4; A Comparative Study
Abstract
:1. Introduction
2. Results and Discussion
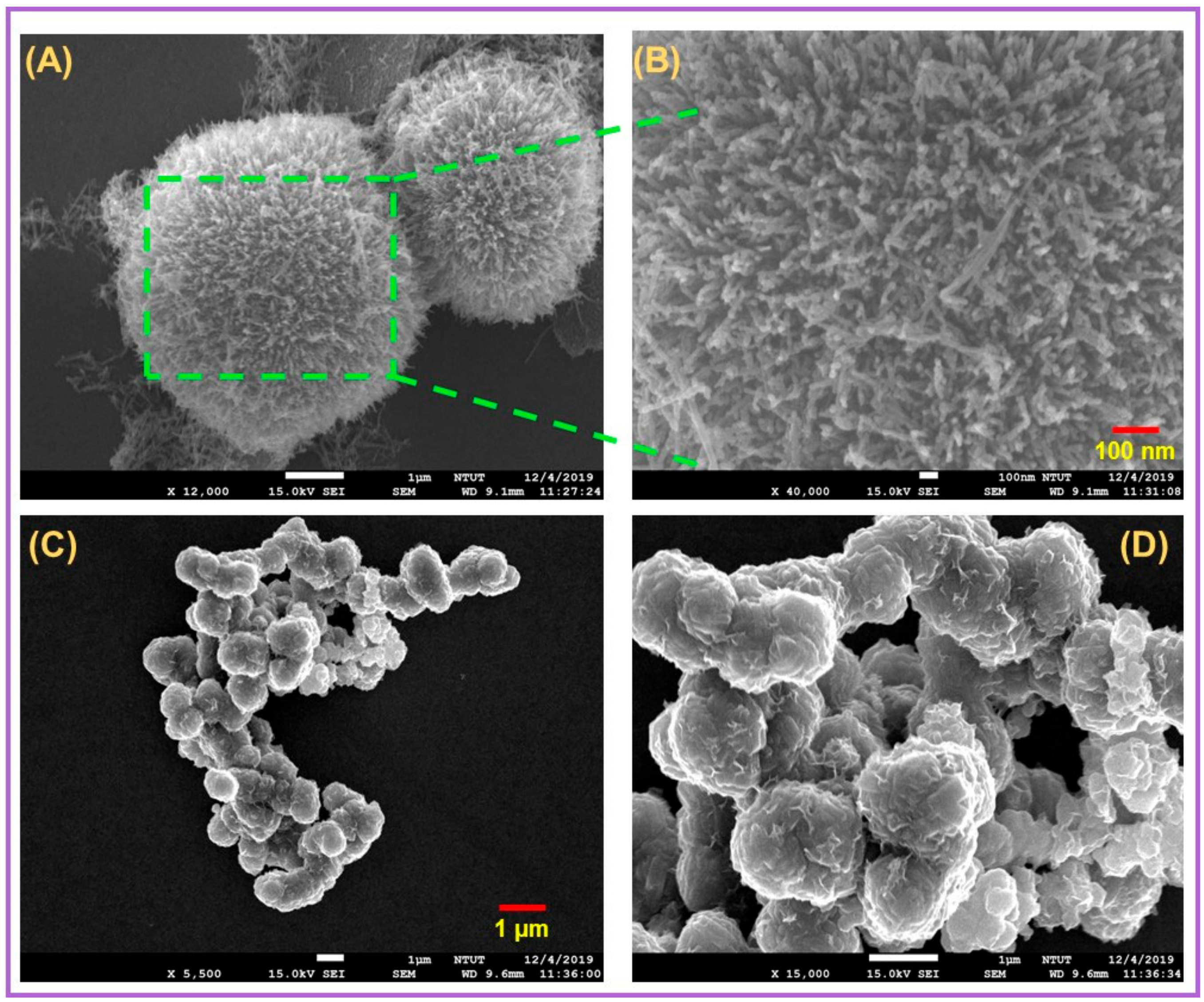
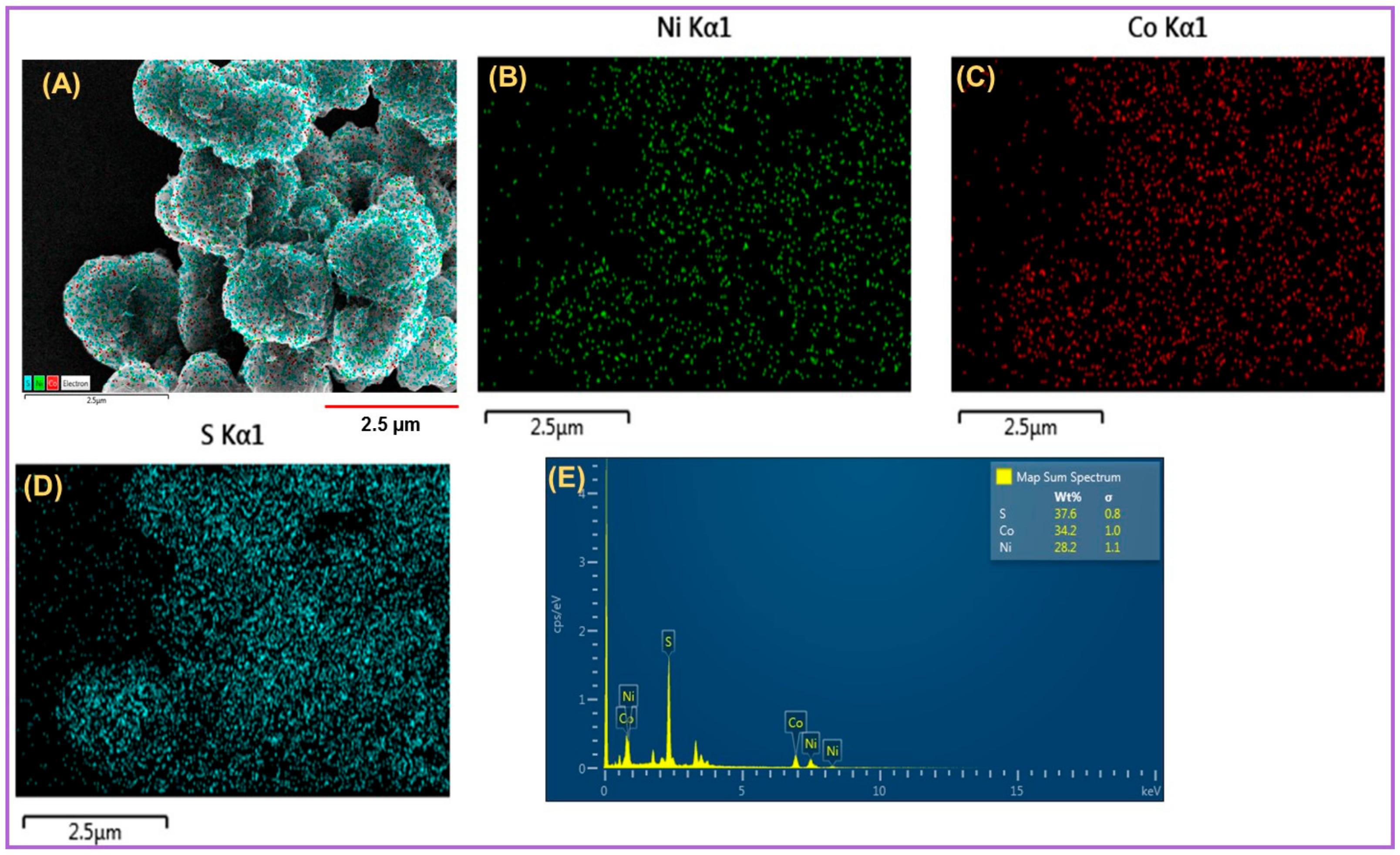
2.1. Microscopic and Elemental Analysis of Synthesized Nanomaterials
2.2. XRD and XPS Analysis of NiCo2S4-Ms and NiCo2O4-MFs
2.3. EIS and Electrochemical Investigation of Different Electrodes
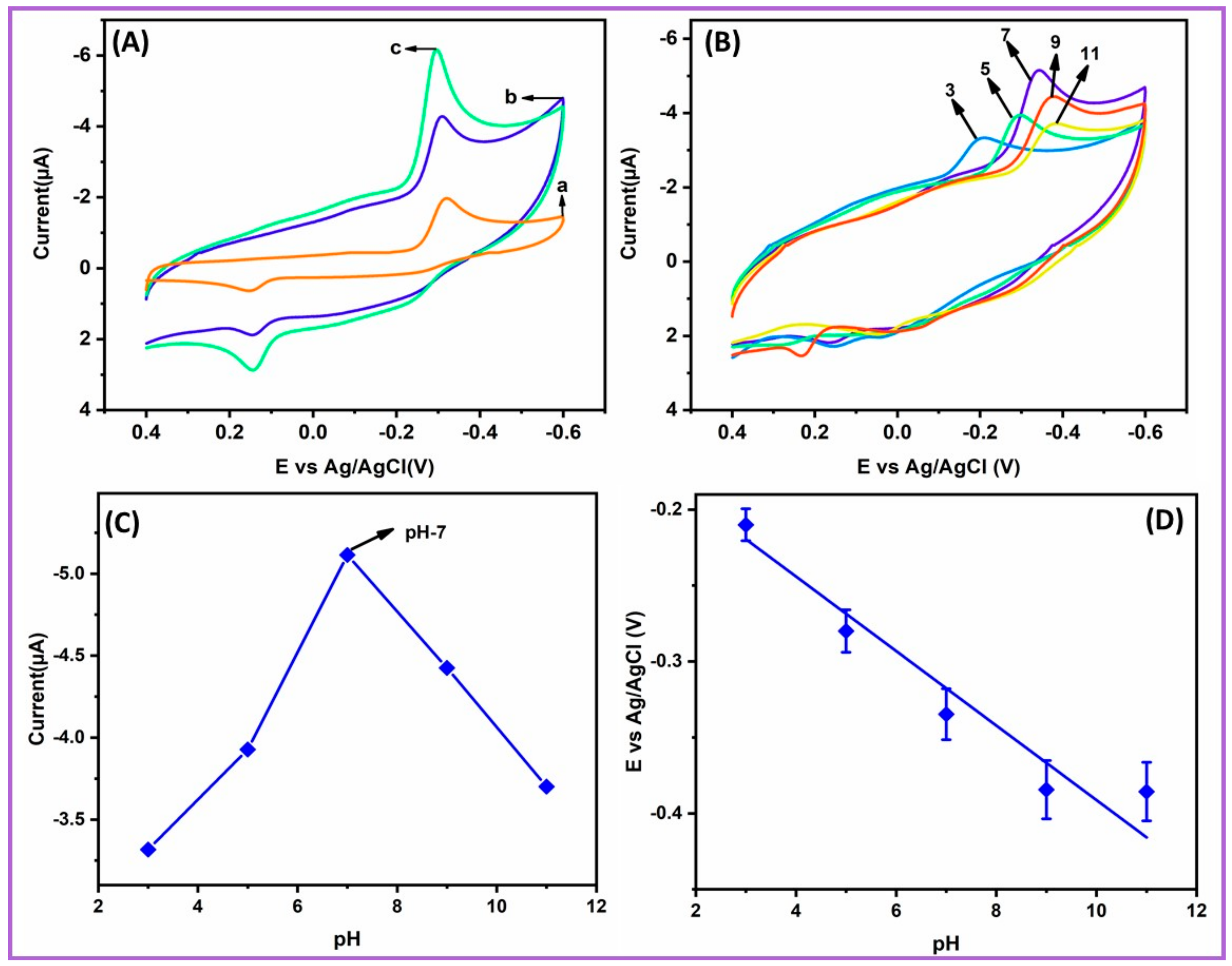
2.4. Electrochemical Activity and Different pH at NiCo2S4-Ms/GCE
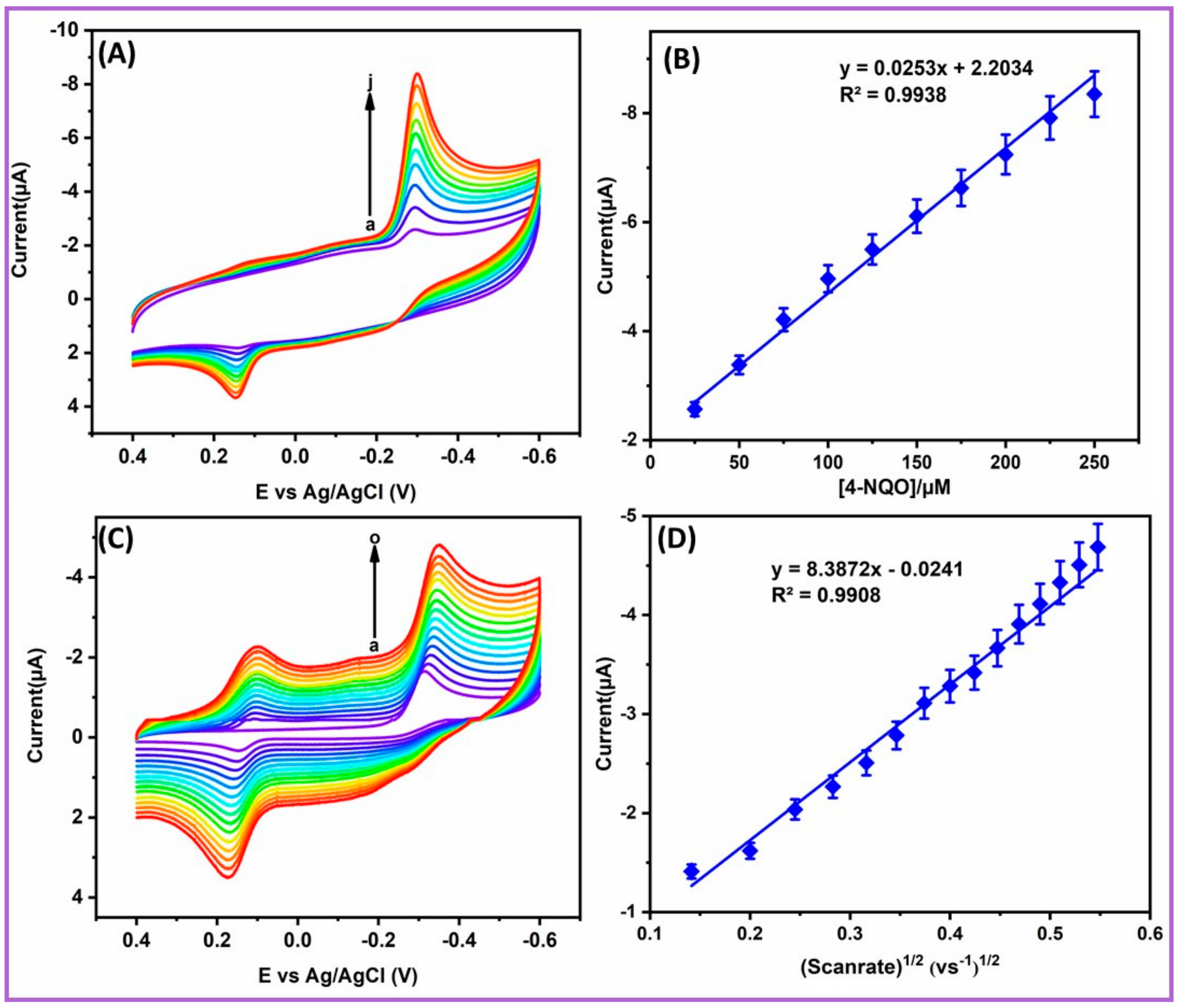
2.5. Influence of Different Concentration and Scan Rate
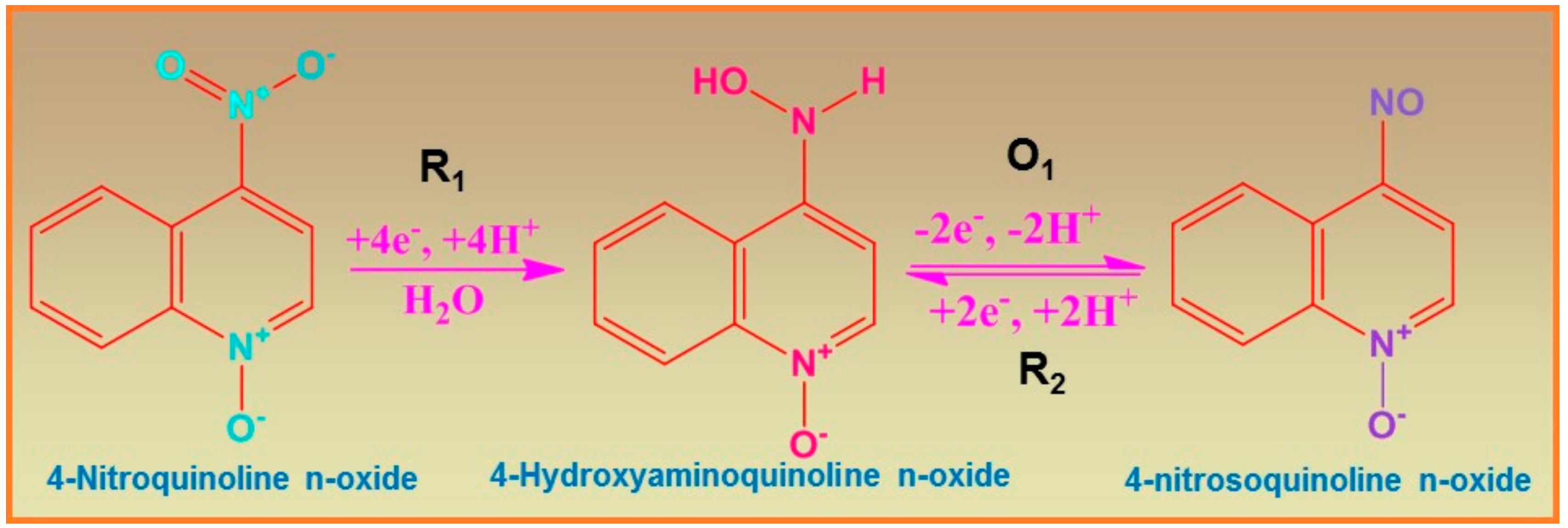
2.6. DPV Analysis of 4-NQO Ions at NiCo2S4-Ms/GCE Techniques
2.7. Interference and Stability Studies of NiCo2S4-Ms/GCE
2.8. Real Sample Analysis
3. Experimental Section
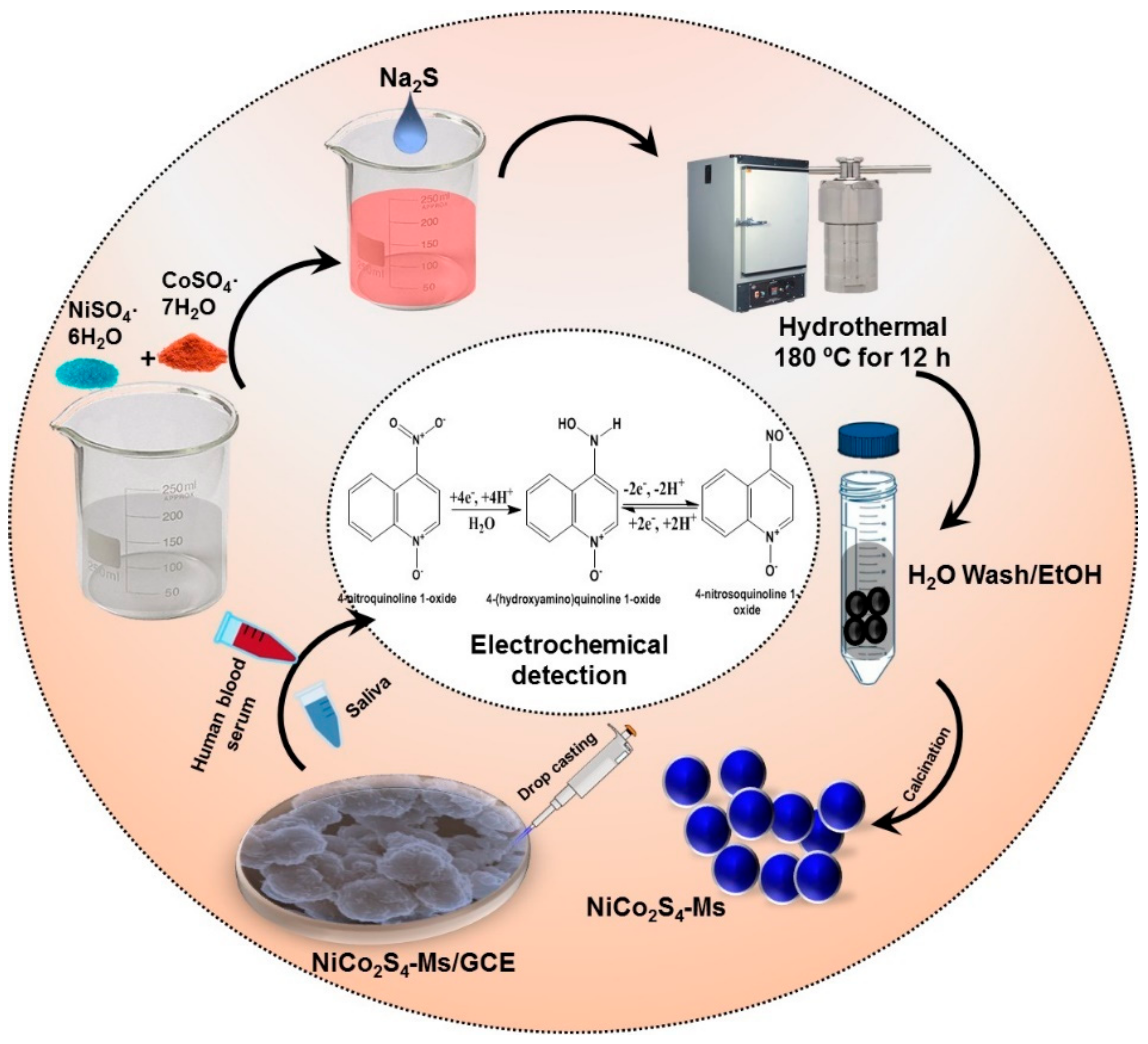
3.1. Synthesis Method for NiCo2S4-Ms and NiCo2O4-MFs
3.2. Fabrication of NiCo2S4-Ms Modified GCE
4. Materials and Reagents
5. Methods
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kim, J.-H.; Kang, S.H.; Zhu, K.; Kim, J.Y.; Neale, N.R.; Frank, A.J. Ni–NiO core–shell inverse opal electrodes for supercapacitors. Chem. Commun. 2011, 47, 5214. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cheng, S.; Ding, Y.; Zhu, X.; Wang, Z.L.; Liu, M. Hierarchical Network Architectures of Carbon Fiber Paper Supported Cobalt Oxide Nanonet for High-Capacity Pseudocapacitors. Nano Lett. 2011, 12, 321–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, M.; Tie, J.; Cheng, G.; Lin, T.; Peng, S.; Deng, F.; Ye, F.; Yu, L. In situ growth of burl-like nickel cobalt sulfide on carbon fibers as high-performance supercapacitors. J. Mater. Chem. A 2015, 3, 1730–1736. [Google Scholar] [CrossRef]
- An, W.; Liu, L.; Gao, Y. Ni 0.9 Co 1.92 Se 4 nanostructures: Binder-free electrode of coral-like bimetallic selenide for supercapacitors. RSC Adv. 2016, 6, 75251–75257. [Google Scholar] [CrossRef]
- Chen, H.C.; Jiang, J.; Zhang, L.; Qi, T.; Xia, D.; Wan, H. Facilely synthesized porous NiCo2O4 flowerlike nanostructure for high-rate supercapacitors. J. Power Sources 2014, 248, 28–36. [Google Scholar] [CrossRef]
- Zhou, Q.; Xing, J.; Gao, Y.; Lv, X.; He, Y.; Guo, Z.; Li, Y. Ordered Assembly of NiCo2O4Multiple Hierarchical Structures for High-Performance Pseudocapacitors. ACS Appl. Mater. Interfaces 2014, 6, 11394–11402. [Google Scholar] [CrossRef]
- Li, L.; Yang, H.; Zhang, L.; Miao, J.; Sun, C.; Huang, W.; Dong, X.; Liu, B. Hierarchical carbon@Ni 3 S 2 @MoS 2 double core–shell nanorods for high-performance supercapacitors. J. Mater. Chem. A 2016, 4, 1319–1325. [Google Scholar] [CrossRef]
- Shen, M.; Ruan, C.; Chen, Y.; Jiang, C.; Ai, K.; Lu, L. Covalent Entrapment of Cobalt–Iron Sulfides in N-Doped Mesoporous Carbon: Extraordinary Bifunctional Electrocatalysts for Oxygen Reduction and Evolution Reactions. ACS Appl. Mater. Interfaces 2015, 7, 1207–1218. [Google Scholar] [CrossRef]
- Wang, X.; Shi, B.; Fang, Y.; Rong, F.; Huang, F.; Que, R.; Shao, M. High capacitance and rate capability of a Ni 3 S 2 @CdS core–shell nanostructure supercapacitor. J. Mater. Chem. A 2017, 5, 7165–7172. [Google Scholar] [CrossRef]
- Ouyang, Y.; Ye, H.; Xia, X.; Jiao, X.; Li, G.; Mutahir, S.; Wang, L.; Mandler, D.; Lei, W.; Hao, Q.; et al. Hierarchical electrodes of NiCo2S4 nanosheets-anchored sulfur-doped Co3O4 nanoneedles with advanced performance for battery-supercapacitor hybrid devices. J. Mater. Chem. A 2019, 7, 3228–3237. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, L.; Bin Wu, H.; Lou, X.W. (David) Formation of NixCo3−xS4Hollow Nanoprisms with Enhanced Pseudocapacitive Properties. Angew. Chem. Int. Ed. 2014, 53, 3711–3714. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Cui, F.; Chu, S.; Wang, T.; Sheng, E.; Wang, Z. Preparation and Electrochemical Characterization of Hollow Hexagonal NiCo2S4 Nanoplates as Pseudocapacitor Materials. ACS Sustain. Chem. Eng. 2013, 2, 809–815. [Google Scholar] [CrossRef]
- Xiao, T.; Li, J.; Zhuang, X.; Zhang, W.; Wang, S.; Chen, X.; Xiang, P.; Jiang, L.; Tan, X. Wide potential window and high specific capacitance triggered via rough NiCo2S4 nanorod arrays with open top for symmetric supercapacitors. Electrochim. Acta 2018, 269, 397–404. [Google Scholar] [CrossRef]
- Padmanathan, N.; Selladurai, S. In AIP Conference Proceeding: Sonochemically precipitated spinel Co[sub 3]O[sub 4] and NiCo[sub 2]O[sub 4] nanostructures as an electrode materials for supercapacitor; AIP Publishing: Melville, NY, USA, 2013; pp. 1216–1217. [Google Scholar]
- Elaiyappillai, E.; Akilarasan, M.; Chen, S.-M.; Kogularasu, S.; Johnson, P.M.; Tamilarasan, E.B. Sonochemically Recovered Aluminum Oxide Nanoparticles from Domestic Aluminum Wastes as a Highly Stable Electrocatalyst for Proton-Pump Inhibitor (Omeprazole) Detection. J. Electrochem. Soc. 2020, 167, 027544. [Google Scholar] [CrossRef]
- Rajaji, U.; Muthumariappan, A.; Chen, S.-M.; Chen, T.-W.; Tseng, T.-W.; Wang, K.; Qi, D.; Jiang, J. Facile sonochemical synthesis of porous and hierarchical manganese(III) oxide tiny nanostructures for super sensitive electrocatalytic detection of antibiotic (chloramphenicol) in fresh milk. Ultrason. Sonochemistry 2019, 58, 104648. [Google Scholar] [CrossRef] [PubMed]
- Pinjari, D.V.; Pandit, A.B. Room temperature synthesis of crystalline CeO2 nanopowder: Advantage of sonochemical method over conventional method. Ultrason. Sonochemistry 2011, 18, 1118–1123. [Google Scholar] [CrossRef]
- Chen, S.-M.; Muthumariappan, A.; Chen, S.-M.; Chen, T.-W.; Ramalingam, R.J. A novel electrochemical sensor for the detection of oxidative stress and cancer biomarker (4-nitroquinoline N-oxide) based on iron nitride nanoparticles with multilayer reduced graphene nanosheets modified electrode. Sens. Actuators B: Chem. 2019, 291, 120–129. [Google Scholar] [CrossRef]
- Chen, T.-W.; Rajaji, U.; Chen, S.-M.; Al Mogren, M.M.; Hochlaf, M.; Al Harbi, S.D.A.; Ramalingam, R.J. A novel nanocomposite with superior electrocatalytic activity: A magnetic property based ZnFe2O4 nanocubes embellished with reduced graphene oxide by facile ultrasonic approach. Ultrason. Sonochemistry 2019, 57, 116–124. [Google Scholar] [CrossRef]
- Nunoshiba, T.; Demple, B. Potent intracellular oxidative stress exerted by the carcinogen 4-nitroquinoline-N-oxide. Cancer Res. 1993, 53, 3250–3252. [Google Scholar]
- Poot, M.; Gollahon, K.A.; Emond, M.J.; Silber, J.R.; Rabinovitch, P.S. Werner syndrome diploid fibroblasts are sensitive to 4-nitroquinoline-N-oxide and 8-methoxypsoralen: Implications for the disease phenotype. FASEB J. 2002, 16, 757–758. [Google Scholar] [CrossRef]
- Muthumariyappan, A.; Rajaji, U.; Chen, S.-M.; Chen, T.-W.; Li, Y.-L.; Ramalingam, R.J. One-pot sonochemical synthesis of Bi2WO6 nanospheres with multilayer reduced graphene nanosheets modified electrode as rapid electrochemical sensing platform for high sensitive detection of oxidative stress biomarker in biological sample. Ultrason. Sonochemistry 2019, 57, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Yasmin, S.; Jeon, S. Effective electrochemical detection of dopamine with highly active molybdenum oxide nanoparticles decorated on 2, 6 diaminopyridine/reduced graphene oxide. Microchem. J. 2020, 153, 104501. [Google Scholar] [CrossRef]
- Li, Y.; Han, X.; Yi, T.-F.; He, Y.-B.; Li, X. Review and prospect of NiCo2O4-based composite materials for supercapacitor electrodes. J. Energy Chem. 2019, 31, 54–78. [Google Scholar] [CrossRef] [Green Version]
- Sui, Y.W.; Zhang, Y.M.; Hou, P.H.; Qi, J.Q.; Wei, F.X.; He, Y.Z.; Meng, Q.K.; Sun, Z. Three-dimensional NiCo2S4 nanosheets as high-performance electrodes materials for supercapacitors. J. Mater. Sci. 2017, 52, 7100–7109. [Google Scholar] [CrossRef]
- Khalid, S.; Caoa, C.; Wang, L.; Zhu, Y. Microwave Assisted Synthesis of Porous NiCo2O4 Microspheres: Application as High Performance Asymmetric and Symmetric Supercapacitors with Large Areal Capacitance. Sci. Rep. 2016, 6, 22699. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, H.; Zhang, J.; Xu, J. IrNi nanoparticle-decorated flower-shaped NiCo2O4 nanostructures: Controllable synthesis and enhanced electrochemical activity for oxygen evolution reaction. Sci. China Mater. 2016, 60, 119–130. [Google Scholar] [CrossRef]
- Wen, Y.; Peng, S.; Wang, Z.; Hao, J.; Qin, T.; Lu, S.; Zhang, J.; He, D.; Fan, X.; Cao, G. Facile synthesis of ultrathin NiCo 2 S 4 nano-petals inspired by blooming buds for high-performance supercapacitors. J. Mater. Chem. A 2017, 5, 7144–7152. [Google Scholar] [CrossRef]
- Abdelkader, A.; Osman, A.I.; Halawy, S.A.; Mohamed, M.A. Preparation and characterization of mesoporous γ-Al2O3 recovered from aluminum cans waste and its use in the dehydration of methanol to dimethyl ether. J. Mater. Cycles Waste Manag. 2018, 20, 1428–1436. [Google Scholar] [CrossRef]
- Muthumariappan, A.; Sakthivel, K.; Chen, S.-M.; Chen, T.-W.; Elgorban, A.M.; Elshikh, M.S.; Marraiki, N. Evaluating an effective electrocatalyst for the rapid determination of triptan drug (Maxalt™) from (mono and binary) transition metal (Co, Mn, CoMn, MnCo) oxides via electrochemical approaches. New J. Chem. 2020, 44, 605–613. [Google Scholar] [CrossRef]




| Method | Linear Range (µM) | LOD (nM) | Ref |
|---|---|---|---|
| Bi2WO6/rGOs·NC/a GCE/b it | 0.025–718 | 6.11 | [22] |
| Fe2 N·NPs@rGOS/c SPCE/d DPV | 0.05–574.2 | 9.24 | [16] |
| ZnFe2O4·NCs/rGO/a GCE/d DPV | 0.025–534.12 | 8.27 | [19] |
| e HPLC | - | 0.157 | [29] |
| NiCo2S4-Ms/GCE/DPV | 0.005–596.64 | 2.29 | This work |
| Real Samples | Added/nM | Found/nM | Recovery/% | * RSD/% |
|---|---|---|---|---|
| Human blood serum | 50 | 47.58 | 95.16 | 2.45 |
| 100 | 96.48 | 96.48 | 2.81 | |
| Saliva | 50 | 49.12 | 98.24 | 2.56 |
| 100 | 94.29 | 94.29 | 2.76 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.-W.; Tamilalagan, E.; Chen, S.-M.; Akilarasan, M.; Maheshwaran, S.; Liu, X. An Ultra-Sensitive Electrochemical Sensor for the Detection of Carcinogen Oxidative Stress 4-Nitroquinoline N-Oxide in Biologic Matrices Based on Hierarchical Spinel Structured NiCo2O4 and NiCo2S4; A Comparative Study. Int. J. Mol. Sci. 2020, 21, 3273. https://doi.org/10.3390/ijms21093273
Chen T-W, Tamilalagan E, Chen S-M, Akilarasan M, Maheshwaran S, Liu X. An Ultra-Sensitive Electrochemical Sensor for the Detection of Carcinogen Oxidative Stress 4-Nitroquinoline N-Oxide in Biologic Matrices Based on Hierarchical Spinel Structured NiCo2O4 and NiCo2S4; A Comparative Study. International Journal of Molecular Sciences. 2020; 21(9):3273. https://doi.org/10.3390/ijms21093273
Chicago/Turabian StyleChen, Tse-Wei, Elayappan Tamilalagan, Shen-Ming Chen, Muthumariappan Akilarasan, Selvarasu Maheshwaran, and Xiaoheng Liu. 2020. "An Ultra-Sensitive Electrochemical Sensor for the Detection of Carcinogen Oxidative Stress 4-Nitroquinoline N-Oxide in Biologic Matrices Based on Hierarchical Spinel Structured NiCo2O4 and NiCo2S4; A Comparative Study" International Journal of Molecular Sciences 21, no. 9: 3273. https://doi.org/10.3390/ijms21093273
APA StyleChen, T. -W., Tamilalagan, E., Chen, S. -M., Akilarasan, M., Maheshwaran, S., & Liu, X. (2020). An Ultra-Sensitive Electrochemical Sensor for the Detection of Carcinogen Oxidative Stress 4-Nitroquinoline N-Oxide in Biologic Matrices Based on Hierarchical Spinel Structured NiCo2O4 and NiCo2S4; A Comparative Study. International Journal of Molecular Sciences, 21(9), 3273. https://doi.org/10.3390/ijms21093273






