Oxidative Stress and Immune System Dysfunction in Autism Spectrum Disorders
Abstract
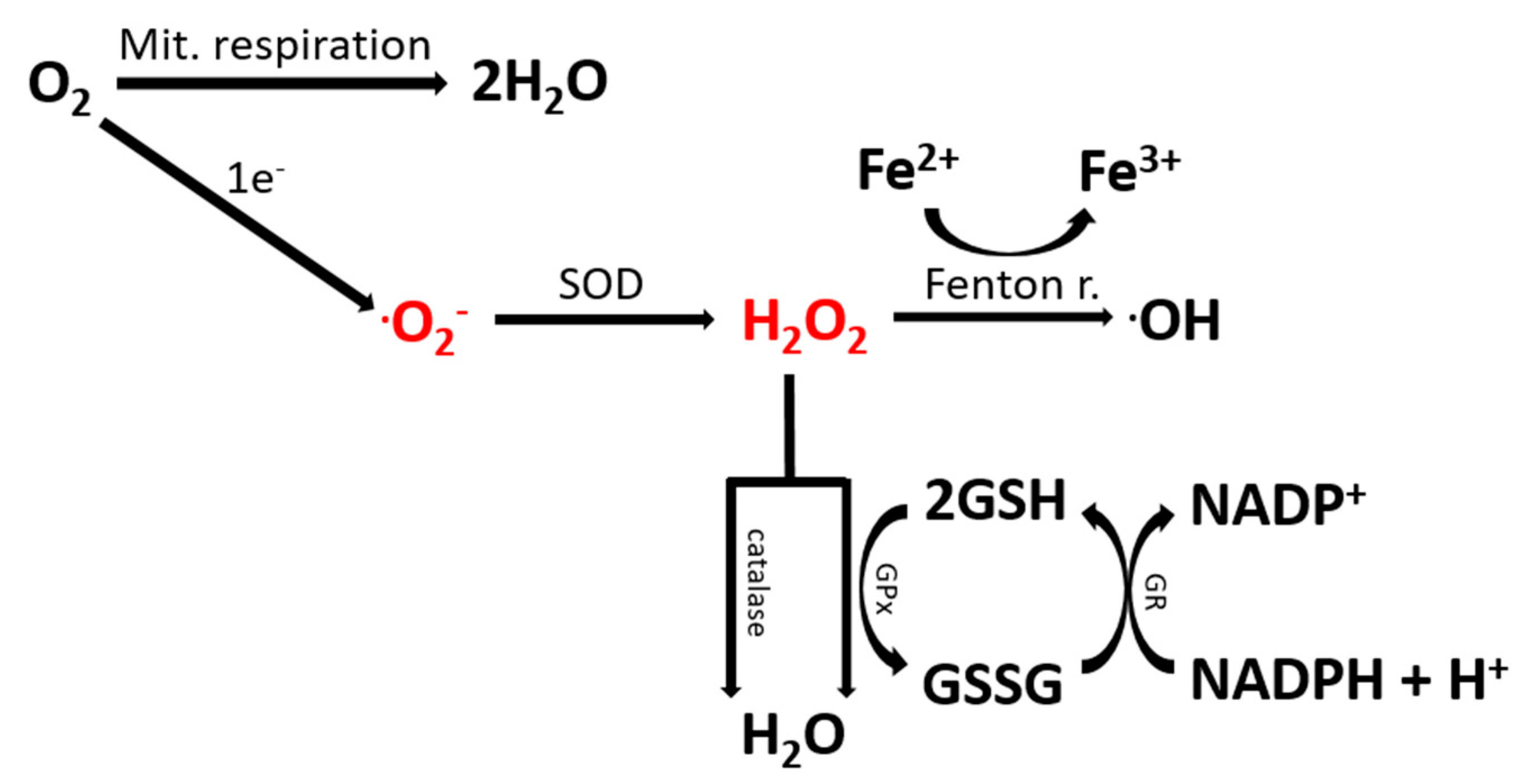
1. Introduction
2. The Contribution of Oxidative Stress to ASD
2.1. Oxidative Stress Is Elevated in ASD Patients
2.2. Oxidative Stress in Human ASD Samples and Mouse Models: A Meta-Analysis
3. Immune System Dysfunction in ASD
4. Infiltration of Immune Cells in the Brain: the Link between ROS, Inflammation and Neurodegeneration
5. Targeting ROS to Treat ASD
6. Conclusions
Funding
Conflicts of Interest
Abbreviations
| ASD | Autism Spectrum Disorders |
| IFN | Interferon |
| GPx | Glutathione peroxidase |
| GR | Glutathione reductase |
| IL | Interleukin |
| NAC | N-acetylcysteine |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| TNF | Tumor necrosis factor |
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Moessner, R.; Marshall, C.R.; Sutcliffe, J.S.; Skaug, J.; Pinto, D.; Vincent, J.; Zwaigenbaum, L.; Fernandez, B.; Roberts, W.; Szatmari, P.; et al. Contribution of SHANK3 Mutations to Autism Spectrum Disorder. Am. J. Hum. Genet. 2007, 81, 1289–1297. [Google Scholar] [CrossRef]
- Jamain, S.; Quach, H.; Betancur, C.; Råstam, M.; Colineaux, C.; Gillberg, I.C.; Söderström, H.; Giros, B.; Leboyer, M.; Gillberg, C.; et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet. 2003, 34, 27–29. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, J.; Wang, Z.; Jia, M.; Lu, T.; Wang, H.; Yue, W.; Zhang, D.; Li, J.; Wang, L. Association between CNTNAP2 polymorphisms and autism: A family-based study in the chinese han population and a meta-analysis combined with GWAS data of psychiatric genomics consortium. Autism Res. 2019, 12, 553–561. [Google Scholar] [CrossRef]
- Buxbaum, J.; Silverman, J.M.; Smith, C.J.; A Greenberg, D.; Kilifarski, M.; Reichert, J.; Jr, E.H.C.; Fang, Y.; Song, C.-Y.; Vitale, R. Association between a GABRB3 polymorphism and autism. Mol. Psychiatry 2002, 7, 311–316. [Google Scholar] [CrossRef]
- Belmonte, M.K.; Bourgeron, T. Fragile X syndrome and autism at the intersection of genetic and neural networks. Nat. Neurosci. 2006, 9, 1221–1225. [Google Scholar] [CrossRef]
- Wiznitzer, M. Autism and Tuberous Sclerosis. J. Child Neurol. 2004, 19, 675–679. [Google Scholar] [CrossRef]
- Rademacher, S.; Eickholt, B.J. PTEN in Autism and Neurodevelopmental Disorders. Cold Spring Harb. Perspect. Med. 2019, 9, a036780. [Google Scholar] [CrossRef]
- Smith, S.E.P.; Zhou, Y.-D.; Zhang, G.; Jin, Z.; Stoppel, D.C.; Anderson, M.P. Increased Gene Dosage of Ube3a Results in Autism Traits and Decreased Glutamate Synaptic Transmission in Mice. Sci. Transl. Med. 2011, 3, 103ra97. [Google Scholar] [CrossRef]
- Swerdlow, R.H. Brain aging, Alzheimer’s disease, and mitochondria. Biochim. Biophys. Acta. 2011, 1812, 1630–1639. [Google Scholar] [CrossRef]
- Reddy, P.H.; Reddy, T.P. Mitochondria as a therapeutic target for aging and neurodegenerative diseases. Curr. Alzheimer Res. 2011, 8, 393–409. [Google Scholar] [CrossRef]
- Georgieva, E.; Ivanova, D.; Zhelev, Z.; Bakalova, R.; Gulubova, M.; Aoki, I. Mitochondrial Dysfunction and Redox Imbalance as a Diagnostic Marker of “Free Radical Diseases. ” Anticancer Res. 2017, 37. [Google Scholar] [CrossRef]
- Devasagayam, T.P.; Tilak, J.C.; Boloor, K.K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R.D. Free radicals and antioxidants in human health: current status and future prospects. J. Assoc. Physicians India 2004, 52, 794–804. [Google Scholar] [PubMed]
- Popa-Wagner, A.; Mitran, S.; Sivanesan, S.; Chang, E.; Buga, A.-M. ROS and Brain Diseases: The Good, the Bad, and the Ugly. Oxidative Med. Cell. Longev. 2013, 2013, 1–14. [Google Scholar] [CrossRef]
- Huang, W.-J.; Zhang, X.; Chen, W.-W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinsons Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef]
- Kumar, A.; Ratan, R.R. Oxidative Stress and Huntington’s Disease: The Good, The Bad, and The Ugly. J. Huntingt. Dis. 2016, 5, 217–237. [Google Scholar] [CrossRef]
- Pollari, E.; Goldsteins, G.; Bart, G.; Koistinaho, J.; Giniatullin, R. The role of oxidative stress in degeneration of the neuromuscular junction in amyotrophic lateral sclerosis. Front. Cell. Neurosci. 2014, 8. [Google Scholar] [CrossRef]
- Ohja, K.; Gozal, E.; Fahnestock, M.; Cai, L.; Cai, J.; Freedman, J.H.; Switala, A.; El-Baz, A.; Barnes, G. Neuroimmunologic and Neurotrophic Interactions in Autism Spectrum Disorders: Relationship to Neuroinflammation. NeuroMolecular Med. 2018, 20, 161–173. [Google Scholar] [CrossRef]
- Zeidán-Chuliá, F.; Salmina, A.B.; Malinovskaya, N.A.; Noda, M.; Verkhratsky, A.; Moreira, J.C.F. The glial perspective of autism spectrum disorders. Neurosci. Biobehav. Rev. 2014, 38, 160–172. [Google Scholar] [CrossRef]
- James, J.; Melnyk, S.; Jernigan, S.; Cleves, M.A.; Halsted, C.H.; Wong, N.H.; Cutler, P.; Bock, K.; Boris, M.; Bradstreet, J.J.; et al. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006, 141, 947–956. [Google Scholar] [CrossRef]
- Rose, S.; Melnyk, S.; Pavliv, O.; Bai, S.; Nick, T.G.; E Frye, R.; James, S.J. Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl. Psychiatry 2012, 2, e134. [Google Scholar] [CrossRef] [PubMed]
- James, J.; Melnyk, S.; Jernigan, S.; Pavliv, O.; Trusty, T.; Lehman, S.; Seidel, L.; Gaylor, D.W.; Cleves, M.A. A functional polymorphism in the reduced folate carrier gene and DNA hypomethylation in mothers of children with autism. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2010, 153, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Al-Gadani, Y.; El-Ansary, A.; Attas, O.; Al-Ayadhi, L. Metabolic biomarkers related to oxidative stress and antioxidant status in Saudi autistic children. Clin. Biochem. 2009, 42, 1032–1040. [Google Scholar] [CrossRef]
- González-Fraguela, M.; Hung, M.-L.D.; Vera, H.; Maragoto, C.; Noris, E.; Blanco, L.; Galvizu, R.; Robinson, M. Oxidative Stress Markers in Children with Autism Spectrum Disorders. Br. J. Med. Med. Res. 2013, 3, 307–317. [Google Scholar] [CrossRef]
- A Meguid, N.; Ghozlan, S.A.S.; Mohamed, M.F.; Ibrahim, M.K.; Dawood, R.M.; El Din, N.B.; Abdelhafez, T.H.; Hemimi, M.; El-Awady, M.K. Expression of Reactive Oxygen Species–Related Transcripts in Egyptian Children With Autism. Biomark. Insights 2017, 12. [Google Scholar] [CrossRef]
- Bolotta, A.; Battistelli, M.; Falcieri, E.; Ghezzo, A.; Manara, M.C.; Manfredini, S.; Marini, M.; Posar, A.; Visconti, P.; Abruzzo, P.M. Oxidative Stress in Autistic Children Alters Erythrocyte Shape in the Absence of Quantitative Protein Alterations and of Loss of Membrane Phospholipid Asymmetry. Oxidative Med. Cell. Longev. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Bauman, M. Medical comorbidities in autism: Challenges to diagnosis and treatment. Neurotherapeutics 2010, 7, 320–327. [Google Scholar] [CrossRef]
- Valiente-Pallejà, A.; Torrell, H.; Muntané, G.; Cortés, M.J.; Martínez-Leal, R.; Abasolo, N.; Alonso, Y.; Vilella, E.; Martorell, L. Genetic and clinical evidence of mitochondrial dysfunction in autism spectrum disorder and intellectual disability. Hum. Mol. Genet. 2018, 27, 891–900. [Google Scholar] [CrossRef]
- Careaga, M.; Schwartzer, J.; Ashwood, P. Inflammatory profiles in the BTBR mouse: how relevant are they to autism spectrum disorders? Brain Behav. Immun. 2014, 43, 11–16. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Attia, S.M.; Alshammari, M.A.; Al-Zahrani, K.S.; Bakheet, S.A. Increased oxidative stress in the cerebellum and peripheral immune cells leads to exaggerated autism-like repetitive behavior due to deficiency of antioxidant response in BTBR T + tf/J mice. Prog. Neuro-Psychopharmacol. Boil. Psychiatry 2018, 89, 245–253. [Google Scholar] [CrossRef]
- Korin, B.; Ben-Shaanan, T.L.; Schiller, M.; Dubovik, T.; Azulay-Debby, H.; Boshnak, N.T.; Koren, T.; Rolls, A. High-dimensional, single-cell characterization of the brain’s immune compartment. Nat. Neurosci. 2017, 20, 1300–1309. [Google Scholar] [CrossRef]
- Lawson, L.; Perry, V.; Gordon, S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience 1992, 48, 405–415. [Google Scholar] [CrossRef]
- Pflieger, F.J.; Hernandez, J.; Schweighöfer, H.; Herden, C.; Rosengarten, B.; Rummel, C. The role of neutrophil granulocytes in immune-to-brain communication. Temperature 2018, 5, 296–307. [Google Scholar] [CrossRef]
- Laurent, C.; Dorothee, G.; Hunot, S.; Martin, E.; Monnet, Y.; Duchamp, M.; Dong, Y.; Légeron, F.-P.; Leboucher, A.; Burnouf, S.; et al. Hippocampal T cell infiltration promotes neuroinflammation and cognitive decline in a mouse model of tauopathy. Brain 2016, 140, 184–200. [Google Scholar] [CrossRef]
- Stubbs, E.G.; Crawford, M.L. Depressed lymphocyte responsiveness in autistic children. J. Autism Child. Schizophr. 1977, 7, 49–55. [Google Scholar] [CrossRef]
- Brigida, A.; Schultz, S.; Cascone, M.; Antonucci, N.; Siniscalco, D. Endocannabinod Signal Dysregulation in Autism Spectrum Disorders: A Correlation Link between Inflammatory State and Neuro-Immune Alterations. Int. J. Mol. Sci. 2017, 18, 1425. [Google Scholar] [CrossRef]
- Siniscalco, D.; Schultz, S.; Brigida, A.; Antonucci, N. Inflammation and Neuro-Immune Dysregulations in Autism Spectrum Disorders. Pharmaceuticals 2018, 11, 56. [Google Scholar] [CrossRef]
- Croonenberghs, J.; Bosmans, E.; Deboutte, D.; Kenis, G.; Maes, M. Activation of the inflammatory response system in autism. Neuropsychobiology 2002, 45, 1–6. [Google Scholar] [CrossRef]
- Ashwood, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Pessah, I.; Van De Water, J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain, Behav. Immun. 2010, 25, 40–45. [Google Scholar] [CrossRef]
- Jácome, M.C.I.; Chacòn, L.M.M.; Cuesta, H.V.; Rizo, C.M.; Santiesteban, M.W.; Hernandez, L.R.; García, E.N.; Fraguela, M.E.G.; I Fernandez, C.; Hurtado, Y.V.; et al. Peripheral Inflammatory Markers Contributing to Comorbidities in Autism. Behav. Sci. 2016, 6, 29. [Google Scholar] [CrossRef]
- Li, X.; Chauhan, A.; Sheikh, A.M.; Patil, S.; Chauhan, V.; Li, X.-M.; Ji, L.; Brown, T.; Malik, M.; Chauhn, A.; et al. Elevated immune response in the brain of autistic patients. J. Neuroimmunol. 2009, 207, 111–116. [Google Scholar] [CrossRef]
- Morgan, J.T.; Chana, G.; Pardo, C.A.; Achim, C.; Semendeferi, K.; Buckwalter, J.; Courchesne, E.; Everall, I.P. Microglial Activation and Increased Microglial Density Observed in the Dorsolateral Prefrontal Cortex in Autism. Boil. Psychiatry 2010, 68, 368–376. [Google Scholar] [CrossRef]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2004, 57, 67–81. [Google Scholar] [CrossRef]
- Gottfried, C.; Bambini-Junior, V.; Francis, F.; Riesgo, R.; Savino, W. The Impact of Neuroimmune Alterations in Autism Spectrum Disorder. Front. Psychol. 2015, 6, 95. [Google Scholar] [CrossRef]
- Masi, A.; Glozier, N.; Dale, R.; Guastella, A.J. The Immune System, Cytokines, and Biomarkers in Autism Spectrum Disorder. Neurosci. Bull. 2017, 33, 194–204. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Arellano, J.J.R.; Parpura, V. Neuroglia in ageing and disease. Cell Tissue Res. 2014, 357, 493–503. [Google Scholar] [CrossRef]
- Careaga, M.; Van De Water, J.; Ashwood, P. Immune dysfunction in autism: A pathway to treatment. Neurotherapeutics 2010, 7, 283–292. [Google Scholar] [CrossRef]
- McDougle, C.J.; Landino, S.M.; Vahabzadeh, A.; O’Rourke, J.; Zürcher, N.R.; Finger, B.C.; Palumbo, M.L.; Helt, J.; Mullett, J.E.; Hooker, J.M.; et al. Toward an immune-mediated subtype of autism spectrum disorder. Brain Res. 2015, 1617, 72–92. [Google Scholar] [CrossRef]
- Chez, M.; Guido-Estrada, N. Immune therapy in autism: Historical experience and future directions with immunomodulatory therapy. Neurotherapeutics 2010, 7, 293–301. [Google Scholar] [CrossRef]
- Masi, A.; Quintana, D.S.; Glozier, N.; Lloyd, A.; Hickie, I.B.; Guastella, A.J. Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Mol. Psychiatry 2014, 20, 440–446. [Google Scholar] [CrossRef]
- Gładysz, D.; Krzywdzińska, A.; Hozyasz, K.K. Immune abnormalities in autism spectrum disorder—Could they hold promise for causative treatment? Mol. Neurobiol. 2018, 55, 6387–6435. [Google Scholar] [CrossRef] [PubMed]
- Ashwood, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Pessah, I.N.; Van De Water, J. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J. Neuroimmunol. 2010, 232, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Heuer, L.; Ashwood, P.; Schauer, J.; Goines, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Croen, L.A.; Pessah, I.N.; Van De Water, J. Reduced levels of immunoglobulin in children with autism correlates with behavioral symptoms. Autism Res. 2008, 1, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Hollander, E.; DelGiudice-Asch, G.; Simon, L.; Schmeidler, J.; Cartwright, C.; DeCaria, C.M.; Kwon, J.; Cunningham-Rundles, C.; Chapman, F.; Zabriskie, J.B. B lymphocyte antigen D8/17 and repetitive behaviors in autism. Am. J. Psychiatry 1999, 156, 317–320. [Google Scholar]
- Mostafa, G.A.; Al-Ayadhi, L. The relationship between the increased frequency of serum antineuronal antibodies and the severity of autism in children. Eur. J. Paediatr. Neurol. 2012, 16, 464–468. [Google Scholar] [CrossRef]
- Piras, I.S.; Haapanen, L.; Napolioni, V.; Sacco, R.; Van De Water, J.; Persico, A.M. Anti-brain antibodies are associated with more severe cognitive and behavioral profiles in Italian children with Autism Spectrum Disorder. Brain Behav. Immun. 2014, 38, 91–99. [Google Scholar] [CrossRef]
- Mostafa, G.A.; Al-Ayadhi, L. Increased serum levels of anti-ganglioside M1 auto-antibodies in autistic children: relation to the disease severity. J. Neuroinflammation 2011, 8, 39. [Google Scholar] [CrossRef]
- Braunschweig, D.; Duncanson, P.; Boyce, R.; Hansen, R.; Ashwood, P.; Pessah, I.N.; Hertz-Picciotto, I.; Van De Water, J. Behavioral correlates of maternal antibody status among children with autism. J. Autism Dev. Disord. 2012, 42, 1435–1445. [Google Scholar] [CrossRef]
- Atladóttir, H.Ó.; Pedersen, M.G.; Thorsen, P.; Mortensen, P.; Deleuran, B.; Eaton, W.W.; Parner, E.; Sutton, R.; Niles, D.E.; Nysaether, J.; et al. Association of Family History of Autoimmune Diseases and Autism Spectrum Disorders. Pediatrics 2009, 124, 687–694. [Google Scholar] [CrossRef]
- Comi, A.M.; Zimmerman, A.W.; Frye, V.H.; Law, P.A.; Peeden, J.N. Familial clustering of autoimmune disorders and evaluation of medical risk factors in autism. J. Child Neurol. 1999, 14, 388–394. [Google Scholar] [CrossRef]
- Keil, A.P.; Daniels, J.L.; Forssén, U.; Hultman, C.; Cnattingius, S.; Söderberg, K.C.; Feychting, M.; Sparén, P. Parental Autoimmune Diseases Associated With Autism Spectrum Disorders in Offspring. Epidemiology 2010, 21, 805–808. [Google Scholar] [CrossRef] [PubMed]
- A Molloy, C.; Morrow, A.L.; Meinzen-Derr, J.; Dawson, G.; Bernier, R.; Dunn, M.; Hyman, S.L.; McMahon, W.M.; Goudie-Nice, J.; Hepburn, S.; et al. Familial Autoimmune Thyroid Disease as a Risk Factor for Regression in Children with Autism Spectrum Disorder: A CPEA Study. J. Autism Dev. Disord. 2006, 36, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Sweeten, T.L.; Bowyer, S.L.; Posey, D.J.; Halberstadt, G.M.; McDougle, C.J. Increased Prevalence of Familial Autoimmunity in Probands With Pervasive Developmental Disorders. Pediatrics 2003, 112. [Google Scholar] [CrossRef] [PubMed]
- Patterson, P.H. Modeling autistic features in animals. Pediatr. Res. 2011, 69, 34R–40R. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; Patterson, P.H. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav. Immun. 2010, 25, 604–615. [Google Scholar] [CrossRef]
- Smith, S.E.P.; Li, J.; Garbett, K.; Mirnics, K.; Patterson, P.H. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 2007, 27, 10695–10702. [Google Scholar] [CrossRef]
- Chan, P.H. Reactive Oxygen Radicals in Signaling and Damage in the Ischemic Brain. Br. J. Pharmacol. 2001, 21, 2–14. [Google Scholar] [CrossRef]
- Hsieh, H.-L.; Yang, C.-M. Role of Redox Signaling in Neuroinflammation and Neurodegenerative Diseases. BioMed Res. Int. 2013, 2013, 1–18. [Google Scholar] [CrossRef]
- Pangrazzi, L.; Meryk, A.; Naismith, E.; Koziel, R.; Lair, J.; Krismer, M.; Trieb, K.; Grubeck-Loebenstein, B. “Inflamm-aging” influences immune cell survival factors in human bone marrow. Eur. J. Immunol. 2017, 47, 481–492. [Google Scholar] [CrossRef]
- Naismith, E.; Pangrazzi, L.; Grasse, M.; Keller, M.; Miggitsch, C.; Weinberger, B.; Trieb, K.; Grubeck-Loebenstein, B. Peripheral antibody concentrations are associated with highly differentiated T cells and inflammatory processes in the human bone marrow. Immun. Ageing 2019, 16, 21. [Google Scholar] [CrossRef]
- Naismith, E.; Pangrazzi, L. The impact of oxidative stress, inflammation, and senescence on the maintenance of immunological memory in the bone marrow in old age. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed]
- Salzano, S.; Checconi, P.; Hanschmann, E.-M.; Lillig, C.H.; Bowler, L.D.; Chan, P.; Vaudry, H.; Mengozzi, M.; Coppo, L.; Sacre, S.; et al. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc. Natl. Acad. Sci. 2014, 111, 12157–12162. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, P. Role of glutathione in immunity and inflammation in the lung. Int. J. Gen. Med. 2011, 4, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, O. Cerebroprotective effect of resveratrol through antioxidant and anti-inflammatory effects in diabetic rats. Naunyn Schmiedebergs Arch. Pharmacol. 2013, 386, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Shemer, A.; Erny, D.; Jung, S.; Prinz, M. Microglia Plasticity During Health and Disease: An Immunological Perspective. Trends Immunol. 2015, 36, 614–624. [Google Scholar] [CrossRef]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2017, 18, 225–242. [Google Scholar] [CrossRef]
- Sierra, A.; Encinas, J.M.; Deudero, J.J.P.; Chancey, J.; Enikolopov, G.N.; Overstreet-Wadiche, L.; Tsirka, S.E.; Maletic-Savatic, M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 2010, 7, 483–495. [Google Scholar] [CrossRef]
- Hagemeyer, N.; Hanft, K.-M.; Akriditou, M.-A.; Unger, N.; Park, E.S.; Stanley, E.R.; Staszewski, O.; Dimou, L.; Prinz, M. Microglia contribute to normal myelinogenesis and to oligodendrocyte progenitor maintenance during adulthood. Acta Neuropathol. 2017, 134, 441–458. [Google Scholar] [CrossRef]
- Streit, W.J.; Graeber, M.B.; Kreutzberg, G.W. Functional plasticity of microglia: A review. Glia 1988, 1, 301–307. [Google Scholar] [CrossRef]
- Rodriguez, J.I.; Kern, J.K. Evidence of microglial activation in autism and its possible role in brain underconnectivity. Neuron Glia Boil. 2011, 7, 205–213. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Maccarrone, M. Chronic Inflammatory Disorders and Their Redox Control: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid. Redox Signal. 2011, 15, 2605–2641. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Maier, O. Interrelation of Oxidative Stress and Inflammation in Neurodegenerative Disease: Role of TNF. Oxidative Med. Cell. Longev. 2015, 2015, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Voet, S.; Prinz, M.; Van Loo, G. Microglia in Central Nervous System Inflammation and Multiple Sclerosis Pathology. Trends Mol. Med. 2019, 25, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Smolders, J.; Heutinck, K.M.; Fransen, N.L.; Remmerswaal, E.B.M.; Hombrink, P.; Berge, I.J.M.T.; Van Lier, R.A.W.; Huitinga, I.; Hamann, J. Tissue-resident memory T cells populate the human brain. Nat. Commun. 2018, 9, 4593. [Google Scholar] [CrossRef]
- Daglas, M.; Draxler, D.F.; Ho, H.; McCutcheon, F.; Galle, A.; Au, A.E.; Larsson, P.; Gregory, J.; Alderuccio, F.; Sashindranath, M.; et al. Activated CD8+ T Cells Cause Long-Term Neurological Impairment after Traumatic Brain Injury in Mice. Cell Rep. 2019, 29, 1178–1191.e6. [Google Scholar] [CrossRef]
- Dulken, B.W.; Buckley, M.T.; Negredo, P.N.; Saligrama, N.; Cayrol, R.; Leeman, D.S.; George, B.M.; Boutet, S.C.; Hebestreit, K.; Pluvinage, J.V.; et al. Single-cell analysis reveals T cell infiltration in old neurogenic niches. Nature 2019, 571, 205–210. [Google Scholar] [CrossRef]
- Manivasagam, T.; Arunadevi, S.; Essa, M.M.; Saravanababu, C.; Borah, A.; Thenmozhi, A.J.; Qoronfleh, M.W. Role of Oxidative Stress and Antioxidants in Autism. Adv. Neurobiol. 2020, 24, 193–206. [Google Scholar] [CrossRef]
- Gvozdjáková, A.; Kucharská, J.; Ostatníková, D.; Babinská, K.; Nakladal, D.; Crane, F.L. Ubiquinol Improves Symptoms in Children with Autism. Oxidative Med. Cell. Longev. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Mousavinejad, E.; Ghaffari, M.A.; Riahi, F.; Hajmohammadi, M.; Tiznobeyk, Z.; Mousavinejad, M. Coenzyme Q10 supplementation reduces oxidative stress and decreases antioxidant enzyme activity in children with autism spectrum disorders. Psychiatry Res. Neuroimaging 2018, 265, 62–69. [Google Scholar] [CrossRef]
- Whillier, S.; Raftos, J.E.; Chapman, B.; Kuchel, P.W. Role ofN-acetylcysteine and cystine in glutathione synthesis in human erythrocytes. Redox Rep. 2009, 14, 115–124. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, W.; Zhai, Q.; Zhang, T.; Wen, X. N-acetylcysteine ameliorates repetitive/stereotypic behavior due to its antioxidant properties without activation of the canonical Wnt pathway in a valproic acid-induced rat model of autism. Mol. Med. Rep. 2017, 16, 2233–2240. [Google Scholar] [CrossRef] [PubMed]
- Hardan, A.Y.; Fung, L.K.; Libove, R.A.; Obukhanych, T.V.; Nair, S.; Herzenberg, L.A.; Frazier, T.W.; Tirouvanziam, R. A randomized controlled pilot trial of oral N-acetylcysteine in children with autism. Boil. Psychiatry 2012, 71, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Çelebi, F.; Koyuncu, A.; Coskun, M. N-acetylcysteine may reduce repetitive behaviors in children with autism: a case series. Psychiatry Clin. Psychopharmacol. 2017, 27, 185–188. [Google Scholar] [CrossRef]
- Naveed, S.; Amray, A.; Waqas, A.; Chaudhary, A.M.; Azeem, M.W. Use of N-Acetylcysteine in Psychiatric Conditions among Children and Adolescents: A Scoping Review. Cureus 2017, 9, 1888. [Google Scholar] [CrossRef]
- Dolske, M.C.; Spollen, J.; McKay, S.; Lancashire, E.; Tolbert, L. A preliminary trial of ascorbic acid as supplemental therapy for autism. Prog. Neuro-Psychopharmacol. Boil. Psychiatry 1993, 17, 765–774. [Google Scholar] [CrossRef]
- Bjørklund, G.; Waly, M.I.; Al-Farsi, Y.; Saad, K.; Dadar, M.; Rahman, M.; Elhoufey, A.; Chirumbolo, S.; Jóźwik-Pruska, J.; Kałużna-Czaplińska, J. The Role of Vitamins in Autism Spectrum Disorder: What Do We Know? J. Mol. Neurosci. 2019, 67, 373–387. [Google Scholar] [CrossRef]
- Zheltova, A.A.; Kharitonova, M.V.; Iezhitsa, I.; Spasov, A.A. Magnesium deficiency and oxidative stress: An update. BioMedicine 2016, 6, 20. [Google Scholar] [CrossRef]
- Mousain-Bosc, M.; Siatka, C.; Bali, J.-P. Magnesium, hyperactivity and autism in children. In Magnesium in the Central Nervous System; Cambridge University Press (CUP): Cambridge, UK, 2012; pp. 283–302. [Google Scholar]
- Ornoy, A.; Weinstein-Fudim, L.; Tfilin, M.; Ergaz, Z.; Yanai, J.; Szyf, M.; Turgeman, G. S-adenosyl methionine prevents ASD like behaviors triggered by early postnatal valproic acid exposure in very young mice. Neurotoxicology Teratol. 2019, 71, 64–74. [Google Scholar] [CrossRef]
- Ehlers, K.; Elmazar, M.M.; Tzimas, G. Methionine Reduces the Valproic Acid-Induced Spina Bifida Rate in Mice without Altering Valproic Acid Kinetics. J. Nutr. 1996, 126, 67–75. [Google Scholar] [CrossRef]
- Villalobos, M.; De La Cruz, J.; Cuerda, M.; Ortiz, P.; Smith-Agreda, J.; De La Cuesta, F.S. Effect of S-adenosyl-L-methionine on rat brain oxidative stress damage in a combined model of permanent focal ischemia and global ischemia-reperfusion. Brain Res. 2000, 883, 31–40. [Google Scholar] [CrossRef]
- Gonzalez-Correa, J.A.; De La Cruz, J.; Aurioles, E.M.; A Lopez-Egea, M.; Ortiz, P.; De La Cuesta, F.S. Effects of S-adenosyl-L-methionine on hepatic and renal oxidative stress in an experimental model of acute biliary obstruction in rats. Hepatology 1997, 26, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cui, J.; Fang, C.; Liu, M.; Min, G.; Li, L. S-Adenosylmethionine Attenuates Oxidative Stress and Neuroinflammation Induced by Amyloid-β Through Modulation of Glutathione Metabolism. J. Alzheimer’s Dis. 2017, 58, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, D.; Frye, R.E. Melatonin in autism spectrum disorders: a systematic review and meta-analysis. Dev. Med. Child Neurol. 2011, 53, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Sadek, A.; Berk, L.S.; Mainess, K.; Daher, N.S. Antioxidants and Autism: Teachers’ Perceptions of Behavioral Changes. Adv. Mind Body Med. 2018, 32, 12–17. [Google Scholar]
- Hafizi, S.; Tabatabaei, D.; Lai, M.-C. Review of Clinical Studies Targeting Inflammatory Pathways for Individuals With Autism. Front. Psychol. 2019, 10, 849. [Google Scholar] [CrossRef]

| Gene | Statistic Test | p_Value | FDR | Expression in ASD Mouse Models |
|---|---|---|---|---|
| SOD1 | 1.24 | 0.11 | 0.26 | no change |
| SOD2 | 3.47 | 0.00 | 0.03 | down |
| SOD3 | 1.68 | 0.05 | 0.19 | down |
| CAT | 0.60 | 0.27 | 0.39 | no change |
| GPX1 | 0.31 | 0.38 | 0.45 | no change |
| GPX2 | 0.05 | 0.48 | 0.49 | no change |
| GPX3 | 2.57 | 0.01 | 0.08 | down |
| GPX4 | 1.69 | 0.05 | 0.19 | down |
| GSTM1 | 1.19 | 0.12 | 0.27 | no change |
| GSR | 0.75 | 0.23 | 0.36 | no change |
| GSTA1 | 2.68 | 0.00 | 0.07 | up |
| GSTA4 | 2.46 | 0.01 | 0.09 | down |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pangrazzi, L.; Balasco, L.; Bozzi, Y. Oxidative Stress and Immune System Dysfunction in Autism Spectrum Disorders. Int. J. Mol. Sci. 2020, 21, 3293. https://doi.org/10.3390/ijms21093293
Pangrazzi L, Balasco L, Bozzi Y. Oxidative Stress and Immune System Dysfunction in Autism Spectrum Disorders. International Journal of Molecular Sciences. 2020; 21(9):3293. https://doi.org/10.3390/ijms21093293
Chicago/Turabian StylePangrazzi, Luca, Luigi Balasco, and Yuri Bozzi. 2020. "Oxidative Stress and Immune System Dysfunction in Autism Spectrum Disorders" International Journal of Molecular Sciences 21, no. 9: 3293. https://doi.org/10.3390/ijms21093293
APA StylePangrazzi, L., Balasco, L., & Bozzi, Y. (2020). Oxidative Stress and Immune System Dysfunction in Autism Spectrum Disorders. International Journal of Molecular Sciences, 21(9), 3293. https://doi.org/10.3390/ijms21093293





