Tonabersat Inhibits Connexin43 Hemichannel Opening and Inflammasome Activation in an In Vitro Retinal Epithelial Cell Model of Diabetic Retinopathy
Abstract
:1. Introduction
2. Results
2.1. Tonabersat Prevented HG + Cyt-Induced ATP Release in a Similar Manner to Peptide5
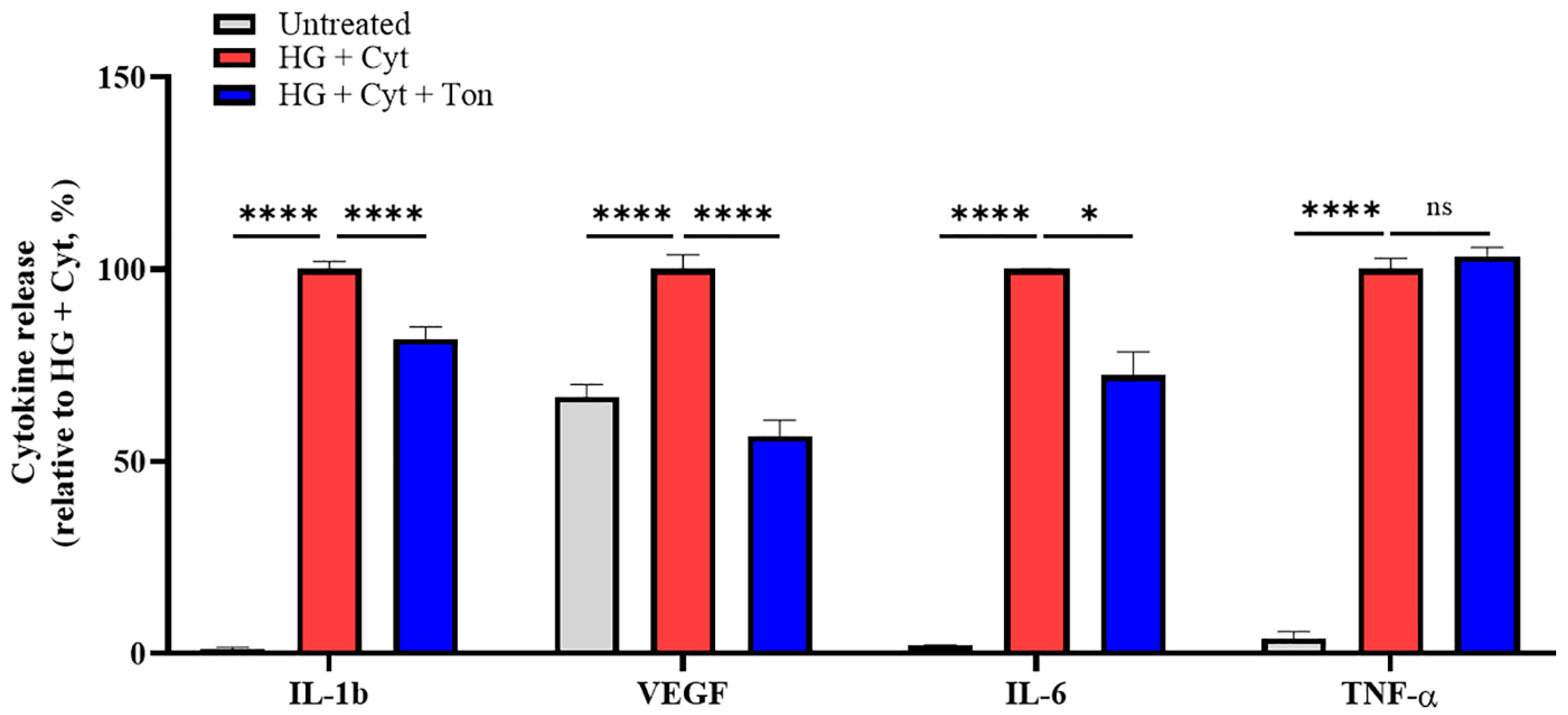
2.2. Tonabersat Treatment Inhibited HG + Cyt-Induced Proinflammatory Cytokine Release
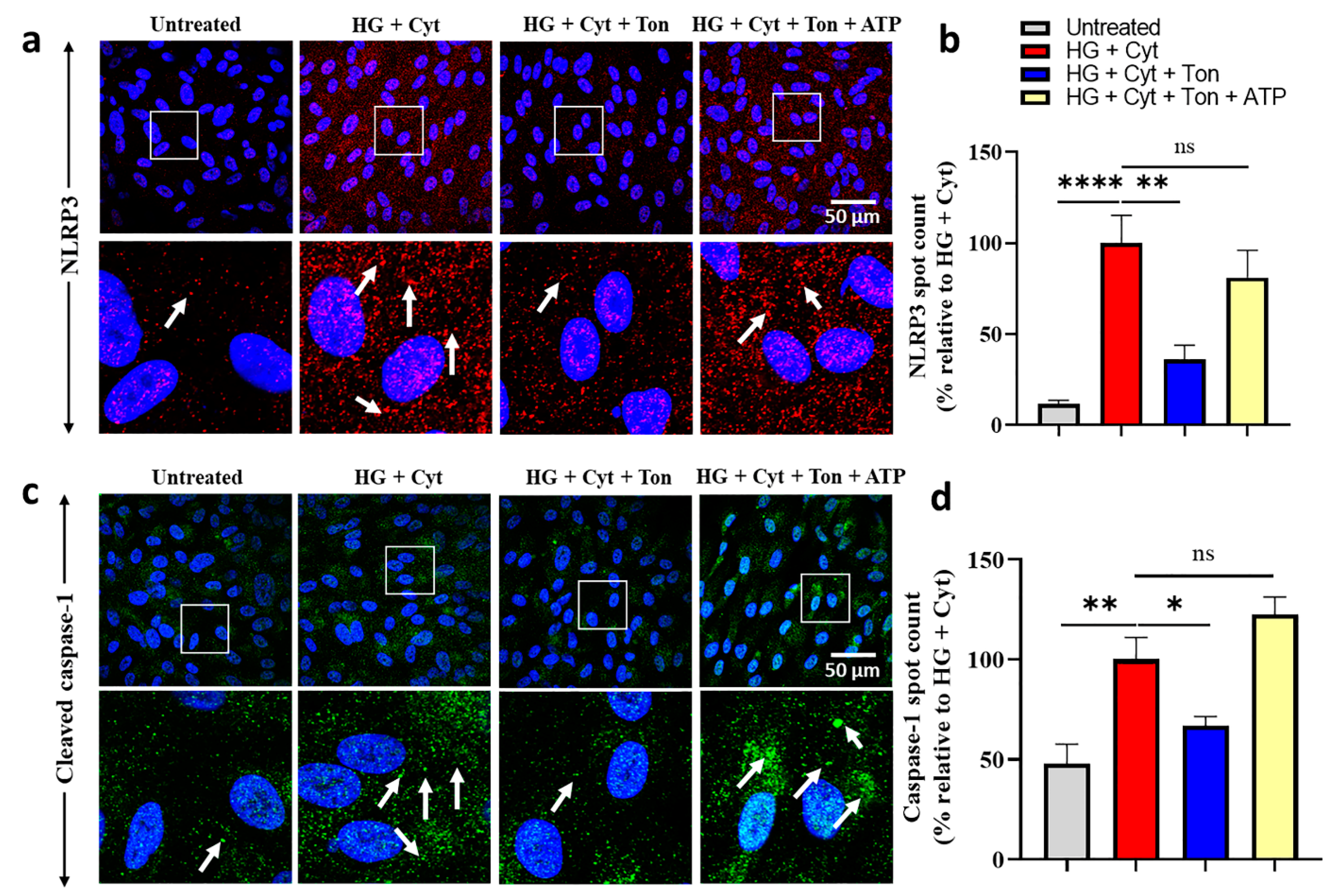
2.3. Tonabersat Treatment Prevented NLRP3 Inflammasome Assembly in an ATP-Dependent Manner
2.4. Tonabersat Treatment Prevented Upregulation of Cleaved Caspase-1 in an ATP-Dependent Manner
2.5. Neither HG + Cyt Challenge Nor Tonabersat Treatment Affected NLRP1 Levels or Localization
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. High Glucose and Cytokine Challenge and Application of Treatments
4.3. ATP Release Assay
4.4. Cytokine Profiling Using the Luminex Bead Array
4.5. Immunocytochemistry
4.6. Image Analysis and Antibody Quantification
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cunha-Vaz, J.; Bernardes, R.; Lobo, C. Blood-retinal barrier. Eur. J. Ophthalmol. 2011, 21 (Suppl. 6), S3–S9. [Google Scholar] [CrossRef]
- Kroll, P.; Rodrigues, E.B.; Hoerle, S. Pathogenesis and classification of proliferative diabetic vitreoretinopathy. Ophthalmologica 2007, 221, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Heng, L.Z.; Comyn, O.; Peto, T.; Tadros, C.; Ng, E.; Sivaprasad, S.; Hykin, P.G. Diabetic retinopathy: Pathogenesis, clinical grading, management and future developments. Diabet. Med. 2013, 30, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Aiello, L.P.; Rosenfeld, P.J. Anti-Vascular Endothelial Growth Factor Agents in the Treatment of Retinal Disease: From Bench to Bedside. Ophthalmology 2016, 123, S78–S88. [Google Scholar] [CrossRef] [PubMed]
- Dhoot, D.S.; Avery, R.L. Vascular Endothelial Growth Factor Inhibitors for Diabetic Retinopathy. Curr. Diabetes Rep. 2016, 16, 122. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.; Munk, M.R.; Ebneter, A.; Wolf, S.; Zinkernagel, M.S. Retinal Ganglion Cell Layer Change in Patients Treated With Anti-Vascular Endothelial Growth Factor for Neovascular Age-related Macular Degeneration. Am. J. Ophthalmol. 2016, 167, 10–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, H.J.; Kim, S.-N.; Chung, H.; Kim, T.-E.; Kim, H.C. Intravitreal Anti–Vascular Endothelial Growth Factor Therapy and Retinal Nerve Fiber Layer Loss in Eyes With Age-Related Macular Degeneration: A Meta-AnalysisIntravitreal Anti-VEGF Therapy and RNFL Loss in AMD. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1798–1806. [Google Scholar] [CrossRef] [Green Version]
- Kniggendorf, V.F.; Novais, E.A.; Kniggendorf, S.L.; Xavier, C.; Cole, E.D.; Regatieri, C.V. Effect of intravitreal anti-VEGF on choroidal thickness in patients with diabetic macular edema using spectral domain OCT. Arq. Bras. Oftalmol. 2016, 79, 155–158. [Google Scholar] [CrossRef] [Green Version]
- Maguire, M.G.; Martin, D.F.; Ying, G.S.; Jaffe, G.J.; Daniel, E.; Grunwald, J.E.; Toth, C.A.; Ferris, F.L., 3rd; Fine, S.L.; Comparison of Age-related Macular Degeneration Treatments Trials Research Group. Five-Year Outcomes with Anti-Vascular Endothelial Growth Factor Treatment of Neovascular Age-Related Macular Degeneration: The Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2016, 123, 1751–1761. [Google Scholar] [CrossRef] [Green Version]
- Munk, M.R.; Ceklic, L.; Ebneter, A.; Huf, W.; Wolf, S.; Zinkernagel, M.S. Macular atrophy in patients with long-term anti-VEGF treatment for neovascular age-related macular degeneration. Acta Ophthalmol. 2016, 94, e757–e764. [Google Scholar] [CrossRef] [Green Version]
- Mugisho, O.O.; Green, C.R.; Kho, D.T.; Zhang, J.; Graham, E.S.; Acosta, M.L.; Rupenthal, I.D. The inflammasome pathway is amplified and perpetuated in an autocrine manner through connexin43 hemichannel mediated ATP release. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 385–393. [Google Scholar] [CrossRef]
- Saez, J.C.; Contreras-Duarte, S.; Gomez, G.I.; Labra, V.C.; Santibanez, C.A.; Gajardo-Gomez, R.; Avendano, B.C.; Diaz, E.F.; Montero, T.D.; Velarde, V.; et al. Connexin 43 Hemichannel Activity Promoted by Pro-Inflammatory Cytokines and High Glucose Alters Endothelial Cell Function. Front. Immunol. 2018, 9, 1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beyer, E.C.; Paul, D.L.; Goodenough, D.A. Connexin family of gap junction proteins. J. Membr. Biol. 1990, 116, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.H. Cell communication across gap junctions: A historical perspective and current developments. Biochem. Soc. Trans. 2015, 43, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.M.; Gilula, N.B. The gap junction communication channel. Cell 1996, 84, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Leybaert, L.; Lampe, P.D.; Dhein, S.; Kwak, B.R.; Ferdinandy, P.; Beyer, E.C.; Laird, D.W.; Naus, C.C.; Green, C.R.; Schulz, R. Connexins in Cardiovascular and Neurovascular Health and Disease: Pharmacological Implications. Pharmacol. Rev. 2017, 69, 396–478. [Google Scholar] [CrossRef]
- Saez, J.C.; Berthoud, V.M.; Branes, M.C.; Martinez, A.D.; Beyer, E.C. Plasma membrane channels formed by connexins: Their regulation and functions. Physiol. Rev. 2003, 83, 1359–1400. [Google Scholar] [CrossRef] [Green Version]
- Danesh-Meyer, H.V.; Zhang, J.; Acosta, M.L.; Rupenthal, I.D.; Green, C.R. Connexin43 in retinal injury and disease. Prog. Retin. Eye Res. 2016, 51, 41–68. [Google Scholar] [CrossRef]
- Kuo, C.; Green, C.R.; Rupenthal, I.D.; Mugisho, O.O. Connexin43 hemichannel block protects against retinal pigment epithelial cell barrier breakdown. Acta Diabetol. 2020, 57, 13–22. [Google Scholar] [CrossRef]
- Mugisho, O.O.; Green, C.R.; Squirrell, D.M.; Bould, S.; Danesh-Meyer, H.V.; Zhang, J.; Acosta, M.L.; Rupenthal, I.D. Connexin43 hemichannel block protects against the development of diabetic retinopathy signs in a mouse model of the disease. J. Mol. Med. 2019, 97, 215–229. [Google Scholar] [CrossRef]
- Kim, Y.; Griffin, J.M.; Nor, M.N.M.; Zhang, J.; Freestone, P.S.; Danesh-Meyer, H.V.; Rupenthal, I.D.; Acosta, M.; Nicholson, L.F.B.; O’Carroll, S.J.; et al. Tonabersat Prevents Inflammatory Damage in the Central Nervous System by Blocking Connexin43 Hemichannels. Neurotherapeutics 2017, 14, 1148–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nor, M.N.M.; Rupenthal, I.D.; Green, C.R.; Acosta, M.L. Connexin Hemichannel Block Using Orally Delivered Tonabersat Improves Outcomes in Animal Models of Retinal Disease. Neurotherapeutics 2020, 17, 371–387. [Google Scholar]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Tupik, J.D.; Nagai-Singer, M.A.; Allen, I.C. To protect or adversely affect? The dichotomous role of the NLRP1 inflammasome in human disease. Mol. Asp. Med. 2020, 76, 100858. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Wu, F.; Zheng, T.; Wang, X.; Chen, Y.; Wu, X. Kaempferol attenuates retinal ganglion cell death by suppressing NLRP1/NLRP3 inflammasomes and caspase-8 via JNK and NF-κB pathways in acute glaucoma. Eye 2019, 33, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.; Li, F.; Chen, H.; Wang, Y.; Zhu, Y.; Yang, X.; Zhu, J.; Wu, F.; Ouyang, H.; Ge, J.; et al. Caspase-8 promotes NLRP1/NLRP3 inflammasome activation and IL-1beta production in acute glaucoma. Proc. Natl. Acad. Sci. USA 2014, 111, 11181–11186. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liu, C.; Wan, X.S.; Li, S.W. NLRP1 deficiency attenuates diabetic retinopathy (DR) in mice through suppressing inflammation response. Biochem. Biophys. Res. Commun. 2018, 501, 351–357. [Google Scholar] [CrossRef]
- Akhtar-Schafer, I.; Wang, L.; Krohne, T.U.; Xu, H.; Langmann, T. Modulation of three key innate immune pathways for the most common retinal degenerative diseases. EMBO Mol. Med. 2018, 10, e8259. [Google Scholar] [CrossRef]
- Tsakiri, N.; Kimber, I.; Rothwell, N.; Pinteaux, E. Interleukin-1-induced interleukin-6 synthesis is mediated by the neutral sphingomyelinase/Src kinase pathway in neurones. Br. J. Pharmacol. 2008, 153, 775–783. [Google Scholar] [CrossRef]
- Bethea, J.R.; Gillespie, G.Y.; Benveniste, E.N. Interleukin-1 beta induction of TNF-alpha gene expression: Involvement of protein kinase C. J. Cell. Physiol. 1992, 152, 264–273. [Google Scholar] [CrossRef]
- Tanaka, T.; Kanai, H.; Sekiguchi, K.; Aihara, Y.; Yokoyama, T.; Arai, M.; Kanda, T.; Nagai, R.; Kurabayashi, M. Induction of VEGF gene transcription by IL-1 beta is mediated through stress-activated MAP kinases and Sp1 sites in cardiac myocytes. J. Mol. Cell. Cardiol. 2000, 32, 1955–1967. [Google Scholar] [CrossRef] [PubMed]
- Gross, O.; Yazdi, A.S.; Thomas, C.J.; Masin, M.; Heinz, L.X.; Guarda, G.; Quadroni, M.; Drexler, S.K.; Tschopp, J. Inflammasome activators induce interleukin-1alpha secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity 2012, 36, 388–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, S.W.; Min, B.W.; Lim, Y.; Lee, Y.H.; Shin, S.Y. Regulatory mechanism of TNFalpha autoregulation in HaCaT cells: The role of the transcription factor EGR-1. Biochem. Biophys. Res. Commun. 2008, 374, 777–782. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyon, H.; Shome, A.; Rupenthal, I.D.; Green, C.R.; Mugisho, O.O. Tonabersat Inhibits Connexin43 Hemichannel Opening and Inflammasome Activation in an In Vitro Retinal Epithelial Cell Model of Diabetic Retinopathy. Int. J. Mol. Sci. 2021, 22, 298. https://doi.org/10.3390/ijms22010298
Lyon H, Shome A, Rupenthal ID, Green CR, Mugisho OO. Tonabersat Inhibits Connexin43 Hemichannel Opening and Inflammasome Activation in an In Vitro Retinal Epithelial Cell Model of Diabetic Retinopathy. International Journal of Molecular Sciences. 2021; 22(1):298. https://doi.org/10.3390/ijms22010298
Chicago/Turabian StyleLyon, Heather, Avik Shome, Ilva D. Rupenthal, Colin R. Green, and Odunayo O. Mugisho. 2021. "Tonabersat Inhibits Connexin43 Hemichannel Opening and Inflammasome Activation in an In Vitro Retinal Epithelial Cell Model of Diabetic Retinopathy" International Journal of Molecular Sciences 22, no. 1: 298. https://doi.org/10.3390/ijms22010298
APA StyleLyon, H., Shome, A., Rupenthal, I. D., Green, C. R., & Mugisho, O. O. (2021). Tonabersat Inhibits Connexin43 Hemichannel Opening and Inflammasome Activation in an In Vitro Retinal Epithelial Cell Model of Diabetic Retinopathy. International Journal of Molecular Sciences, 22(1), 298. https://doi.org/10.3390/ijms22010298





