Revisiting Platinum-Based Anticancer Drugs to Overcome Gliomas
Abstract
:1. Clinical Treatment for Overcoming Brain Tumors
2. Characteristics of Platinum-Based Anticancer Drugs
2.1. Anticancer Action Mechanism of Platinum-Based Anticancer Drugs
2.2. Efficacy and Advantages of Platinum-Based Anticancer Drugs
2.3. Limitations of Platinum-Based Anticancer Drugs
3. Application of Platinum-Based Anticancer Drugs in Clinical Treatment
3.1. Clinical Trials of Cisplatin in Brain Tumor Treatment
3.2. Clinical Trials of Carboplatin in Brain Tumor Treatment
3.3. Clinical Trials of Oxaliplatin in Brain Tumor Treatment
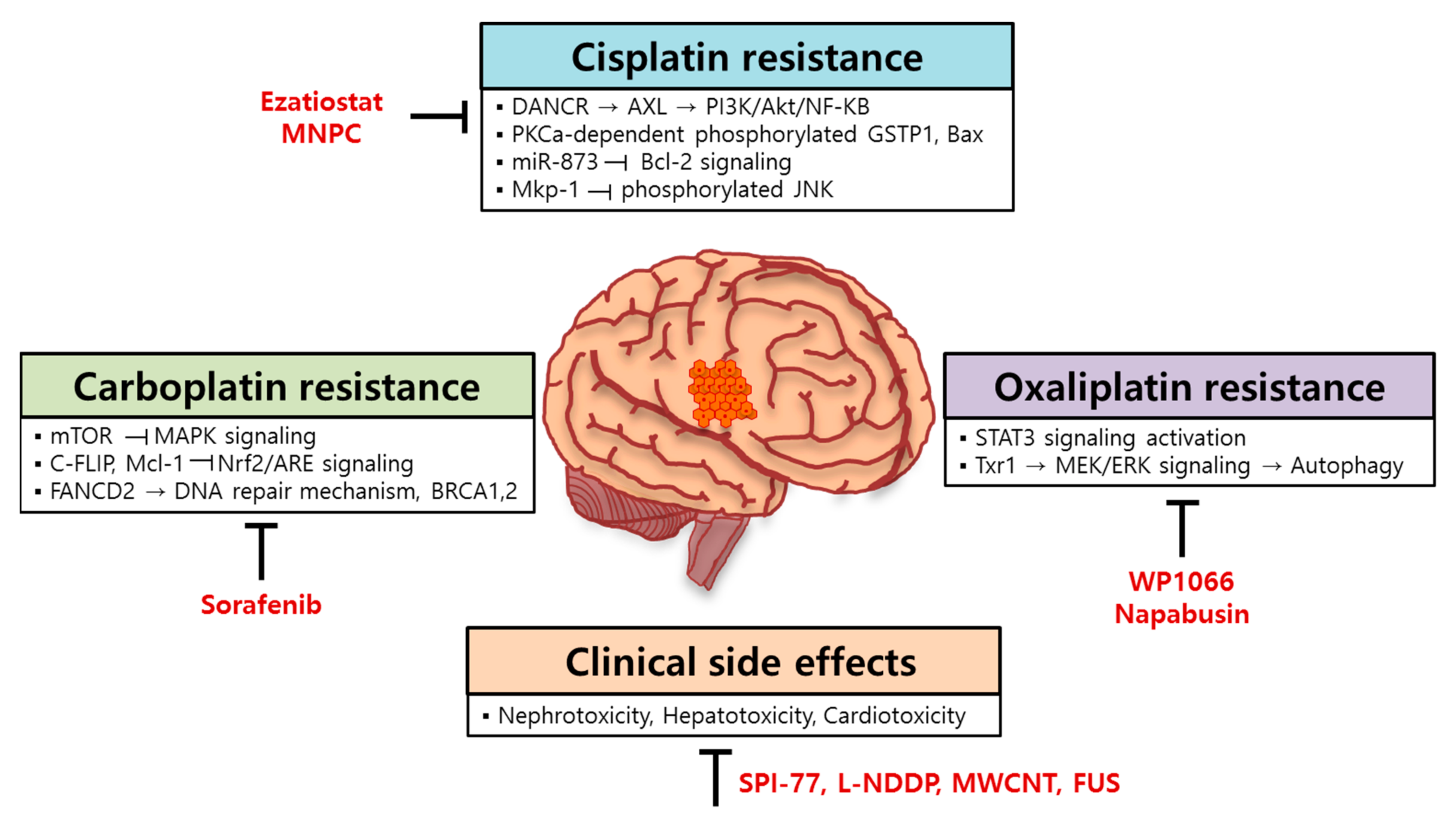
4. Resistance Mechanism of Platinum-Based Anticancer Drugs in Brain Tumors
4.1. Molecular Mechanisms for Cisplatin Resistance in Brain Tumors
4.2. Molecular Mechanisms for Carboplatin Resistance in Brain Tumors
4.3. Molecular Mechanisms for Oxaliplatin Resistance in Brain Tumors
5. A Strategy for Improving the Efficacy of Platinum-Based Anticancer Drugs in Brain Tumors
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Vecchione-Koval, T.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro-Oncology 2017, 19, v1–v88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rape, A.; Ananthanarayanan, B.; Kumar, S. Engineering strategies to mimic the glioblastoma microenvironment. Adv. Drug Deliv. Rev. 2014, 79, 172–183. [Google Scholar] [CrossRef] [Green Version]
- Weller, M.; Wick, W.; Aldape, K.; Brada, M.; Berger, M.; Pfister, S.M.; Nishikawa, R.; Rosenthal, M.; Wen, P.Y.; Stupp, R. Glioma. Nat. Rev. Dis. Primers 2015, 1, 1–18. [Google Scholar] [CrossRef]
- Brodbelt, A.; Greenberg, D.; Winters, T.; Williams, M.; Vernon, S.; Collins, V.P. Glioblastoma in England: 2007–2011. Eur. J. Cancer 2015, 51, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Tseng, Y.-Y.; Wang, Y.-C.; Su, C.-H.; Yang, T.-C.; Chang, T.-M.; Kau, Y.-C.; Liu, S.-J. Concurrent delivery of carmustine, irinotecan, and cisplatin to the cerebral cavity using biodegradable nanofibers: In vitro and in vivo studies. Colloids Surf. B Biointerfaces 2015, 134, 254–261. [Google Scholar] [CrossRef]
- Huang, D.; Lin, C.; Wen, X.; Gu, S.; Zhao, P. A potential nanofiber membrane device for filling surgical residual cavity to prevent glioma recurrence and improve local neural tissue reconstruction. PLoS ONE 2016, 11, e0161435. [Google Scholar] [CrossRef] [Green Version]
- Wijaya, J.; Fukuda, Y.; Schuetz, J.D. Obstacles to brain tumor therapy: Key ABC transporters. Int. J. Mol. Sci. 2017, 18, 2544. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.-X.; Cui, W.-W.; Yang, X.; Tao, A.-B.; Lan, T.; Li, T.-S.; Luo, L. Mesenchymal Stem Cells for Mitigating Radiotherapy Side Effects. Cells 2021, 10, 294. [Google Scholar] [CrossRef]
- Wang, D.; Wang, C.; Wang, L.; Chen, Y. A comprehensive review in improving delivery of small-molecule chemotherapeutic agents overcoming the blood-brain/brain tumor barriers for glioblastoma treatment. Drug Deliv. 2019, 26, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Kelley, K.; Knisely, J.; Symons, M.; Ruggieri, R. Radioresistance of brain tumors. Cancers 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rycaj, K.; Tang, D.G. Cancer stem cells and radioresistance. Int. J. Radiat. Biol. 2014, 90, 615–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Poppel, M.; Klimo, P.; Dewire, M.; Sanford, R.A.; Boop, F.; Broniscer, A.; Wright, K.; Gajjar, A.J. Resection of infantile brain tumors after neoadjuvant chemotherapy: The St. Jude experience. J. Neurosurg. Pediatrics 2011, 8, 251–256. [Google Scholar] [CrossRef]
- Wang, C.-C.; Liu, A.; Zhang, J.-T.; Sun, B.; Zhao, Y.-L. Surgical management of brain-stem cavernous malformations: Report of 137 cases. Surg. Neurol. 2003, 59, 444–454. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. The microenvironmental landscape of brain tumors. Cancer Cell 2017, 31, 326–341. [Google Scholar] [CrossRef] [Green Version]
- Cha, J.; Kang, S.-G.; Kim, P. Strategies of mesenchymal invasion of patient-derived brain tumors: Microenvironmental adaptation. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.K.; Clarke, I.D.; Terasaki, M.; Bonn, V.E.; Hawkins, C.; Squire, J.; Dirks, P.B. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003, 63, 5821–5828. [Google Scholar]
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of human brain tumour initiating cells. Nature 2004, 432, 396–401. [Google Scholar] [CrossRef]
- Galli, R.; Binda, E.; Orfanelli, U.; Cipelletti, B.; Gritti, A.; De Vitis, S.; Fiocco, R.; Foroni, C.; Dimeco, F.; Vescovi, A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004, 64, 7011–7021. [Google Scholar] [CrossRef] [Green Version]
- Gilbertson, R.J.; Rich, J.N. Making a tumour’s bed: Glioblastoma stem cells and the vascular niche. Nat. Rev. Cancer 2007, 7, 733–736. [Google Scholar] [CrossRef]
- Shahmabadi, H.E.; Movahedi, F.; Esfahani, M.K.M.; Alavi, S.E.; Eslamifar, A.; Anaraki, G.M.; Akbarzadeh, A. Efficacy of Cisplatin-loaded polybutyl cyanoacrylate nanoparticles on the glioblastoma. Tumor Biol. 2014, 35, 4799–4806. [Google Scholar] [CrossRef]
- Dréan, A.; Goldwirt, L.; Verreault, M.; Canney, M.; Schmitt, C.; Guehennec, J.; Delattre, J.-Y.; Carpentier, A.; Idbaih, A. Blood-brain barrier, cytotoxic chemotherapies and glioblastoma. Expert Rev. Neurother. 2016, 16, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.M.; Stevens, D.C.; Rubin, J.; Ames, M.M. Pharmacokinetics of 3-methyl-(triazen-1-yl) imidazole-4-carboximide following administration of temozolomide to patients with advanced cancer. Clin. Cancer Res. 1997, 3, 2393–2398. [Google Scholar]
- Moody, C.L.; Wheelhouse, R.T. The medicinal chemistry of imidazotetrazine prodrugs. Pharmaceuticals 2014, 7, 797–838. [Google Scholar] [CrossRef] [Green Version]
- Quirt, I.; Verma, S.; Petrella, T.; Bak, K.; Charette, M. Temozolomide for the treatment of metastatic melanoma: A systematic review. Oncologist 2007, 12, 1114–1123. [Google Scholar] [CrossRef]
- Hart, M.G.; Garside, R.; Rogers, G.; Stein, K.; Grant, R. Temozolomide for high grade glioma. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef] [Green Version]
- Cohen, M.H.; Johnson, J.R.; Pazdur, R. Food and Drug Administration Drug approval summary: Temozolomide plus radiation therapy for the treatment of newly diagnosed glioblastoma multiforme. Clin. Cancer Res. 2005, 11, 6767–6771. [Google Scholar] [CrossRef] [Green Version]
- Stupp, R.; Mason, W.P.; Van Den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Lee, S.Y. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso, M.M.; Gomez-Manzano, C.; Bekele, B.N.; Yung, W.A.; Fueyo, J. Adenovirus-based strategies overcome temozolomide resistance by silencing the O6-methylguanine-DNA methyltransferase promoter. Cancer Res. 2007, 67, 11499–11504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanzawa, T.; Bedwell, J.; Kondo, Y.; Kondo, S.; Germano, I.M. Inhibition of DNA repair for sensitizing resistant glioma cells to temozolomide. J. Neurosurg. 2003, 99, 1047–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montaldi, A.P.; Godoy, P.R.; Sakamoto-Hojo, E.T. APE1/REF-1 down-regulation enhances the cytotoxic effects of temozolomide in a resistant glioblastoma cell line. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2015, 793, 19–29. [Google Scholar] [CrossRef]
- Giannini, C.; Sarkaria, J.N.; Saito, A.; Uhm, J.H.; Galanis, E.; Carlson, B.L.; Schroeder, M.A.; James, C.D. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro-Oncology 2005, 7, 164–176. [Google Scholar] [CrossRef]
- Kitange, G.J.; Carlson, B.L.; Schroeder, M.A.; Grogan, P.T.; Lamont, J.D.; Decker, P.A.; Wu, W.; James, C.D.; Sarkaria, J.N. Induction of MGMT expression is associated with temozolomide resistance in glioblastoma xenografts. Neuro-Oncology 2009, 11, 281–291. [Google Scholar] [CrossRef] [Green Version]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; Van Den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Ramirez, Y.P.; Weatherbee, J.L.; Wheelhouse, R.T.; Ross, A.H. Glioblastoma multiforme therapy and mechanisms of resistance. Pharmaceuticals 2013, 6, 1475–1506. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorganic Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Kallu, J.; Banerjee, T.; Sulthana, S.; Darji, S.; Higginbotham, R.; Fletcher, C.; Gerasimchuk, N.N.; Santra, S. Nanomedicine-assisted combination therapy of NSCLC: New platinum-based anticancer drug synergizes the therapeutic efficacy of ganetespib. Nanotheranostics 2019, 3, 120. [Google Scholar] [CrossRef] [Green Version]
- Johnstone, T.C.; Park, G.Y.; Lippard, S.J. Understanding and improving platinum anticancer drugs–phenanthriplatin. Anticancer Res. 2014, 34, 471–476. [Google Scholar]
- Massimino, M.; Spreafico, F.; Cefalo, G.; Riccardi, R.; Tesoro-Tess, J.D.; Gandola, L.; Riva, D.; Ruggiero, A.; Valentini, L.; Mazza, E. High response rate to cisplatin/etoposide regimen in childhood low-grade glioma. J. Clin. Oncol. 2002, 20, 4209–4216. [Google Scholar] [CrossRef]
- Silvani, A.; Eoli, M.; Salmaggi, A.; Lamperti, E.; Maccagnano, E.; Broggi, G.; Boiardi, A. Phase II trial of cisplatin plus temozolomide, in recurrent and progressive malignant glioma patients. J. Neuro-Oncol. 2004, 66, 203–208. [Google Scholar] [CrossRef]
- Wang, J.L.; Barth, R.F.; Cavaliere, R.; Puduvalli, V.K.; Giglio, P.; Lonser, R.R.; Elder, J.B. Phase I trial of intracerebral convection-enhanced delivery of carboplatin for treatment of recurrent high-grade gliomas. PLoS ONE 2020, 15, e0244383. [Google Scholar] [CrossRef]
- Nellan, A.; Wright, E.; Campbell, K.; Davies, K.D.; Donson, A.M.; Amani, V.; Judd, A.; Hemenway, M.S.; Raybin, J.; Foreman, N.K. Retrospective analysis of combination carboplatin and vinblastine for pediatric low-grade glioma. J. Neuro-Oncol. 2020, 148, 569–575. [Google Scholar] [CrossRef]
- McDannold, N.; Zhang, Y.; Supko, J.G.; Power, C.; Sun, T.; Peng, C.; Vykhodtseva, N.; Golby, A.J.; Reardon, D.A. Acoustic feedback enables safe and reliable carboplatin delivery across the blood-brain barrier with a clinical focused ultrasound system and improves survival in a rat glioma model. Theranostics 2019, 9, 6284. [Google Scholar] [CrossRef]
- Dréan, A.; Lemaire, N.; Bouchoux, G.; Goldwirt, L.; Canney, M.; Goli, L.; Bouzidi, A.; Schmitt, C.; Guehennec, J.; Verreault, M. Temporary blood–brain barrier disruption by low intensity pulsed ultrasound increases carboplatin delivery and efficacy in preclinical models of glioblastoma. J. Neuro-Oncol. 2019, 144, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Ashrafzadeh, M.S.; Akbarzadeh, A.; Heydarinasab, A.; Ardjmand, M. In vivo Glioblastoma Therapy Using Targeted Liposomal Cisplatin. Int. J. Nanomed. 2020, 15, 7035. [Google Scholar] [CrossRef]
- Rosenberg, B.; Van Camp, L.; Krigas, T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef]
- Rosenberg, B.; Vancamp, L.; Trosko, J.E.; Mansour, V.H. Platinum compounds: A new class of potent antitumour agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef]
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef]
- Kelland, L.R.; Sharp, S.Y.; O’Neill, C.F.; Raynaud, F.I.; Beale, P.J.; Judson, I.R. Mini-review: Discovery and development of platinum complexes designed to circumvent cisplatin resistance. J. Inorg. Biochem. 1999, 77, 111–115. [Google Scholar] [CrossRef]
- Fuertes, M.A.; Alonso, C.; Pérez, J.M. Biochemical modulation of cisplatin mechanisms of action: Enhancement of antitumor activity and circumvention of drug resistance. Chem. Rev. 2003, 103, 645–662. [Google Scholar] [CrossRef]
- Jamieson, E.R.; Lippard, S.J. Structure, recognition, and processing of cisplatin-DNA adducts. Chem. Rev. 1999, 99, 2467–2498. [Google Scholar] [CrossRef]
- Chaney, S.G.; Campbell, S.L.; Bassett, E.; Wu, Y. Recognition and processing of cisplatin-and oxaliplatin-DNA adducts. Crit. Rev. Oncol. Hematol. 2005, 53, 3–11. [Google Scholar] [CrossRef]
- Ho, Y.P.; Au-Yeung, S.C.; To, K.K. Platinum-based anticancer agents: Innovative design strategies and biological perspectives. Med. Res. Rev. 2003, 23, 633–655. [Google Scholar] [CrossRef]
- Sedletska, Y.; Giraud-Panis, M.-J.; Malinge, J.-M. Cisplatin is a DNA-damaging antitumour compound triggering multifactorial biochemical responses in cancer cells: Importance of apoptotic pathways. Curr. Med. Chem.-Anti-Cancer Agents 2005, 5, 251–265. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [Green Version]
- Barnes, K.R.; Kutikov, A.; Lippard, S.J. Synthesis, characterization, and cytotoxicity of a series of estrogen-tethered platinum (IV) complexes. Chem. Biol. 2004, 11, 557–564. [Google Scholar] [CrossRef] [Green Version]
- Giandomenico, C.M.; Abrams, M.J.; Murrer, B.A.; Vollano, J.F.; Rheinheimer, M.I.; Wyer, S.B.; Bossard, G.E.; Higgins, J.D. Carboxylation of kinetically inert platinum (IV) hydroxy complexes. An entr. acte. ee into orally active platinum (IV) antitumor agents. Inorg. Chem. 1995, 34, 1015–1021. [Google Scholar] [CrossRef]
- Zdraveski, Z.Z.; Mello, J.A.; Farinelli, C.K.; Essigmann, J.M.; Marinus, M.G. MutS preferentially recognizes cisplatin-over oxaliplatin-modified DNA. J. Biol. Chem. 2002, 277, 1255–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kartalou, M.; Essigmann, J.M. Recognition of cisplatin adducts by cellular proteins. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2001, 478, 1–21. [Google Scholar] [CrossRef]
- Cohen, S.M.; Lippard, S.J. Cisplatin: From DNA damage to cancer chemotherapy. Prog. Nucleic Acid Res. Mol. Biol. 2001, 67, 93–130. [Google Scholar] [PubMed]
- Woźniak, K.; Błasiak, J. Recognition and repair of DNA-cisplatin adducts. Acta Biochim. Pol. 2002, 49, 583–596. [Google Scholar] [CrossRef]
- Rajeswari, M.R.; Jain, A. High-mobility-group chromosomal proteins, HMGA1 as potential tumour markers. Curr. Sci. 2002, 82, 838–844. [Google Scholar]
- Swanson, P.C. Fine structure and activity of discrete RAG-HMG complexes on V (D) J recombination signals. Mol. Cell. Biol. 2002, 22, 1340–1351. [Google Scholar] [CrossRef] [Green Version]
- Aidinis, V.; Bonaldi, T.; Beltrame, M.; Santagata, S.; Bianchi, M.E.; Spanopoulou, E. The RAG1 homeodomain recruits HMG1 and HMG2 to facilitate recombination signal sequence binding and to enhance the intrinsic DNA-bending activity of RAG1-RAG2. Mol. Cell. Biol. 1999, 19, 6532–6542. [Google Scholar] [CrossRef] [Green Version]
- Bustin, M. At the crossroads of necrosis and apoptosis: Signaling to multiple cellular targets by HMGB1. Sci. Signal. 2002, 2002, pe39. [Google Scholar] [CrossRef]
- Jayaraman, L.; Moorthy, N.C.; Murthy, K.G.; Manley, J.L.; Bustin, M.; Prives, C. High mobility group protein-1 (HMG-1) is a unique activator of p53. Genes Dev. 1998, 12, 462–472. [Google Scholar] [CrossRef] [Green Version]
- Yuan, F.; Gu, L.; Guo, S.; Wang, C.; Li, G.-M. Evidence for involvement of HMGB1 protein in human DNA mismatch repair. J. Biol. Chem. 2004, 279, 20935–20940. [Google Scholar] [CrossRef] [Green Version]
- Losa, J.H.; Cobo, C.P.; Viniegra, J.G.; Lobo, V.J.S.-A.; y Cajal, S.R.; Sanchez-Prieto, R. Role of the p38 MAPK pathway in cisplatin-based therapy. Oncogene 2003, 22, 3998–4006. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Boehm, J.; Lee, J.C. p38 MAP kinases: Key signalling molecules as therapeutic targets for inflammatory diseases. Nat. Rev. Drug Discov. 2003, 2, 717–726. [Google Scholar] [CrossRef]
- Reedijk, J. Why does cisplatin reach guanine-N7 with competing S-donor ligands available in the cell? Chem Rev. 1999, 99, 2499–2510. [Google Scholar] [CrossRef]
- Qu, Y.; Farrell, N. Interaction of bis (platinum) complexes with the mononucleotide 5′-guanosine monophosphate. Effect of diamine linker and the nature of the bis (platinum) complex on product formation. J. Am. Chem. Soc. 1991, 113, 4851–4857. [Google Scholar] [CrossRef]
- Kraker, A.J.; Hoeschele, J.D.; Elliott, W.L.; Showalter, H.H.; Sercel, A.D.; Farrell, N.P. Anticancer activity in murine and human tumor cell lines of bis (platinum) complexes incorporating straight-chain aliphatic diamine linker groups. J. Med. Chem. 1992, 35, 4526–4532. [Google Scholar] [CrossRef]
- Brabec, V.; Kasparkova, J. Molecular aspects of resistance to antitumor platinum drugs. Drug Resist. Updates 2002, 5, 147–161. [Google Scholar] [CrossRef]
- van Zutphen, S.; Reedijk, J. Targeting platinum anti-tumour drugs: Overview of strategies employed to reduce systemic toxicity. Coord. Chem. Rev. 2005, 249, 2845–2853. [Google Scholar] [CrossRef]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzym. Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef]
- Boulikas, T. Low toxicity and anticancer activity of a novel liposomal cisplatin (Lipoplatin) in mouse xenografts. Oncol. Rep. 2004, 12, 3–12. [Google Scholar] [CrossRef]
- Vail, D.M.; Kurzman, I.D.; Glawe, P.C.; O’Brien, M.G.; Chun, R.; Garrett, L.D.; Obradovich, J.E.; Fred, R.M.; Khanna, C.; Colbern, G.T. STEALTH liposome-encapsulated cisplatin (SPI-77) versus carboplatin as adjuvant therapy for spontaneously arising osteosarcoma (OSA) in the dog: A randomized multicenter clinical trial. Cancer Chemother. Pharmacol. 2002, 50, 131–136. [Google Scholar] [CrossRef]
- Terwogt, J.M.M.; Groenewegen, G.; Pluim, D.; Maliepaard, M.; Tibben, M.M.; Huisman, A.; Wim, W.; Schot, M.; Welbank, H.; Voest, E.E. Phase I and pharmacokinetic study of SPI-77, a liposomal encapsulated dosage form of cisplatin. Cancer Chemother. Pharmacol. 2002, 49, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Veal, G.; Griffin, M.; Price, E.; Parry, A.; Dick, G.; Little, M.; Yule, S.; Morland, B.; Estlin, E.; Hale, J. A phase I study in paediatric patients to evaluate the safety and pharmacokinetics of SPI-77, a liposome encapsulated formulation of cisplatin. Br. J. Cancer 2001, 84, 1029–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddik, Z.H.; Newell, D.R.; Boxall, F.E.; Harrap, K.R. The comparative pharmacokinetics of carboplatin and cisplatin in mice and rats. Biochem. Pharmacol. 1987, 36, 1925–1932. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Q.; Zhang, X.-Y.; Chen, J.; Yin, J.-Y.; Li, X.-P. ATP7B rs9535826 is associated with gastrointestinal toxicity of platinum-based chemotherapy in nonsmall cell lung cancer patients. J. Cancer Res. Ther. 2018, 14, 881. [Google Scholar] [CrossRef] [PubMed]
- Ghisoni, E.; Casalone, V.; Giannone, G.; Mittica, G.; Tuninetti, V.; Valabrega, G. Role of Mediterranean diet in preventing platinum based gastrointestinal toxicity in gynecolocological malignancies: A single Institution experience. World J. Clin. Oncol. 2019, 10, 391. [Google Scholar] [CrossRef]
- Pezeshki, Z.; Khosravi, A.; Nekuei, M.; Khoshnood, S.; Zandi, E.; Eslamian, M.; Talebi, A. Time course of cisplatin-induced nephrotoxicity and hepatotoxicity. J. Nephropathol. 2017, 6, 163. [Google Scholar] [CrossRef] [Green Version]
- Hassan, I.; Chibber, S.; Naseem, I. Ameliorative effect of riboflavin on the cisplatin induced nephrotoxicity and hepatotoxicity under photoillumination. Food Chem. Toxicol. 2010, 48, 2052–2058. [Google Scholar] [CrossRef]
- Hoek, J.; Bloemendal, K.M.; Van der Velden, L.-A.A.; Van Diessen, J.N.; Van Werkhoven, E.; Klop, W.; Tesselaar, M.E. Nephrotoxicity as a dose-limiting factor in a high-dose cisplatin-based chemoradiotherapy regimen for head and neck carcinomas. Cancers 2016, 8, 21. [Google Scholar] [CrossRef] [Green Version]
- Topal, İ.; Bilgin, A.Ö.; Çimen, F.K.; Kurt, N.; Süleyman, Z.; Bilgin, Y.; Özçiçek, A.; Altuner, D. The effect of rutin on cisplatin-induced oxidative cardiac damage in rats. Anatol. J. Cardiol. 2018, 20, 136. [Google Scholar] [CrossRef]
- Rademaker-Lakhai, J.M.; Crul, M.; Zuur, L.; Baas, P.; Beijnen, J.H.; Simis, Y.J.; Van Zandwijk, N.; Schellens, J.H. Relationship between cisplatin administration and the development of ototoxicity. J. Clin. Oncol. 2006, 24, 918–924. [Google Scholar] [CrossRef]
- Crawford, J.; Becker, P.S.; Armitage, J.O.; Blayney, D.W.; Chavez, J.; Curtin, P.; Dinner, S.; Fynan, T.; Gojo, I.; Griffiths, E.A. Myeloid growth factors, version 2.2017, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 1520–1541. [Google Scholar] [CrossRef] [Green Version]
- Sheleg, S.V.; Korotkevich, E.A.; Zhavrid, E.A.; Muravskaya, G.V.; Smeyanovich, A.F.; Shanko, Y.G.; Yurkshtovich, T.L.; Bychkovsky, P.B.; Belyaev, S.A. Local chemotherapy with cisplatin-depot for glioblastoma multiforme. J. Neuro-Oncol. 2002, 60, 53–59. [Google Scholar] [CrossRef]
- Feun, L.G.; Stewart, D.J.; Maor, M.; Leavens, M.; Savaraj, N.; Burgess, M.A.; Yung, W.-K.A.; Benjamin, R.S. A pilot study of cis-diamminedichloroplatinum and radiation therapy in patients with high grade astrocytomas. J. Neuro-Oncol. 1983, 1, 109–113. [Google Scholar] [CrossRef]
- Stewart, D.J.; Leavens, M.; Maor, M.; Feun, L.; Luna, M.; Bonura, J.; Caprioli, R.; Loo, T.L.; Benjamin, R.S. Human central nervous system distribution of cis-diamminedichloroplatinum and use as a radiosensitizer in malignant brain tumors. Cancer Res. 1982, 42, 2474–2479. [Google Scholar]
- Hall, P.E.; Lewis, R.; Syed, N.; Shaffer, R.; Evanson, J.; Ellis, S.; Williams, M.; Feng, X.; Johnston, A.; Thomson, J.A. A phase I study of Pegylated arginine deiminase (Pegargiminase), cisplatin, and Pemetrexed in Argininosuccinate Synthetase 1-deficient recurrent high-grade glioma. Clin. Cancer Res. 2019, 25, 2708–2716. [Google Scholar] [CrossRef] [Green Version]
- Gaynon, P.S.; Ettinger, L.J.; Baum, E.S.; Siegel, S.E.; Krailo, M.D.; Hammond, G.D. Carboplatin in childhood brain tumors: A Children’s Cancer Study Group phase II trial. Cancer 1990, 66, 2465–2469. [Google Scholar] [CrossRef]
- Walker, R.W.; Allen, J.C. Cisplatin in the treatment of recurrent childhood primary brain tumors. J. Clin. Oncol. 1988, 6, 62–66. [Google Scholar] [CrossRef]
- Bertolone, S.J.; Baum, E.S.; Krivit, W.; Hammond, G.D. A phase II study of cisplatin therapy in recurrent childhood brain tumors. J. Neuro-Oncol. 1989, 7, 5–11. [Google Scholar] [CrossRef]
- Allen, J.; Siffert, J.; Donahue, B.; Nirenberg, A.; Jakacki, R.; Robertson, P.; DaRosso, R.; Thoron, L.; Rosovsky, M.; Pinto, R. A phase I/II study of carboplatin combined with hyperfractionated radiotherapy for brainstem gliomas. Cancer Interdiscip. Int. J. Am. Cancer Soc. 1999, 86, 1064–1069. [Google Scholar] [CrossRef]
- Levin, V.; Yung, W.; Bruner, J.; Kyritsis, A.; Leeds, N.; Gleason, M.; Hess, K.; Meyers, C.; Ictech, S.; Chang, E. Phase II study of accelerated fractionation radiation therapy with carboplatin followed by PCV chemotherapy for the treatment of anaplastic gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 58–66. [Google Scholar] [CrossRef]
- Sweetman, S.C. Martindale: The Complete Drug Reference; Pharmaceutical Press: London, UK, 2009; Volume 3709. [Google Scholar]
- Zarate, R.; Rodriguez, J.; Bandres, E.; Patiño-García, A.; Ponz-Sarvise, M.; Viudez, A.; Ramirez, N.; Bitarte, N.; Chopitea, A.; Gacia-Foncillas, J. Oxaliplatin, irinotecan and capecitabine as first-line therapy in metastatic colorectal cancer (mCRC): A dose-finding study and pharmacogenomic analysis. Br. J. Cancer 2010, 102, 987–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faivre, S.; Chan, D.; Salinas, R.; Woynarowska, B.; Woynarowski, J.M. DNA strand breaks and apoptosis induced by oxaliplatin in cancer cells. Biochem. Pharmacol. 2003, 66, 225–237. [Google Scholar] [CrossRef]
- Raymond, E.; Faivre, S.; Chaney, S.; Woynarowski, J.; Cvitkovic, E. Cellular and molecular pharmacology of oxaliplatin1. Mol. Cancer Ther. 2002, 1, 227–235. [Google Scholar]
- Fouladi, M.; Blaney, S.M.; Poussaint, T.Y.; Freeman, B.B., III; McLendon, R.; Fuller, C.; Adesina, A.M.; Hancock, M.L.; Danks, M.K.; Stewart, C. Phase II study of oxaliplatin in children with recurrent or refractory medulloblastoma, supratentorial primitive neuroectodermal tumors, and atypical teratoid rhabdoid tumors: A pediatric brain tumor consortium study. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2006, 107, 2291–2297. [Google Scholar] [CrossRef]
- Maindrault-Goebel, F.; De Gramont, A.; Louvet, C.; Andre, T.; Carola, E.; Mabro, M.; Artru, P.; Gilles, V.; Lotz, J.; Izrael, V. High-dose intensity oxaliplatin added to the simplified bimonthly leucovorin and 5-fluorouracil regimen as second-line therapy for metastatic colorectal cancer (FOLFOX 7). Eur. J. Cancer 2001, 37, 1000–1005. [Google Scholar] [CrossRef]
- Giacchetti, S.; Perpoint, B.; Zidani, R.; Le Bail, N.; Faggiuolo, R.; Focan, C.; Chollet, P.; Llory, J.; Letourneau, Y.; Coudert, B. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil–leucovorin as first-line treatment of metastatic colorectal cancer. J. Clin. Oncol. 2000, 18, 136–147. [Google Scholar] [CrossRef]
- Macy, M.E.; Duncan, T.; Whitlock, J.; Hunger, S.P.; Boklan, J.; Narendran, A.; Herzog, C.; Arceci, R.J.; Bagatell, R.; Trippett, T. A multi-center phase Ib study of oxaliplatin (NSC# 266046) in combination with fluorouracil and leucovorin in pediatric patients with advanced solid tumors. Pediatric Blood Cancer 2013, 60, 230–236. [Google Scholar]
- Stordal, B.; Davey, M. Understanding cisplatin resistance using cellular models. IUBMB Life 2007, 59, 696–699. [Google Scholar] [CrossRef] [Green Version]
- Yi, D.Y.; Su, Q.; Zhang, F.C.; Fu, P.; Zhang, Q.; Cen, Y.C.; Zhao, H.Y.; Xiang, W. Effect of microRNA-128 on cisplatin resistance of glioma SHG-44 cells by targeting JAG1. J. Cell. Biochem. 2018, 119, 3162–3173. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, G.; Li, M.; Hu, D.; Zhang, L.; Liu, P.; Lin, K. Long noncoding RNA DANCR mediates cisplatin resistance in glioma cells via activating AXL/PI3K/Akt/NF-κB signaling pathway. Neurochem. Int. 2018, 118, 233–241. [Google Scholar] [CrossRef]
- Jiang, N.; Wang, X.; Xie, X.; Liao, Y.; Liu, N.; Liu, J.; Miao, N.; Shen, J.; Peng, T. lncRNA DANCR promotes tumor progression and cancer stemness features in osteosarcoma by upregulating AXL via miR-33a-5p inhibition. Cancer Lett. 2017, 405, 46–55. [Google Scholar] [CrossRef]
- Jin, L.; Fu, H.; Quan, J.; Pan, X.; He, T.; Hu, J.; Li, Y.; Li, H.; Yang, Y.; Ye, J. Overexpression of long non-coding RNA differentiation antagonizing non-protein coding RNA inhibits the proliferation, migration and invasion and promotes apoptosis of renal cell carcinoma. Mol. Med. Rep. 2017, 16, 4463–4468. [Google Scholar] [CrossRef]
- Sha, S.; Yuan, D.; Liu, Y.; Han, B.; Zhong, N. Targeting long non-coding RNA DANCR inhibits triple negative breast cancer progression. Biol. Open 2017, 6, 1310–1316. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Lin, T.; Che, H.; Wang, X. Long noncoding RNA DANCR knockdown inhibits proliferation, migration and invasion of glioma by regulating miR-135a-5p/BMI1. Cancer Cell Int. 2020, 20, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Yu, J.; Gao, G.; Lu, G.; Zhang, Y.; Ma, P. LncRNA DANCR functions as a competing endogenous RNA to regulate RAB1A expression by sponging miR-634 in glioma. Biosci. Rep. 2018, 38, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Cesana, M.; Cacchiarelli, D.; Legnini, I.; Santini, T.; Sthandier, O.; Chinappi, M.; Tramontano, A.; Bozzoni, I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 2011, 147, 358–369. [Google Scholar] [CrossRef] [Green Version]
- Fan, Q.; Aksoy, O.; Wong, R.A.; Ilkhanizadeh, S.; Novotny, C.J.; Gustafson, W.C.; Truong, A.Y.-Q.; Cayanan, G.; Simonds, E.F.; Haas-Kogan, D. A kinase inhibitor targeted to mTORC1 drives regression in glioblastoma. Cancer Cell 2017, 31, 424–435. [Google Scholar] [CrossRef] [Green Version]
- Giampazolias, E.; Zunino, B.; Dhayade, S.; Bock, F.; Cloix, C.; Cao, K.; Roca, A.; Lopez, J.; Ichim, G.; Proïcs, E. Mitochondrial permeabilization engages NF-κB-dependent anti-tumour activity under caspase deficiency. Nat. Cell Biol. 2017, 19, 1116–1129. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Ali-Osman, F. Serine-phosphorylation of the GSTP1 protein by PKC\#945; increases GSTP1-mediated cisplatin metabolism and decreases cisplatin-induced DNA interstrand cross link formation and cisplatin sensitivity in human glioma cells. In Proceedings of the 100th AACR Annual Meeting, Denver, CO, USA, 18–22 April 2009. [Google Scholar]
- Lo, H.-W.; Antoun, G.R.; Ali-Osman, F. The human glutathione S-transferase P1 protein is phosphorylated and its metabolic function enhanced by the Ser/Thr protein kinases, cAMP-dependent protein kinase and protein kinase C, in glioblastoma cells. Cancer Res. 2004, 64, 9131–9138. [Google Scholar] [CrossRef] [Green Version]
- Siddik, Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, T.; Ali-Osman, F. Glutathione-associated cis-diamminedichloroplatinum (II) metabolism and ATP-dependent efflux from leukemia cells. Molecular characterization of glutathione-platinum complex and its biological significance. J. Biol. Chem. 1993, 268, 20116–20125. [Google Scholar] [CrossRef]
- Singh, S.; Okamura, T.; Ali-Osman, F. Serine phosphorylation of glutathione S-transferase P1 (GSTP1) by PKCα enhances GSTP1-dependent cisplatin metabolism and resistance in human glioma cells. Biochem. Pharmacol. 2010, 80, 1343–1355. [Google Scholar] [CrossRef]
- Fruehauf, J.P.; Brem, H.; Brem, S.; Sloan, A.; Barger, G.; Huang, W.; Parker, R. In vitro drug response and molecular markers associated with drug resistance in malignant gliomas. Clin. Cancer Res. 2006, 12, 4523–4532. [Google Scholar] [CrossRef] [Green Version]
- Cullen, K.J.; Newkirk, K.A.; Schumaker, L.M.; Aldosari, N.; Rone, J.D.; Haddad, B.R. Glutathione S-transferase π amplification is associated with cisplatin resistance in head and neck squamous cell carcinoma cell lines and primary tumors. Cancer Res. 2003, 63, 8097–8102. [Google Scholar]
- Patnaik, S.; Mallick, R.; Kannisto, E.; Sharma, R.; Bshara, W.; Yendamuri, S.; Dhillon, S.S. MiR-205 and MiR-375 microRNA assays to distinguish squamous cell carcinoma from adenocarcinoma in lung cancer biopsies. J. Thorac. Oncol. 2015, 10, 446–453. [Google Scholar] [CrossRef] [Green Version]
- Lai, N.; Wu, D.; Fang, X.; Lin, Y.; Chen, S.; Li, Z.; Xu, S. Serum microRNA-210 as a potential noninvasive biomarker for the diagnosis and prognosis of glioma. Br. J. Cancer 2015, 112, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yan, K.; Fang, J.; Qu, Q.; Zhou, M.; Chen, F. Let-7b expression determines response to chemotherapy through the regulation of cyclin D1 in glioblastoma. J. Exp. Clin. Cancer Res. 2013, 32, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhang, Y.; Shi, Y.; Lian, H.; Tu, H.; Han, S.; Peng, B.; Liu, W.; He, X. MiR-873 acts as a novel sensitizer of glioma cells to cisplatin by targeting Bcl-2. Int. J. Oncol. 2015, 47, 1603–1611. [Google Scholar] [CrossRef]
- Rocha, C.R.R.; Garcia, C.C.M.; Vieira, D.B.; Quinet, A.; de Andrade-Lima, L.; Munford, V.; Belizário, J.E.; Menck, C.F.M. Glutathione depletion sensitizes cisplatin-and temozolomide-resistant glioma cells in vitro and in vivo. Cell Death Dis. 2014, 5, e1505. [Google Scholar] [CrossRef]
- Yu, H.; Park, J.; Lee, J.; Choi, K.; Choi, C. Constitutive expression of MAP kinase phosphatase-1 confers multi-drug resistance in human glioblastoma cells. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2012, 44, 195. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.I.; Brummer, T.; O’brien, P.M.; Daly, R.J. Dual-specificity phosphatases: Critical regulators with diverse cellular targets. Biochem. J. 2009, 418, 475–489. [Google Scholar] [CrossRef] [Green Version]
- Bermudez, O.; Pagès, G.; Gimond, C. The dual-specificity MAP kinase phosphatases: Critical roles in development and cancer. Am. J. Physiol. Cell Physiol. 2010, 299, C189–C202. [Google Scholar] [CrossRef]
- Keyse, S.M. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev. 2008, 27, 253–261. [Google Scholar] [CrossRef]
- Small, G.W.; Shi, Y.Y.; Higgins, L.S.; Orlowski, R.Z. Mitogen-activated protein kinase phosphatase-1 is a mediator of breast cancer chemoresistance. Cancer Res. 2007, 67, 4459–4466. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Kho, D.; Zhou, J.-Y.; Davis, R.J.; Wu, G.S. MKP-1 suppresses PARP-1 degradation to mediate cisplatin resistance. Oncogene 2017, 36, 5939–5947. [Google Scholar] [CrossRef] [Green Version]
- Blommaert, F.A.; van Dijk-Knijnenburg, H.C.; Dijt, F.J.; den Engelse, L.; Baan, R.A.; Berends, F.; Fichtinger-Schepman, A.M.J. Formation of DNA adducts by the anticancer drug carboplatin: Different nucleotide sequence preferences in vitro and in cells. Biochemistry 1995, 34, 8474–8480. [Google Scholar] [CrossRef]
- Lokich, J.; Anderson, N. Carboplatin versus cisplatin in solid tumors: An analysis of the literature. Ann. Oncol. 1998, 9, 13–21. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [Green Version]
- Poore, B.; Yuan, M.; Arnold, A.; Price, A.; Alt, J.; Rubens, J.A.; Slusher, B.S.; Eberhart, C.G.; Raabe, E.H. Inhibition of mTORC1 in pediatric low-grade glioma depletes glutathione and therapeutically synergizes with carboplatin. Neuro-Oncology 2019, 21, 252–263. [Google Scholar] [CrossRef]
- Albert, L.; Karsy, M.; Murali, R.; Jhanwar-Uniyal, M. Inhibition of mTOR activates the MAPK pathway in glioblastoma multiforme. Cancer Genom. Proteom. 2009, 6, 255–261. [Google Scholar]
- Seo, S.U.; Cho, H.K.; Min, K.-j.; Woo, S.M.; Kim, S.; Park, J.-W.; Kim, S.H.; Choi, Y.H.; Keum, Y.S.; Hyun, J.W. Thioridazine enhances sensitivity to carboplatin in human head and neck cancer cells through downregulation of c-FLIP and Mcl-1 expression. Cell Death Dis. 2017, 8, e2599. [Google Scholar] [CrossRef]
- Arlt, A.; Bauer, I.; Schafmayer, C.; Tepel, J.; Müerköster, S.S.; Brosch, M.; Röder, C.; Kalthoff, H.; Hampe, J.; Moyer, M. Increased proteasome subunit protein expression and proteasome activity in colon cancer relate to an enhanced activation of nuclear factor E2-related factor 2 (Nrf2). Oncogene 2009, 28, 3983–3996. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1–Nrf2 system. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Sarlette, A.; Krampfl, K.; Grothe, C.; von Neuhoff, N.; Dengler, R.; Petri, S. Nuclear erythroid 2-related factor 2-antioxidative response element signaling pathway in motor cortex and spinal cord in amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 2008, 67, 1055–1062. [Google Scholar] [CrossRef]
- Metselaar, D.S.; Meel, M.H.; Benedict, B.; Waranecki, P.; Koster, J.; Kaspers, G.J.; Hulleman, E. Celastrol-induced degradation of FANCD2 sensitizes pediatric high-grade gliomas to the DNA-crosslinking agent carboplatin. EBioMedicine 2019, 50, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Niedernhofer, L.J.; Lalai, A.S.; Hoeijmakers, J.H. Fanconi anemia (cross) linked to DNA repair. Cell 2005, 123, 1191–1198. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Huang, J. The Fanconi anemia pathway and DNA interstrand cross-link repair. Protein Cell 2011, 2, 704–711. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.-C.; Li, Z.; Lopez-Martinez, D.; Nicholson, W.V.; Vénien-Bryan, C.; Cohn, M.A. The FANCD2–FANCI complex is recruited to DNA interstrand crosslinks before monoubiquitination of FANCD2. Nat. Commun. 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Garcia-Higuera, I.; Taniguchi, T.; Ganesan, S.; Meyn, M.S.; Timmers, C.; Hejna, J.; Grompe, M.; D’Andrea, A.D. Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Mol. Cell 2001, 7, 249–262. [Google Scholar] [CrossRef]
- Patil, A.A.; Sayal, P.; Depondt, M.-L.; Beveridge, R.D.; Roylance, A.; Kriplani, D.H.; Myers, K.N.; Cox, A.; Jellinek, D.; Fernando, M. FANCD2 re-expression is associated with glioma grade and chemical inhibition of the Fanconi Anaemia pathway sensitises gliomas to chemotherapeutic agents. Oncotarget 2014, 5, 6414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, N.B.; Alqazzaz, A.; Hwang, J.R.; Qi, X.; Keegan, A.D.; Kim, A.J.; Winkles, J.A.; Woodworth, G.F. Oxaliplatin disrupts pathological features of glioma cells and associated macrophages independent of apoptosis induction. J. Neuro-Oncol. 2018, 140, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Cross-Knorr, S.; Lu, S.; Perez, K.; Guevara, S.; Brilliant, K.; Pisano, C.; Quesenberry, P.J.; Resnick, M.B.; Chatterjee, D. RKIP phosphorylation and STAT3 activation is inhibited by oxaliplatin and camptothecin and are associated with poor prognosis in stage II colon cancer patients. BMC Cancer 2013, 13, 463. [Google Scholar] [CrossRef] [Green Version]
- Lesterhuis, W.J.; Punt, C.J.; Hato, S.V.; Eleveld-Trancikova, D.; Jansen, B.J.; Nierkens, S.; Schreibelt, G.; de Boer, A.; Van Herpen, C.M.; Kaanders, J.H. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J. Clin. Investig. 2011, 121, 3100–3108. [Google Scholar] [CrossRef]
- Hato, S.V.; de Vries, I.J.M.; Lesterhuis, W.J. STATing the importance of immune modulation by platinum chemotherapeutics. Oncoimmunology 2012, 1, 234–236. [Google Scholar] [CrossRef] [Green Version]
- Bruno, P.M.; Liu, Y.; Park, G.Y.; Murai, J.; Koch, C.E.; Eisen, T.J.; Pritchard, J.R.; Pommier, Y.; Lippard, S.J.; Hemann, M.T. A subset of platinum-containing chemotherapeutic agents kills cells by inducing ribosome biogenesis stress. Nat. Med. 2017, 23, 461–471. [Google Scholar] [CrossRef]
- Kepp, O.; Menger, L.; Vacchelli, E.; Locher, C.; Adjemian, S.; Yamazaki, T.; Martins, I.; Sukkurwala, A.Q.; Michaud, M.; Senovilla, L. Crosstalk between ER stress and immunogenic cell death. Cytokine Growth Factor Rev. 2013, 24, 311–318. [Google Scholar] [CrossRef]
- Roberts, N.B.; Wadajkar, A.S.; Winkles, J.A.; Davila, E.; Kim, A.J.; Woodworth, G.F. Repurposing platinum-based chemotherapies for multi-modal treatment of glioblastoma. Oncoimmunology 2016, 5, e1208876. [Google Scholar] [CrossRef] [Green Version]
- Martins, I.; Kepp, O.; Schlemmer, F.; Adjemian, S.; Tailler, M.; Shen, S.; Michaud, M.; Menger, L.; Gdoura, A.; Tajeddine, N. Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene 2011, 30, 1147–1158. [Google Scholar] [CrossRef] [Green Version]
- Roberts, N. Repurposing Oxaliplatin for the Treatment of Glioblastoma. Ph.D. Thesis, University of Maryland, Baltimore, MD, USA, 2018. [Google Scholar]
- Luwor, R.B.; Stylli, S.S.; Kaye, A.H. The role of Stat3 in glioblastoma multiforme. J. Clin. Neurosci. 2013, 20, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Iwamaru, A.; Szymanski, S.; Iwado, E.; Aoki, H.; Yokoyama, T.; Fokt, I.; Hess, K.; Conrad, C.; Madden, T.; Sawaya, R. A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo. Oncogene 2007, 26, 2435–2444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agudelo-Garcia, P.A.; De Jesus, J.K.; Williams, S.P.; Nowicki, M.O.; Chiocca, E.A.; Liyanarachchi, S.; Li, P.-K.; Lannutti, J.J.; Johnson, J.K.; Lawler, S.E. Glioma cell migration on three-dimensional nanofiber scaffolds is regulated by substrate topography and abolished by inhibition of STAT3 signaling. Neoplasia 2011, 13, 831–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, G.K.; McFarland, B.C.; Nozell, S.E.; Benveniste, E.N. NF-κB and STAT3 in glioblastoma: Therapeutic targets coming of age. Expert Rev. Neurother. 2014, 14, 1293–1306. [Google Scholar] [CrossRef] [Green Version]
- Jackson, C.; Ruzevick, J.; Amin, A.G.; Lim, M. Potential role for STAT3 inhibitors in glioblastoma. Neurosurg. Clin. 2012, 23, 379–389. [Google Scholar] [CrossRef]
- Chi, H.-m.; Du, J.-d.; Cheng, J.; Mao, H.-d. Taxol-Resistant Gene 1 (Txr1) Mediates Oxaliplatin Resistance by Inducing Autophagy in Human Nasopharyngeal Carcinoma Cells. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 475. [Google Scholar] [CrossRef]
- Chen, S.; Rehman, S.K.; Zhang, W.; Wen, A.; Yao, L.; Zhang, J. Autophagy is a therapeutic target in anticancer drug resistance. Biochim. Biophys. Acta (BBA) Rev. Cancer 2010, 1806, 220–229. [Google Scholar] [CrossRef]
- Barth, S.; Glick, D.; Macleod, K.F. Autophagy: Assays and artifacts. J. Pathol. 2010, 221, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef] [Green Version]
- Hindler, K.; Cleeland, C.S.; Rivera, E.; Collard, C.D. The role of statins in cancer therapy. Oncologist 2006, 11, 306–315. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Zhang, Z.; Zhang, Y.; Chen, X.; Guo, S.; Lei, Y.; Xu, Y.; Ji, C.; Bi, Z.; Wang, K. HMGB1-mediated autophagy modulates sensitivity of colorectal cancer cells to oxaliplatin via MEK/ERK signaling pathway. Cancer Biol. Ther. 2015, 16, 511–517. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.K.; Oza, A.M.; Siu, L.L. The statins as anticancer agents. Clin. Cancer Res. 2003, 9, 10–19. [Google Scholar] [PubMed]
- Brabec, V.; Kasparkova, J. Modifications of DNA by platinum complexes: Relation to resistance of tumors to platinum antitumor drugs. Drug Resist. Updates 2005, 8, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Torigoe, T.; Izumi, H.; Ishiguchi, H.; Yoshida, Y.; Tanabe, M.; Yoshida, T.; Igarashi, T.; Niina, I.; Wakasugi, T.; Imaizumi, T. Cisplatin resistance and transcription factors. Curr. Med. Chem. Anti-Cancer Agents 2005, 5, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, D.; Sutton, G.R. Ezatiostat hydrochloride for the treatment of myelodysplastic syndromes. Expert Opin. Investig. Drugs 2015, 24, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Adler, V.; Yin, Z.; Fuchs, S.Y.; Benezra, M.; Rosario, L.; Tew, K.D.; Pincus, M.R.; Sardana, M.; Henderson, C.J.; Wolf, C.R. Regulation of JNK signaling by GSTp. EMBO J. 1999, 18, 1321–1334. [Google Scholar] [CrossRef] [PubMed]
- Widersten, M.; Kolm, R.H.; Björnestedt, R.; Mannervik, B. Contribution of five amino acid residues in the glutathione-binding site to the function of human glutathione transferase P1-1. Biochem. J. 1992, 285, 377–381. [Google Scholar] [CrossRef]
- Ali-Osman, F.; Okamura, T.; Turley, R.; Barker, A.; Keck, J.G.; Laborde, E.; Cai, D.; Mascata, R. Abstract B229: Novel Ezatiostat Analogues Disrupt Binding of GSTP1 to All Three Major MAP Kinases (JNK, ERK and p38) and Exhibit Context-Dependent Antitumor Activity. Mol. Cancer Ther. 2011, 12–16. [Google Scholar]
- Raza, A.; Galili, N.; Callander, N.; Ochoa, L.; Piro, L.; Emanuel, P.; Williams, S.; Burris, H.; Faderl, S.; Estrov, Z. Phase 1-2a multicenter dose-escalation study of ezatiostat hydrochloride liposomes for injection (Telintra®, TLK199), a novel glutathione analog prodrug in patients with myelodysplastic syndrome. J. Hematol. Oncol. 2009, 2, 20. [Google Scholar] [CrossRef] [Green Version]
- Lei, K.; Gu, X.; Alvarado, A.G.; Du, Y.; Luo, S.; Ahn, E.H.; Kang, S.S.; Ji, B.; Liu, X.; Mao, H. Discovery of a dual inhibitor of NQO1 and GSTP1 for treating glioblastoma. J. Hematol. Oncol. 2020, 13, 1–21. [Google Scholar] [CrossRef]
- Hinko, C.N.; Seibert, K.; Crider, A. Anticonvulsant activity of the nipecotic acid ester, (±)-m-nitrophenyl-3-piperidinecarboxylate. Neuropharmacology 1984, 23, 1009–1014. [Google Scholar] [CrossRef]
- Méndez-Sánchez, N.; Vásquez-Fernández, F.; Zamora-Valdés, D. Sorafenib, a systemic therapy for hepatocellular carcinoma. Ann. Hepatol. 2008, 7, 46–51. [Google Scholar] [CrossRef]
- Llovet, J.M.; Bruix, J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology 2008, 48, 1312–1327. [Google Scholar] [CrossRef] [Green Version]
- Keating, G.M.; Santoro, A. Sorafenib. Drugs 2009, 69, 223–240. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, L.; Xia, L.; Chen, G.-q.; Chen, Z.; Miller, W.H.; Waxman, S. Combined effect of all-trans retinoic acid and arsenic trioxide in acute promyelocytic leukemia cells in vitro and in vivo. Blood J. Am. Soc. Hematol. 2001, 97, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Xia, L.; Gabrilove, J.; Waxman, S.; Jing, Y. Sorafenib inhibition of Mcl-1 accelerates ATRA-induced apoptosis in differentiation-responsive AML cells. Clin. Cancer Res. 2016, 22, 1211–1221. [Google Scholar] [CrossRef] [Green Version]
- Ke, Y.; Xiang, C. Transferrin receptor-targeted HMSN for sorafenib delivery in refractory differentiated thyroid cancer therapy. Int. J. Nanomed. 2018, 13, 8339. [Google Scholar] [CrossRef] [Green Version]
- Mandal, R.; Becker, S.; Strebhardt, K. Stamping out RAF and MEK1/2 to inhibit the ERK1/2 pathway: An emerging threat to anticancer therapy. Oncogene 2016, 35, 2547–2561. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Tang, Y.; Wang, S.; Wang, C.; Li, Y.; Su, X.; Tian, J.; Tian, Y.; Pan, J. Synergistic chemo–photothermal therapy of breast cancer by mesenchymal stem cell-encapsulated yolk–shell GNR@ HPMO-PTX nanospheres. ACS Appl. Mater. Interfaces 2016, 8, 17927–17935. [Google Scholar] [CrossRef]
- Escudier, B.; Eisen, T.; Stadler, W.M.; Szczylik, C.; Oudard, S.; Siebels, M.; Negrier, S.; Chevreau, C.; Solska, E.; Desai, A.A. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 2007, 356, 125–134. [Google Scholar] [CrossRef]
- Escudier, B.; Eisen, T.; Stadler, W.M.; Szczylik, C.; Oudard, S.; Staehler, M.; Negrier, S.; Chevreau, C.; Desai, A.A.; Rolland, F. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J. Clin. Oncol. 2009, 27, 3312–3318. [Google Scholar] [CrossRef] [PubMed]
- Schindler, C.; Darnell, J., Jr. Transcriptional responses to polypeptide ligands: The JAK-STAT pathway. Annu. Rev. Biochem. 1995, 64, 621–652. [Google Scholar] [CrossRef] [PubMed]
- Darnell, J.E. STATs and gene regulation. Science 1997, 277, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, A.; Asano, T.; Kuroda, K.; Sato, A.; Asakuma, J.; Ito, K.; Hayakawa, M.; Sumitomo, M. STAT3 inhibitor WP1066 as a novel therapeutic agent for renal cell carcinoma. Br. J. Cancer 2010, 102, 1592–1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.E.; Lee, H.G.; Cho, I.H.; Chung, D.H.; Yoon, S.H.; Yang, Y.M.; Lee, J.W.; Choi, S.; Park, J.W.; Ye, S.K. STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. FASEB J. 2005, 19, 1296–1298. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Fone, P.D.; GANDOUR-EDWARDS, R.; White, R.W.d.; LOW, R.K. Immunohistochemical analysis of BCL-2 protein expression in renal cell carcinoma. J. Urol. 1999, 162, 610–613. [Google Scholar] [CrossRef]
- Han, D.; Yu, T.; Dong, N.; Wang, B.; Sun, F.; Jiang, D. Napabucasin, a novel STAT3 inhibitor suppresses proliferation, invasion and stemness of glioblastoma cells. J. Exp. Clin. Cancer Res. 2019, 38, 289. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Hubbard, J.M.; Grothey, A. Napabucasin: An update on the first-in-class cancer stemness inhibitor. Drugs 2017, 77, 1091–1103. [Google Scholar] [CrossRef]
- Jonker, D.J.; Nott, L.; Yoshino, T.; Gill, S.; Shapiro, J.; Ohtsu, A.; Zalcberg, J.; Vickers, M.M.; Wei, A.C.; Gao, Y. Napabucasin versus placebo in refractory advanced colorectal cancer: A randomised phase 3 trial. Lancet Gastroenterol. Hepatol. 2018, 3, 263–270. [Google Scholar] [CrossRef]
- Harper, B.W.; Krause-Heuer, A.M.; Grant, M.P.; Manohar, M.; Garbutcheon-Singh, K.B.; Aldrich-Wright, J.R. Advances in platinum chemotherapeutics. Chem. Eur. J. 2010, 16, 7064–7077. [Google Scholar] [CrossRef]
- Haxton, K.J.; Burt, H.M. Polymeric drug delivery of platinum-based anticancer agents. J. Pharm. Sci. 2009, 98, 2299–2316. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Z. Targeting and delivery of platinum-based anticancer drugs. Chem. Soc. Rev. 2013, 42, 202–224. [Google Scholar] [CrossRef]
- Sapra, P.; Allen, T. Ligand-targeted liposomal anticancer drugs. Prog. Lipid Res. 2003, 42, 439–462. [Google Scholar] [CrossRef]
- Hannon, M.J. Metal-based anticancer drugs: From a past anchored in platinum chemistry to a post-genomic future of diverse chemistry and biology. Pure Appl. Chem. 2007, 79, 2243–2261. [Google Scholar] [CrossRef]
- Zahednezhad, F.; Zakeri-Milani, P.; Shahbazi Mojarrad, J.; Valizadeh, H. The latest advances of cisplatin liposomal formulations: Essentials for preparation and analysis. Expert Opin. Drug Deliv. 2020, 17, 523–541. [Google Scholar] [CrossRef]
- Kim, E.S.; Lu, C.; Khuri, F.R.; Tonda, M.; Glisson, B.S.; Liu, D.; Jung, M.; Hong, W.K.; Herbst, R.S. A phase II study of STEALTH cisplatin (SPI-77) in patients with advanced non-small cell lung cancer. Lung Cancer 2001, 34, 427–432. [Google Scholar] [CrossRef]
- Liu, L.; Ye, Q.; Lu, M.; Lo, Y.-C.; Hsu, Y.-H.; Wei, M.-C.; Chen, Y.-H.; Lo, S.-C.; Wang, S.-J.; Bain, D.J. A new approach to reduce toxicities and to improve bioavailabilities of platinum-containing anti-cancer nanodrugs. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Heger, M. Amgen Deal Triggers Watchful Waiting in Targeted Nanomedicine. Nat. Med. 2013, 19, 120–131. [Google Scholar] [CrossRef]
- Chow, E.K.-H.; Ho, D. Cancer nanomedicine: From drug delivery to imaging. Sci. Transl. Med. 2013, 5, rv214–rv216. [Google Scholar] [CrossRef]
- Zamboni, W.C.; Torchilin, V.; Patri, A.K.; Hrkach, J.; Stern, S.; Lee, R.; Nel, A.; Panaro, N.J.; Grodzinski, P. Best practices in cancer nanotechnology: Perspective from NCI nanotechnology alliance. Clin. Cancer Res. 2012, 18, 3229–3241. [Google Scholar] [CrossRef] [Green Version]
- Cabral, H.; Nishiyama, N.; Okazaki, S.; Koyama, H.; Kataoka, K. Preparation and biological properties of dichloro (1, 2-diaminocyclohexane) platinum (II)(DACHPt)-loaded polymeric micelles. J. Control. Release 2005, 101, 223–232. [Google Scholar] [CrossRef]
- Wu, H.; Cabral, H.; Toh, K.; Mi, P.; Chen, Y.-C.; Matsumoto, Y.; Yamada, N.; Liu, X.; Kinoh, H.; Miura, Y. Polymeric micelles loaded with platinum anticancer drugs target preangiogenic micrometastatic niches associated with inflammation. J. Control Release 2014, 189, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Oberoi, H.S.; Nukolova, N.V.; Kabanov, A.V.; Bronich, T.K. Nanocarriers for delivery of platinum anticancer drugs. Adv. Drug Deliv. Rev. 2013, 65, 1667–1685. [Google Scholar] [CrossRef] [Green Version]
- Ajima, K.; Yudasaka, M.; Murakami, T.; Maigné, A.; Shiba, K.; Iijima, S. Carbon nanohorns as anticancer drug carriers. Mol. Pharm. 2005, 2, 475–480. [Google Scholar] [CrossRef]
- Nainwal, N. Recent advances in transcranial focused ultrasound (FUS) triggered brain delivery. Curr. Drug Targets 2017, 18, 1225–1232. [Google Scholar] [CrossRef]
- Kato, S.; Subbiah, V.; Kurzrock, R. Counterpoint: Successes in the pursuit of precision medicine: Biomarkers take credit. J. Natl. Compr. Cancer Netw. 2017, 15, 863–866. [Google Scholar] [CrossRef] [Green Version]
- Hoadley, K.A.; Yau, C.; Wolf, D.M.; Cherniack, A.D.; Tamborero, D.; Ng, S.; Leiserson, M.D.; Niu, B.; McLellan, M.D.; Uzunangelov, V. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell 2014, 158, 929–944. [Google Scholar] [CrossRef] [Green Version]
- Sicklick, J.K.; Kato, S.; Okamura, R.; Schwaederle, M.; Hahn, M.E.; Williams, C.B.; De, P.; Krie, A.; Piccioni, D.E.; Miller, V.A. Molecular profiling of cancer patients enables personalized combination therapy: The I-PREDICT study. Nat. Med. 2019, 25, 744–750. [Google Scholar] [CrossRef]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Dilruba, S.; Kalayda, G.V. Platinum-based drugs: Past, present and future. Cancer Chemother. Pharmacol. 2016, 77, 1103–1124. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.D.; Mellor, H.R.; Callaghan, R.; Hambley, T.W. Basis for design and development of platinum (IV) anticancer complexes. J. Med. Chem. 2007, 50, 3403–3411. [Google Scholar] [CrossRef] [PubMed]
| Drug | Tumor Type | Number of Clinical Trial Participants | Major Findings | Reference |
|---|---|---|---|---|
| Cisplatin | Glioblastoma | 30 | On MRI, CT images, tumor growth was suppressed, and median survival was increased by 7–14 months. | [93,94,95,96] |
| High-grade gliomas | 10 | |||
| Carboplatin | Child brain Tumor | 95 | 4 weeks, a good response was shown in 40 brain tumor patients. The median overall survival rate was 12 months. | [97,98,100,101] |
| Gliomas | 34 | |||
| Recurrent glioblastomas | 122 | |||
| Oxaliplatin | Refractory pediatric brain tumors | 43 | 3 weeks, there was no CR, and PR (15 patients, 13.3%) was seen in the MRI images. | [102,104,105,106,109] |
| Medulloblastoma, diffuse cranial glioma | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, J.; Lee, S.; Kim, H.; Kang, H.; Youn, H.; Jo, S.; Youn, B.; Kim, H.Y. Revisiting Platinum-Based Anticancer Drugs to Overcome Gliomas. Int. J. Mol. Sci. 2021, 22, 5111. https://doi.org/10.3390/ijms22105111
Jeon J, Lee S, Kim H, Kang H, Youn H, Jo S, Youn B, Kim HY. Revisiting Platinum-Based Anticancer Drugs to Overcome Gliomas. International Journal of Molecular Sciences. 2021; 22(10):5111. https://doi.org/10.3390/ijms22105111
Chicago/Turabian StyleJeon, Jaewan, Sungmin Lee, Hyunwoo Kim, Hyunkoo Kang, HyeSook Youn, Sunmi Jo, BuHyun Youn, and Hae Yu Kim. 2021. "Revisiting Platinum-Based Anticancer Drugs to Overcome Gliomas" International Journal of Molecular Sciences 22, no. 10: 5111. https://doi.org/10.3390/ijms22105111






