A Comprehensive Review on the Role of Non-Coding RNAs in the Pathophysiology of Bipolar Disorder
Abstract
:1. Introduction
2. CircRNAs and BD
3. LncRNAs and BD
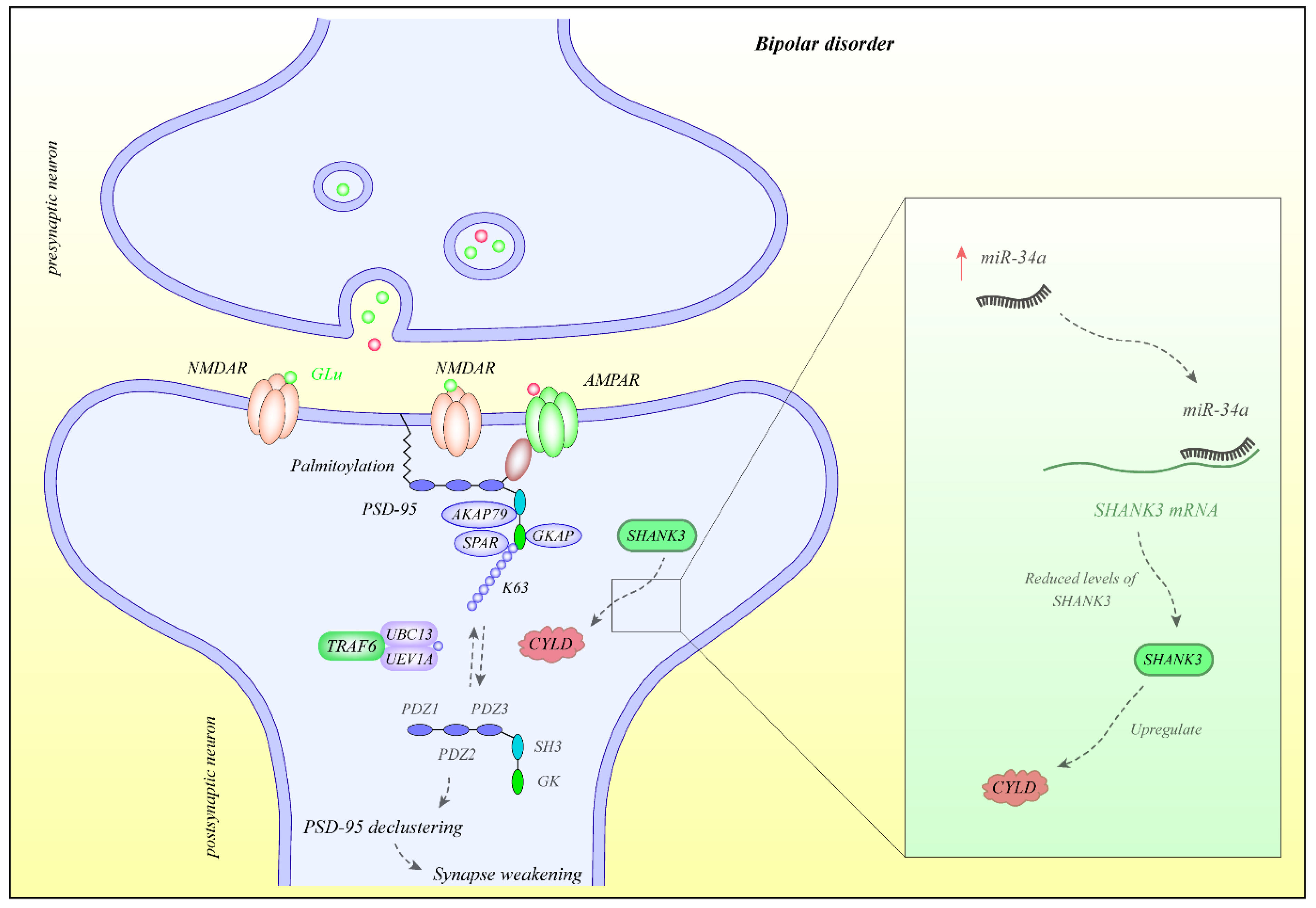
4. miRNAs and BD
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krahn, G.L. WHO World Report on Disability: A review. Disabil. Health J. 2011, 4, 141–142. [Google Scholar] [CrossRef]
- World Health Organization. World Report on Disability 2011; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Hayes, J.; Miles, J.; Walters, K.; King, M.; Osborn, D. A systematic review and meta-analysis of premature mortality in bipolar affective disorder. Acta Psychiatr. Scand. 2015, 131, 417–425. [Google Scholar] [CrossRef]
- Crump, C.; Sundquist, K.; Winkleby, M.A.; Sundquist, J. Comorbidities and mortality in bipolar disorder: A Swedish national cohort study. JAMA Psychiatry 2013, 70, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Rowland, T.A.; Marwaha, S. Epidemiology and risk factors for bipolar disorder. Ther. Adv. Psychopharmacol. 2018, 8, 251–269. [Google Scholar] [CrossRef] [PubMed]
- Luykx, J.J.; Giuliani, F.; Giuliani, G.; Veldink, J. Coding and Non-Coding RNA Abnormalities in Bipolar Disorder. Genes 2019, 10, 946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, L.J.; Schönfeld, B.; Chen, X.S. The epigenetics of non-coding RNA. Handb. Epigenet. 2011, 49–61. Available online: https://www.semanticscholar.org/paper/the-epigenetics-of-non-coding-rnA-Collins-Sch%C3%B6nfeld/aba3bdd0159c0af9496b580c61bcdcdf826eb37f (accessed on 1 May 2021).
- Lee, S.-Y.; Lu, R.-B.; Wang, L.-J.; Chang, C.-H.; Lu, T.; Wang, T.-Y.; Tsai, K.W. Serum miRNA as a possible biomarker in the diagnosis of bipolar II disorder. Sci. Rep. 2020, 10, 1131. [Google Scholar] [CrossRef] [Green Version]
- Sekar, S.; Liang, W.S. Circular RNA expression and function in the brain. Noncoding RNA Res. 2019, 4, 23–29. [Google Scholar] [CrossRef]
- Rybak-Wolf, A.; Stottmeister, C.; Glažar, P.; Jens, M.; Pino, N.; Giusti, S.; Hanan, M.; Behm, M.; Bartok, O.; Ashwal-Fluss, R.; et al. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol. Cell. 2015, 58, 870–885. [Google Scholar] [CrossRef] [Green Version]
- You, X.; Vlatkovic, I.; Babic, A.; Will, T.; Epstein, I.; Tushev, G.; Akbalik, G.; Wang, M.; Glock, C.; Quedenau, C.; et al. Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nat. Neurosci. 2015, 18, 603. [Google Scholar] [CrossRef] [Green Version]
- Dines, M.; Lamprecht, R. The role of Ephs and ephrins in memory formation. Int. J. Neuropsychopharmacol. 2016, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dines, M.; Lamprecht, R. EphrinA4 mimetic peptide targeted to EphA binding site impairs the formation of long-term fear memory in lateral amygdala. Transl. Psychiatry 2014, 4, 450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attwood, B.K.; Bourgognon, J.-M.; Patel, S.; Mucha, M.; Schiavon, E.; Skrzypiec, A.E.; Young, K.W.; Shiosaka, S.; Korostynski, M.; Piechota, M.; et al. Neuropsin cleaves EphB2 in the amygdala to control anxiety. Nature 2011, 473, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, A.J.; Hafez, A.K.; Amoah, S.K.; Rodriguez, B.A.; Dell’Orco, M.; Lozano, E.; Hartley, B.J.; Alural, B.; Lalonde, J.; Chander, P.; et al. A psychiatric disease-related circular RNA controls synaptic gene expression and cognition. Mol. Psychiatry 2020, 25, 2712–2727. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Xu, J.; Pang, L.; Zhao, H.; Li, F.; Deng, Y.; Liu, L.; Lan, Y.; Zhang, X.; Zhao, T.; et al. Systematically characterizing dysfunctional long intergenic non-coding RNAs in multiple brain regions of major psychosis. Oncotarget 2016, 7, 71087–71098. [Google Scholar] [CrossRef] [Green Version]
- Ji, B.; Higa, K.K.; Kelsoe, J.R.; Zhou, X. Over-expression of XIST, the Master Gene for X Chromosome Inactivation, in Females With Major Affective Disorders. EBioMedicine 2015, 2, 909–918. [Google Scholar] [CrossRef] [Green Version]
- Son, H.J.; Choi, E.J.; Yoo, N.J.; Lee, S.H. Somatic mutations in long-non-coding RNA RMRP in acute leukemias. Pathol. Res. Pract. 2019, 215, 152647. [Google Scholar] [CrossRef]
- Sayad, A.; Taheri, M.; Omrani, M.D.; Fallah, H.; Kholghi Oskooei, V.; Ghafouri-Fard, S. Peripheral expression of long non-coding RNAs in bipolar patients. J. Affect Disord. 2019, 249, 169–174. [Google Scholar] [CrossRef]
- Naghavi-Gargari, B.; Zahirodin, A.; Ghaderian, S.M.H.; Shirvani-Farsani, Z. Significant increasing of DISC2 long non-coding RNA expression as a potential biomarker in bipolar disorder. Neurosci. Lett. 2019, 696, 206–211. [Google Scholar] [CrossRef]
- Ghafelehbashi, H.; Pahlevan Kakhki, M.; Kular, L.; Moghbelinejad, S.; Ghafelehbashi, S.H. Decreased Expression of IFNG-AS1, IFNG and IL-1B Inflammatory Genes in Medicated Schizophrenia and Bipolar Patients. Scand. J. Immunol. 2017, 86, 479–485. [Google Scholar] [CrossRef]
- Shirvani Farsani, Z.; Zahirodin, A.; Ghaderian, S.M.H.; Shams, J.; Naghavi Gargari, B. The role of long non-coding RNA MALAT1 in patients with bipolar disorder. Metab. Brain Dis. 2020, 35, 1077–1083. [Google Scholar] [CrossRef]
- Squassina, A.; Niola, P.; Lopez, J.P.; Cruceanu, C.; Pisanu, C.; Congiu, D.; Severino, G.; Ardau, R.; Chillotti, C.; Alda, M.; et al. MicroRNA expression profiling of lymphoblasts from bipolar disorder patients who died by suicide, pathway analysis and integration with postmortem brain findings. Eur. Neuropsychopharmacol. 2020, 34, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Pogue, A.I.; Lukiw, W.J. Up-regulated pro-inflammatory microRNAs (miRNAs) in Alzheimer’s disease (AD) and age-related macular degeneration (AMD). Cell. Mol. Neurobiol. 2018, 38, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.L.; Kao, P.F.; Itriago, E.; Zhan, Y.; Kozubek, J.A.; Hoss, A.G.; Banigan, M.G.; Vanderburg, C.R.; Rezvani, A.H.; Latourelle, J.C.; et al. miR-149 and miR-29c as candidates for bipolar disorder biomarkers. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2017, 174, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Kim, S.; Kang, H.; Yun, K.N.; Lee, Y.; Zhang, Y.; Kim, Y.; Kim, J.Y.; Han, K. Shank3 regulates striatal synaptic abundance of Cyld, a deubiquitinase specific for Lys63-linked polyubiquitin chains. J. Neurochem. 2019, 150, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Pang, K.; Kim, J.Y.; Ryu, J.R.; Kang, H.; Liu, Z.; Kim, W.K.; Sun, W.; Kim, H.; Han, K. Post-transcriptional regulation of SHANK3 expression by microRNAs related to multiple neuropsychiatric disorders. Mol. Brain 2015, 8, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q.; Ruan, H.; Peng, L.; Zhang, M.; Gack, M.U.; Yao, W.-D. Proteasome-independent polyubiquitin linkage regulates synapse scaffolding, efficacy, and plasticity. Proc. Natl. Acad. Sci. USA 2017, 114, 8760–8769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisanu, C.; Merkouri Papadima, E.; Melis, C.; Congiu, D.; Loizedda, A.; Orru, N.; Calza, S.; Orrù, S.; Carcassi, C.; Severino, G.; et al. Whole Genome Expression Analyses of miRNAs and mRNAs Suggest the Involvement of miR-320a and miR-155-3p and their Targeted Genes in Lithium Response in Bipolar Disorder. Int. J. Mol. Sci. 2019, 20, 6040. [Google Scholar] [CrossRef] [Green Version]
- Amoah, S.K.; Rodriguez, B.A.; Logothetis, C.N.; Chander, P.; Sellgren, C.M.; Weick, J.P.; Sheridan, S.D.; Jantzie, L.L.; Webster, M.J.; Mellios, N. Exosomal secretion of a psychosis-altered miRNA that regulates glutamate receptor expression is affected by antipsychotics. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2020, 45, 656–665. [Google Scholar] [CrossRef]
- Ceylan, D.; Tufekci, K.U.; Keskinoglu, P.; Genc, S.; Ozerdem, A. Circulating exosomal microRNAs in bipolar disorder. J. Affect. Disord. 2020, 262, 99–107. [Google Scholar] [CrossRef]
- Camkurt, M.A.; Karababa, I.F.; Erdal, M.E.; Kandemir, S.B.; Fries, G.R.; Bayazit, H.; Ay, M.E.; Kandemir, H.; Ay, Ö.I.; Coşkun, S.; et al. MicroRNA dysregulation in manic and euthymic patients with bipolar disorder. J. Affect. Disord. 2020, 261, 84–90. [Google Scholar] [CrossRef]
- Tabano, S.; Caldiroli, A.; Terrasi, A.; Colapietro, P.; Grassi, S.; Carnevali, G.S.; Fontana, L.; Serati, M.; Vaira, V.; Altamura, A.C.; et al. A miRNome analysis of drug-free manic psychotic bipolar patients versus healthy controls. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fries, G.R.; Lima, C.N.C.; Valvassori, S.S.; Zunta-Soares, G.; Soares, J.C.; Quevedo, J. Preliminary investigation of peripheral extracellular vesicles’ microRNAs in bipolar disorder. J. Affect. Disord. 2019, 255, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Tyryshkin, K.; Elmi, N.; Feilotter, H.; Andreazza, A.C. Examining redox modulation pathways in the post-mortem frontal cortex in patients with bipolar disorder through data mining of microRNA expression datasets. J. Psychiatr. Res. 2018, 99, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Maffioletti, E.; Cattaneo, A.; Rosso, G.; Maina, G.; Maj, C.; Gennarelli, M.; Tardito, D.; Bocchio-Chiavetto, L. Peripheral whole blood microRNA alterations in major depression and bipolar disorder. J. Affect. Disord. 2016, 200, 250–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, R.M.; Rybka, J.; Anderson, S.M.; Torrance, H.S.; Boxall, R.; Sussmann, J.E.; Porteous, D.J.; McIntosh, A.M.; Evans, K.L. Preliminary investigation of miRNA expression in individuals at high familial risk of bipolar disorder. J. Psychiatr. Res. 2015, 62, 48–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bavamian, S.; Mellios, N.; Lalonde, J.; Fass, D.M.; Wang, J.; Sheridan, S.D.; Madison, J.M.; Zhou, F.; Rueckert, E.H.; Barker, D.; et al. Dysregulation of miR-34a links neuronal development to genetic risk factors for bipolar disorder. Mol. Psychiatry 2015, 20, 573–584. [Google Scholar] [CrossRef]
- Smalheiser, N.R.; Lugli, G.; Zhang, H.; Rizavi, H.; Cook, E.H.; Dwivedi, Y. Expression of microRNAs and other small RNAs in prefrontal cortex in schizophrenia, bipolar disorder and depressed subjects. PLoS ONE 2014, 9, e86469. [Google Scholar] [CrossRef]
- Banigan, M.G.; Kao, P.F.; Kozubek, J.A.; Winslow, A.R.; Medina, J.; Costa, J.; Schmitt, A.; Schneider, A.; Cabral, H.; Cagsal-Getkin, O.; et al. Differential expression of exosomal microRNAs in prefrontal cortices of schizophrenia and bipolar disorder patients. PLoS ONE 2013, 8, e48814. [Google Scholar] [CrossRef] [Green Version]
- Miller, B.H.; Zeier, Z.; Xi, L.; Lanz, T.A.; Deng, S.; Strathmann, J.; Willoughby, D.; Kenny, P.J.; Elsworth, J.D.; Lawrence, M.S.; et al. MicroRNA-132 dysregulation in schizophrenia has implications for both neurodevelopment and adult brain function. Proc. Natl. Acad. Sci. USA 2012, 109, 3125–3130. [Google Scholar] [CrossRef] [Green Version]
- Kim, A.H.; Reimers, M.; Maher, B.; Williamson, V.; McMichael, O.; McClay, J.L.; van den Oord, E.J.; Riley, B.P.; Kendler, K.S.; Vladimirov, V.I. MicroRNA expression profiling in the prefrontal cortex of individuals affected with schizophrenia and bipolar disorders. Schizophr. Res. 2010, 124, 183–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Wang, N.; Burmeister, M.; McInnis, M.G. MicroRNA expression changes in lymphoblastoid cell lines in response to lithium treatment. Int. J. Neuropsychopharmacol. 2009, 12, 975–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bame, M.; McInnis, M.G.; O’Shea, K.S. MicroRNA Alterations in Induced Pluripotent Stem Cell-Derived Neurons from Bipolar Disorder Patients: Pathways Involved in Neuronal Differentiation, Axon Guidance, and Plasticity. Stem Cells Dev. 2020, 29, 1145–1159. [Google Scholar] [CrossRef]
- Zhang, H.P.; Liu, X.L.; Chen, J.J.; Cheng, K.; Bai, S.J.; Zheng, P.; Zhou, C.J.; Wang, W.; Wang, H.Y.; Zhong, L.M.; et al. Circulating microRNA 134 sheds light on the diagnosis of major depressive disorder. Transl. Psychiatry 2020, 10, 95. [Google Scholar] [CrossRef]
- Banach, E.; Dmitrzak-Weglarz, M.; Pawlak, J.; Kapelski, P.; Szczepankiewicz, A.; Rajewska-Rager, A.; Slopien, A.; Skibinska, M.; Czerski, P.; Hauser, J. Dysregulation of miR-499, miR-708 and miR-1908 during a depression episode in bipolar disorders. Neurosci. Lett. 2017, 654, 117–119. [Google Scholar] [CrossRef]
- Kim, Y.; Zhang, Y.; Pang, K.; Kang, H.; Park, H.; Lee, Y.; Lee, B.; Lee, H.J.; Kim, W.K.; Geum, D.; et al. Bipolar Disorder Associated microRNA, miR-1908-5p, Regulates the Expression of Genes Functioning in Neuronal Glutamatergic Synapses. Exp. Neurobiol. 2016, 25, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, J.A.; Carter, B.S.; Meng, F.; Turner, D.L.; Dai, M.; Schatzberg, A.F.; Barchas, J.D.; Jones, E.G.; Bunney, W.E.; Myers, R.M.; et al. The microRNA network is altered in anterior cingulate cortex of patients with unipolar and bipolar depression. J. Psychiatr. Res. 2016, 82, 58–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, H.; Liu, T.B.; Yang, K.J.; Yang, H.C.; Wu, D.H.; Liao, C.P.; Hong, F.; Yang, H.Z.; Wan, F.; Ye, X.Y.; et al. MicroRNA-134 plasma levels before and after treatment for bipolar mania. J. Psychiatr. Res. 2011, 45, 92–95. [Google Scholar] [CrossRef]
- Zhu, Y.; Kalbfleisch, T.; Brennan, M.D.; Li, Y. A MicroRNA gene is hosted in an intron of a schizophrenia-susceptibility gene. Schizophr. Res. 2009, 109, 86–89. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Shi, J.; Liu, H.; Wang, Q.; Chen, X.; Tang, H.; Yan, R.; Lu, Q.; Yao, Z. Plasma microRNA Array Analysis Identifies Overexpressed miR-19b-3p as a Biomarker of Bipolar Depression Distinguishing From Unipolar Depression. Front. Psychiatry 2020, 11, 757. [Google Scholar] [CrossRef]
- Lim, C.H.; Zainal, N.Z.; Kanagasundram, S.; Zain, S.M.; Mohamed, Z. Preliminary examination of microRNA expression profiling in bipolar disorder I patients during antipsychotic treatment. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2016, 171, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Kidnapillai, S.; Wade, B.; Bortolasci, C.C.; Panizzutti, B.; Spolding, B.; Connor, T.; Crowley, T.; Jamain, S.; Gray, L.; Leboyer, M.; et al. Drugs used to treat bipolar disorder act via microRNAs to regulate expression of genes involved in neurite outgrowth. J. Psychopharmacol. 2020, 34, 370–379. [Google Scholar] [CrossRef]
- Gawryluk, J.W.; Young, L.T. Signal Transduction Pathways in the Pathophysiology of Bipolar Disorder. Behavioral Neurobiology of Bipolar Disorder and Its Treatment; Springer: Berlin/Heidelberg, Germany, 2010; pp. 139–165. [Google Scholar]
- Lener, M.S.; Niciu, M.J.; Ballard, E.D.; Park, M.; Park, L.T.; Nugent, A.C.; Zarate, C.A., Jr. Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biol. Psychiatry 2017, 81, 886–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stahl, E.A.; Breen, G.; Forstner, A.J.; McQuillin, A.; Ripke, S.; Trubetskoy, V.; Mattheisen, M.; Wang, Y.; Coleman, J.R.; Gaspar, H.A.; et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat. Genet. 2019, 51, 793–803. [Google Scholar] [CrossRef]
- Kim, S.; Hwang, Y.; Webster, M.; Lee, D. Differential activation of immune/inflammatory response-related co-expression modules in the hippocampus across the major psychiatric disorders. Mol. Psychiatry 2016, 21, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Akula, N.; Barb, J.; Jiang, X.; Wendland, J.R.; Choi, K.H.; Sen, S.K.; Hou, L.; Chen, D.T.; Laje, G.; Johnson, K.; et al. RNA-sequencing of the brain transcriptome implicates dysregulation of neuroplasticity, circadian rhythms and GTPase binding in bipolar disorder. Mol. Psychiatry 2014, 19, 1179–1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruceanu, C.; Tan, P.P.C.; Rogic, S.; Lopez, J.P.; Torres-Platas, S.G.; Gigek, C.O.; Alda, M.; Rouleau, G.A.; Pavlidis, P.; Turecki, G. Transcriptome sequencing of the anterior cingulate in bipolar disorder: Dysregulation of G protein-coupled receptors. Am. J. Psychiatry 2015, 172, 1131–1140. [Google Scholar] [CrossRef]
- Li, J.; Hao, M.; Yang, B.; Shi, T.; Zhang, Y.; Feng, J.; Chen, J. Long non-coding RNAs expression profile and functional analysis of acute ischemic stroke. Medicine 2020, 99, e22964. [Google Scholar] [CrossRef] [PubMed]
- Sajja, V.; Jablonska, A.; Haughey, N.; Bulte, J.W.M.; Stevens, R.D.; Long, J.B.; Walczak, P.; Janowski, M. Sphingolipids and microRNA Changes in Blood following Blast Traumatic Brain Injury: An Exploratory Study. J. Neurotrauma 2018, 35, 353–361. [Google Scholar] [CrossRef]
- Shi, D.; Han, M.; Liu, W.; Tao, J.; Chen, L. Circulating MicroRNAs as Diagnostic Biomarkers of Clinical Cognitive Impairment: A Meta-Analysis. Am. J. Alzheimer’s Dis. Dement. 2020, 35, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Sheinerman, K.S.; Toledo, J.B.; Tsivinsky, V.G.; Irwin, D.; Grossman, M.; Weintraub, D.; Hurtig, H.I.; Chen-Plotkin, A.; Wolk, D.A.; McCluskey, L.F.; et al. Circulating brain-enriched microRNAs as novel biomarkers for detection and differentiation of neurodegenerative diseases. Alzheimers Res. Ther. 2017, 9, 89. [Google Scholar] [CrossRef] [PubMed]
| circRNA | Number of Clinical Samples | Type of Study | False Discovery Rate | Function | Ref |
|---|---|---|---|---|---|
| cNEBL | postmortem human medial frontal gyrus tissues from BPD cases (n = 4) and normal subjects (n = 4) | High throughput analysis | FDR < 0.05 | - | [6] |
| cEPHA3 | High throughput analysis | FDR < 0.1 | EPHA3 participates in the neurodevelopment. |
| circRNA | Number of Clinical Samples | Targets/Regulators | p Value | Function | Ref |
|---|---|---|---|---|---|
| circHomer1a | Human OFC postmortem brain tissues of BD patients (n = 32) and healthy controls (n = 34) | RNA-binding protein HuD | p < 0.01 | circHomer1a is originated from HOMER1, a gene regulating neuronal excitability and synaptic plasticity. This gene is down-regulated in the OFC and stem cells-originated neurons of BD patients. | [15] |
| lncRNA | Number of Clinical Samples | Assessed Cell Line | Targets/Regulators | p Value | Type of Study | Function | Ref |
|---|---|---|---|---|---|---|---|
| PTCSC3 | Whole blood sample of BD patients with manic episode (n =13) | - | - | p = 2.39 × 10−4 | High throughput analysis | PTCSC3 gene is reported to be associated with thyroid cancer. | [18] |
| CCAT2 | Peripheral blood specimens BD patients (n = 50) and healthy subjects (n = 50) | - | - | p = 0.006 | Candidate molecule analysis | CCAT2 is an oncogenic lncRNA in numerous neoplasms that enhances cell proliferation and suppresses apoptosis. | [19] |
| TUG1 | - | - | p < 0.001 | Candidate molecule analysis | TUG2 is an oncogenic lncRNA in numerous neoplasms that enhances cell proliferation and suppresses apoptosis. | [19] | |
| PANDA | - | - | p = 0.004 | Candidate molecule analysis | PANDA is an oncogenic lncRNA in numerous neoplasms that enhances cell proliferation and suppresses apoptosis. | [19] | |
| DISC2 | Peripheral blood mononuclear cells (PBMCs) of BD patients (n = 50) and controls (n = 50) | - | hsa-miR-92a-2-5p, hsa-miR363-5p, hsa-miR-1285-3p and hsa-miR-1268a | p = 0.0015 | Candidate molecule analysis | DISC2 may regulate DISC1 expression. | [20] |
| XIST | Lymphoblastoid cells were from healthy females (n = 36) and female patients with either BD or recurrent major depression (n = 60) | Lymphoblastoid cell lines | TSIX, FTX, JPX | p = 1 × 10−7 | Candidate molecule analysis | XIST is the master gene for XCI. | [17] |
| FTX | Lymphoblastoid cell lines | XIST | p < 0.1 | Candidate molecule analysis | FTX is a positive regulator of XIST expression. | [17] |
| lncRNA | Number of Clinical Samples | Assessed Cell Line | Targets/Regulators | Signaling Pathways | p Value | Type of Study | Function | Ref |
|---|---|---|---|---|---|---|---|---|
| OIP5-AS1 | Peripheral blood samples of BD patients (n = 50) and healthy controls (n = 50) | - | - | - | p = 0.001 | Candidate genes analysis | OIP5-AS1 is as an oncogene that enhances cell proliferation and suppresses of apoptosis. | [19] |
| IFNG-AS1 | Blood samples of BD patients (n = 30) and healthy control individuals (n = 32) | - | IFNG | - | p < 0.0001 | High throughput analysis | IFNG-AS1 facilitates IFN-γ expression through association with the WDR methyltransferases and subsequent increase in H3K4 methylation at the IFNG locus. | [21] |
| ENSG00000228794 | Post-mortem brain samples of patients with SZ and BD and control subjects (n = 82) | - | - | Calcium signaling | p < 0.05 | High throughput analysis | ENSG00000228794 is located in a genomic region linked with BD and partakes in calcium ion transport. | [16] |
| TSIX | Lymphoblastoid cells were from healthy females (n = 36) and female patients with either BD or recurrent major depression (n = 60) | Lymphoblastoid cell lines | XIST | - | p < 0.01 | Candidate genes analysis | TSIX is a negative regulator of XIST expression. | [17] |
| MALAT1 | Peripheral blood samples of BD patients (n = 50) and healthy controls (n = 50) | PBMCs | hsa-miR-17-5p, hsa-miR-106a-5p, hsa-miR-30c-5p, hsa-miR-20b-5p, hsa-miR-503, hsa-miR-92b-3p, hsa-miR-1224-3p | - | p < 0.0001 | Candidate molecule analysis | MALAT1 takes part in the regulation of genes involved in synaptogenesis. | [22] |
| microRNA | Number of Clinical Samples | Assessed Cell Line | Targets/Regulators | Signaling Pathways | p Value | Type of Study | Function | Ref |
|---|---|---|---|---|---|---|---|---|
| miR-7-5p | Whole blood samples of BD-II patients (n = 102) and controls (n = 118) | - | BDNF | GABAergic and glutamatergic synapses and TGF-beta, Hippo, and FoxO signaling | p < 0.001 | High throughput analysis | miR-7 has a role in inhibition of the repair of peripheral nerve damage by affecting the migration and proliferation of neural stem cells. | [8] |
| miR-142-3p | - | TNF-α | p < 0.0001 | High throughput analysis | miR-142-3p may modulate the BMAL1 gene and regulate circadian functions. | [8] | ||
| miR-221-5p | - | - | p < 0.0001 | High throughput analysis | miR-221 is potentially involved in atherosclerosis. | [8] | ||
| miR-370-3p | - | - | p < 0.0001 | High throughput analysis | miR-370 is reduced in brain tissue of depressed animals. | [8] | ||
| miR-23b-3p | - | - | p = 0.006 | High throughput analysis | miR-23b may have an anti-inflammatory role in central nervous system inflammation. | [8] | ||
| miR-4286 | Lymphoblastoid cell line cultures from patients with BD who died by suicide (SC, n = 7) and with low risk of suicide (LR, n = 11) and 12, non-suicidal controls | Lymphoblastoid cell lines (LCLs) | PRKAB2, PTPRF, PIK3R3, CREB1, PPARGC1B, PIK3R1, CREB3L2, PTPA, PTEN RELA, PRKAG1, PTPN11, PRKCB, SOCS3, INSR, PYGB, PPARA | Insulin resistance signaling pathway | p = 0.000043 | High throughput analysis | miR-4286 might be a specific biomarker of suicide. | [23] |
| hsa-miR-155-3p | Lymphoblastoid cell line cultures from BD patients excellent responders (ER, n = 12) and non-responders (NR, n = 12) to lithium | Lymphoblastoid cell lines | SP4 | - | p = 0.0003 | High throughput analysis | hsa-miR-155-3p was up-regulated in ER. It partakes in inflammatory response and modulates differentiation and activation of innate and adaptive immune systems. | [29] |
| miR-223 | Orbitofrontal cortex of SZ (n = 29; 20 males and 9 females), BD (n = 26; 12 males and 14 females), and unaffected controls (n = 25; 21 males and 4 females) | - | GRIN2B, GRIA2 | - | p < 0.001 | High throughput analysis | miR-223 regulates glutamate receptors. miR-223 expression is negatively correlated with levels of its targets GRIN2B and GRIA2. | [30] |
| miR-193b-3p | - | - | - | p < 0.05 | High throughput analysis | miR-193a-3p was upregulated in both BD and SZ. | [30] | |
| miR-330-3p | - | - | - | p < 0.05 | High throughput analysis | miR-330-3p has been over-expressed in the blood of subjects with BD and monopolar depression. | [30] | |
| miR-28a-3p | - | - | - | 0.05 < p <0.10 | High throughput analysis | miR-28a-3p is in the same family as miR-708, a miRNA that is associated with risk of BD. | [30] | |
| miR-1260 | - | - | - | p < 0.05 | High throughput analysis | - | [30] | |
| miR-185-5p | Plasma samples of patients with BD type I (n = 69; 15 depressed, 27 manic, 27 euthymic) and healthy controls (n = 41) | - | Tyrosine kinase receptor type 2 | PI3K-Akt | p = 0.001 | High throughput analysis | miR-185-5p is a target miRNA for depression. | [31] |
| miR-29a-3p | Peripheral blood of BD I patients (n = 58, 19 manic, 39 euthymic) and healthy controls (n = 51) | - | - | PI3K-Akt, TGF-beta | p = 0.035 | Candidate analysis | - | [32] |
| miR125a-3p | p = 0.014 | |||||||
| miR-106b-5p | - | IL-10 | PI3K-Akt, TGF-beta | p = 0.014 | Candidate analysis | miR-106 might be involved in immunomodulatory aspects of BD. | [32] | |
| miR-107 | - | GRIN2A, SLC1A4 | PI3K-Akt, TGF-beta | p = 0.011 | Candidate analysis | miR-107 is up-regulated in manic and euthymic patients. | [32] | |
| hsa-miR-150-5p, hsa-miR-25-3p, hsa-miR-451a, hsa-miR-144-3p | Plasma samples from drug-free psychotic bipolar patients (n = 15) and HC (n = 9) | - | - | - | p < 0.01 | High throughput analysis | These miRNAs were upregulated in patients. | [33] |
| hsa-miR-4516, hsa-miR-6808-5p, hsa-miR-7977, hsa-miR-1185-2-3p, hsa-miR-6791-5p, hsa-miR-3194-5p, hsa-miR-6090, hsa-miR-3135b | Peripheral blood EV of BD patients (n = 20) and age- and sex-matched normal controls (n = 21) | - | - | Axon guidance mediated by netrin, endothelin signaling pathway, 5HT2 type receptor-mediated signaling, beta1 and beta2 adrenergic receptor and the androgen receptor signaling pathways | p < 0.05 | High throughput data mining | These miRNAs are related to neuron development. | [34] |
| hsa-miR-29c-3p | - | - | p = 0.010514 | High throughput data mining | Increased levels of miR-29c have been detected in EVs isolated from post-mortem prefrontal cortex (BA9) of patients. | [34] | ||
| hsa-miR-7975 | - | - | p = 0.048192 | High throughput data mining | hsa-miR-7975 is associated with the brain. | [34] | ||
| hsa-miR-21-5p | NTN1, NTNG1, | - | p = 0.028475 | High throughput data mining | The increased levels of miR-22 in EVs are supported by findings of upregulation of these miRNAs in the prefrontal cortex of patients with BD. | [34] | ||
| hsa-miR-142-3p | NTN3, NTNG1, NTNG2 | - | p = 0.019266 | High throughput data mining | NTN3, NTNG1, NTNG2 are targeted by hsa-miR-142-3p. | [34] | ||
| hsa-miR-22-3p, hsa-miR-92a-3p | NTN3, NTN4, NTNG1, NTNG2 | - | p < 0.05 | High throughput data mining | NTN3, NTN4, NTNG1, NTNG2 are targeted by hsa-miR-22-3p, hsa-miR-92a-3p. | [34] | ||
| hsa-miR-198 | 3 frontal cortex miRNA expression datasets (N for BD = 30–36 per dataset, N for controls = 28–34 per dataset) | - | GPX4 | Redox modulation pathways | p < 0.05 | High throughput data mining | These are the top 10th percentile of up-regulated miRNAs that target redox modulators ranked for their ability to discriminate between BD and controls in Vladimirov dataset. | [35] |
| hsa-miR-601 | - | ATP2B4 | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-659 | - | SOD2, ATP5A1 | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-192 | - | TXN2, UQCRC2, ATP5L, PXDN, TXNIP, COX5A, TXN2 | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-346 | - | NDUFA1, COX5A | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-9* | - | ATP5F1 | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-301a | - | ATP5B, COX10, TXNIP, BCL2L11, NDFUA7, TXNRD3, COX7A2, OXA1L, MGST1, PXDN, SOD2, COX5B, NDUFA5, UQCRQ | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-199a-3p | - | ATP5B, UQCRC2, COX10, TXNIP, BCL2L11, PTGS2, GLRX2, NDUFA2, NDUFC2, NDUFA12 | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-34a | - | TXNIP, NDUFS1, SOD2, PRDX5, NDUFV1 | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-145 | - | NDUFS1, NDUFA4 | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-27a | - | PPA1, TXNIP, ATP2B4, ATP5SL, PRDX1, PRDX4, FOXO3, NDUFA8, CAT, PXDN, SOD1, FOXO1, NDUC2, GSTO1, ATP5G3, NDUFV3, NDUFS2, NDUFS4, NDUFV1, PRDX3, PPA1, GLRX5 | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-92a-1* | - | BCS1L, NDUFS1, SDHB, OXA1L | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-103 | - | MGST1, TXN2, NDUFS8, ATP5B, PRDX4, ATP5A1, OXA1L, NDUFS2, NOS3, COX5A, TXNRD3 | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-196b | - | ATP5G3, MT-ATP6, BCL2L2, BCL2L12, ATP2B4, GLRX3, NDUFC2, OXA1L, NDUFV3 | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-449a | - | ATP5H, TXNIP, FOXO1, BCL2L11, ATP6V0A2, NDUFS1 | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-196a | - | GPX1, ATP5G3, UQCRC2, TXR1, MTATP8, MT-ATP6, BCL2L12, ATP2B4, GLRX3, FOXO1, OXA1L, NDUFV3, NDUFC2, GSTK1 | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-675 | - | SOD2, PXDN | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-184 | - | BCL2A1 | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-200c | - | MT-ATP6, MGST1 | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-200b | - | BCL2, GLRX5 | p < 0.05 | High throughput data mining | [35] | |||
| miR-149 | Extracellular vesicles (EVs) extracted from Human Postmortem Anterior Cingulate Cortices (BA24) Diagnosed With BD (n = 4) and Non-Psychiatric Control Cases (n = 6) | Neuronal and glial cells | - | AKT1 | p = 0.0046 | Candidate analysis | miR-149 suppresses glial proliferation. | [25] |
| miR-30d-5p | Blood samples of MD patienst (n = 20) and BD patienst (n =20, 10 type I and 10 type II) and healthy controls (n = 20, 15 females, 5 males) | - | - | - | p = 0.028 | High throughput analysis | The blood expression of miR-30d-5p was increased also in MD patients after AD treatment. | [36] |
| miR140-3p | - | - | - | p = 0.027 | High throughput analysis | The blood expression of miR140-3p was increased also in MD patients after AD treatment. | [36] | |
| miR-330-5p | - | HTR2C, MAOA, DRD1, CAMKK2, NTRK3, CLOCK, CREB1, GABRA2, CNR1, MTHFR | - | p = 0.030 | High throughput analysis | miR-330-5p regulates many targets participating in neuronal plasticity and neurodevelopment. | [36] | |
| miR-21-3p | - | - | - | p = 0.043 | High throughput analysis | miR-21-3p is decreased in MD fibroblast cultures. | [36] | |
| miR-378a-5p | - | - | - | p = 0.042 | High throughput analysis | miR-378a-5p is mainly involved in lipid and metabolism homeostasis. | [36] | |
| hsa-miR-345-5p | - | HTR2C, MAOA, DRD1, CAMKK2, NTRK3, CLOCK, CREB1, GABRA2, CNR1, MTHFR | - | p = 0.010 | High throughput analysis | miR-345- 5p is predicted to regulate several target genes with a putative role in the shared pathogenetic mechanisms between MD and BD. | [36] | |
| miR-15b | Blood of unaffected individuals at higher genetic risk of developing a mood disorder (n = 34) and control subjects (n = 46) | - | - | PI3K/Akt, PTEN | p = 0.0166 | Candidate gene analysis (20 miRNAs) | miR-15b was over-expressed in the high-risk persons. It is involved in metabolism, angiogenesis, stress response, cancer, cardiovascular disease and neurodegenerative conditions. | [37] |
| miR-132 | - | - | PI3K/Akt | p = 0.0249 | Candidate gene analysis (20 miRNAs) | miR-132 was over-expressed in the high-risk persons. miR-132 is transcribed from a cluster of miRNAs that partake in neuronal development and function. | [37] | |
| miR-652 | - | GABARB2, GABARB3, 5-HT1D, DISC1 | - | p = 0.01076 | Candidate gene analysis (20 miRNAs) | miR-652 was up-regulated in the high-risk individuals. miR-652 plays a central role in myeloid development. | [37] | |
| miR-34a | Postmortem human brain samples from the cerebellum (lateral cerebellar hemisphere; 34 control and 29 BD samples) | - | ANK3, CACNB3, DDN, SHANK3 | WNT, cadherin | p < 0.01 | Candidate analysis | miR-34a expression is inversely associated with expression of ANK3 and CACNB3. | [27,38] |
| miR-17-5p | Human prefrontal cortex (Brodmann area 10) of 15 SZ, 15 MDD, 15 BD, and 15 controls | - | - | - | p = 0.0028 | High throughput analysis | - | [39] |
| miR29c-3p | p = 0.049 | |||||||
| miR-106b-5p | p = 0.021 | |||||||
| miR-579 | p = 0.0092 | |||||||
| miR-29c | Postmortem Human Prefrontal Cortex (Brodmann area 9, BA9) 8 SZ, 9 BD, and 13 controls | - | - | Wnt | p = 0.0237 | High throughput analysis | miR29c is induced by canonical Wnt signaling. | [40] |
| hsa-miR-188-5p, hsa-miR-196b, hsa-miR-32*, hsa-miR-187, hsa-miR-383, hsa-miR-297, hsa-miR-876-3p, hsa-miR-490-5p, hsa-miR-449b, hsa-miR-513-5p | Dorsolateral prefrontal cortex tissue of control (n = 34), bipolar (n = 31), and schizophrenic (SZ, n = 35) subjects | - | - | - | p < 0.05 | High throughput analysis | - | [41] |
| hsa-miR-504 | Postmortem DLPFC sections from 35 cases with schizophrenia 35 cases with BD | - | - | - | p = 0.00003 | High throughput analysis | - | [42] |
| hsa-miR-145* | p = 0.00080 | |||||||
| hsa-miR-22* | p = 0.00106 | |||||||
| hsa-miR-145 | p = 0.00177 | |||||||
| hsa-miR-133b | p = 0.00190 | |||||||
| hsa-miR-154* | p = 0.00195 | |||||||
| hsa-miR-889 | p = 0.00321 | |||||||
| miR-34a | 20 LCLs derived from bipolar I disorder (BPI) family members with and without LiCl treatment in culture | Lymphoblastoid cell lines (LCLs) | AP2A1, AP2S1, CD2AP, EIF1, and VCL | - | p = 0.023917 | Candidate analysis (13 miRNAs) | miR-34a, miR-152, miR-155, and miR-221 were consistently up-regulated at treatment time point day 4 and day 16. | [43] |
| miR-152 | p = 0.000405 | |||||||
| miR-155 | p = 0.012045 | |||||||
| miR-221 | p = 0.000073 | |||||||
| miR-195-5p, | Skin biopsies of 3 control and 3 BP patient | Pluripotent Stem Cell-derived neurons | AXIN2, BDNF, CACNA1E, MIB1, NLGN1 and RELN | Axon guidance, Mapk, Ras, Hippo, Neurotrophin and Wnt signaling pathway | p < 0.05 | Candidate molecule analysis(58 miRNAs) | - | [44] |
| miR-382-5p | SYT4 | |||||||
| miR-128-3p, miR-138-2-3p, miR-487b-3p, miR-744-3p | - |
| microRNA | Number of Clinical Samples | Assessed Cell Line | Targets/Regulators | Signaling Pathways | p Value | Type of Study | Function | Ref |
|---|---|---|---|---|---|---|---|---|
| miR-320a | BD patients (excellent responders, n = 12; non-responders, n = 12) to lithium | Lymphoblastoid cell lines | CAPNS1 | - | p < 0.0001 | High throughput analysis | Participates in response to lithium | [29] |
| miR-134 | Whole blood samples of BD (n = 50) and controls (n = 50) | - | cAMP response element-binding protein (CREB) | - | p = 2.25 × 10−5 | Candidate molecule analysis | miR-134 regulates dendritic spine development and plasticity. | [45] |
| miR-186–5p | LCLs from patients with BD who deceased by suicide (SC, n = 7) and with low risk of suicide (LR, n = 11) and 12, non-suicidal controls | Lymphoblastoid cell lines (LCLs) | - | - | p = 0.032 | High throughput analysis | miR-186–5p was lower in lithium-treated LCLs from SC compared to controls. | [23] |
| miR-484 | Plasma samples of patients with BD type I and healthy controls (n = 41) | - | - | PI3K-Akt | p < 0.001 | High throughput analysis | miR-484 is linked with neurogenesis, mitochondrial network and redox modulations | [31] |
| miR-142-3p | - | - | PI3K-Akt | p = 0.001 | High throughput analysis | miR-142-3p regulates signaling pathways during embryonic development and homeostasis. | [31] | |
| miR-652-3p | - | - | PI3K-Akt | p < 0.001 | High throughput analysis | miR-652 is linked with immune system and oxidative stress. | [31] | |
| hsa-miR-363-3p, hsa-miR-4454 + has-miR-7975, hsa-miR-873-3p, hsa-miR-548al, hsa-miR-598-3p, hsa-miR-4443, hsa-miR-551a, hsa-miR-6721-5p | Plasma samples from drug-free psychotic BD cases (n = 15) and HC (n = 9) | - | - | - | p < 0.01 | High throughput analysis | These miRNAs were downregulated in patients. | [33] |
| hsa-miR-1281, hsa-miR-6068, hsa-miR-8060, hsa-miR-4433a-5p, hsa-miR-1268b, hsa-miR-1238-3p, hsa-miR-188-5p, hsa-miR-6775-5p, hsa-miR-6800-3p, hsa-miR-3620-5p, hsa-miR-451a, hsa-miR-1227-5p, hsa-miR-7108-5p, hsa-miR-671-5p, hsa-miR-6727-5p, hsa-miR-6125, hsa-miR-6821-5p | Peripheral blood EVs from BD patients (n = 20) and age- and sex matched normal subjects (n = 21) | - | - | Axon guidance mediated by netrin, endothelin signaling, 5HT2 type receptor-mediated signaling, beta1 and beta2 adrenergic receptor pathways, and the androgen receptor signaling pathway | p < 0.05 | High throughput analysis | These miRNAs were nominally downregulated between patients and controls. Pathway analyses identified some brain-relevant mechanisms enriched in these miRNAs, including axon guidance by netrin and the serotonin receptor pathway. | [34] |
| hsa-miR-5739 | - | - | - | p = 0.024667 | High throughput analysis | miR-5739 is suggested to be highly associated with the brain. | [34] | |
| hsa-miR-133a-3p | Peripheral blood EVs from BD type I (n = 20) and age- and sex matched healthy controls (n = 21) | - | NTN1, NTN3, NTNG1, NTNG2 | - | p < 0.05 | High throughput data mining | - | [35] |
| hsa-miR-299-5p | 3 frontal cortex miRNA expression datasets | - | SOD2, GPX4 | Redox modulation pathways | p < 0.05 | High throughput data mining | These are the top 10th percentile of decreased miRNAs that target redox modulators ranked for their ability to discriminate between BD and controls in Miller dataset. | [35] |
| hsa-miR-197 | - | SOD1, GCLC, TXN, COX8A, ATP2B4 | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-23a | - | NDUFA2, PPA1, GCLM, PTGS1, SOD2, PRDX4, PXDN, TTN, UQCRQ, NDUFV1, PRDX3, NDUFA3, TXNIP, ATP5O, TXNRD1 | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-450a | - | GCLC, NDUFA10,ATP5C1 | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-17 | - | ATP5B, TXN, NDUFA10, TXNIP, MTATP6, BCL2L11, NDUFS1, OXA1L, ATP2B4, BCl2L13, TXN2, SOD2, SDHB, PXDN, FOXO1, BCL2, UQCRFS1, RXNRD2, GPX2, TXNRD2 | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-944 | - | FOXO1 | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-19b | - | GCLC, ATP2B4, NDUFB2, COX6A1, FOXO3, PXDN, NDUFS3, COX10, NDUFB2 | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-503 | - | COX10, NDUFS1,PXDN | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-7 | - | NDUFA4, SDHC, ATP5S, FOXO6, NDUFS1, GCLM, COX4I1, ATP2B4, TXN2, GSR, ATP5F1, SDHB, NDUFC2, PPA1, PRDX1 | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-199a-5p | - | NDUFA13, MGST2 | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-484 | - | NOS3, PRDX1, COX7A2L, UQCRQ, GSTO1, UQCRFS1, ATP5J, BCL2L1, COX8A, PRDX1, MTATP6, PRDX4, COX5A, UQCRQ | p < 0.05 | High throughput data mining | [35] | |||
| hsa-miR-424 | - | NDUFS1, COX7A2L, BCL2L11, UQCRH | p < 0.05 | High throughput data mining | [35] | |||
| miR-499 | Peripheral blood of adult women only, 17 UP (age: 50 ± 17) and 15 BP (age: 33 ± 13) patients | - | - | - | p = 0.008 | Candidate molecule analysis | miR-499 is down-regulated in depression episodes of the BD patients compared with remission phase. | [46] |
| miR-708 | - | - | - | p = 0.02 | Candidate molecule analysis | miR-708 is down-regulated in depression episodes of the BD patients compared with remission phase. | [46] | |
| miR-1908 | - | KLC2 | - | p = 0.004 | Candidate molecule analysis | miR-1908 is down-regulated in depression episodes of the BD patients compared with remission phase. It is involved in lipid metabolism. Overexpression of miR-1908 in multipotent adipose-derived stem cells suppressed adipogenic differentiation and increased cell proliferation. | [46] | |
| miR-1908-5p | Two human NPC lines derived from dermal fibroblasts of either a control or a BD subject, treated with vehicle or 1 mM lithium or valproate for a week | Human neural progenitor cells (NPCs) | DLGAP4, GRIN1, STX1A, CLSTN1, GRM4 | NF-kappaB | p < 0.05 | Candidate molecule analysis | miR-1908 is an intronic miRNA of the fatty acid desaturase 1 (FADS1) gene. | [47] |
| miR-132 | Human post-mortem anterior cingulate cortex (AnCg) tissue. (n = 8, BP; n = 15, MDD; n = 14, Control) | - | - | - | p < 0.05 | Candidate molecule analysis (29 miRNAs) | - | [48] |
| miR-133a | - | - | - | p < 0.05 | Candidate molecule analysis (29 miRNAs) | While miR-133b levels did not change, miR-133a was differentially expressed in the AnCg of cohort of BP patients. | [48] | |
| miR-212 | - | - | - | p < 0.05 | Candidate molecule analysis (29 miRNAs) | miR-132 and miR-212 have been previously identified as differentially expressed in the DLPFC of SZ patients. | [48] | |
| miR-34a | - | NCOA1, PDE4B | - | p < 0.05 | Candidate molecule analysis (29 miRNAs) | miR-34a expression is dysregulated in SZ and BP patients. miR-34a has been linked to acute responses to stress. | [48] | |
| miR-145-5p | Human prefrontal cortex (Brodmann area 10) of 15 SZ, 15 MDD, 15 BD, and 15 controls | - | - | - | p = 0.0069 | High throughput | - | [39] |
| miR-485-5p | p = 0.036 | |||||||
| miR-370 | p = 0.041 | |||||||
| miR-500a-5p | p = 0.041 | |||||||
| miR-34a-5p | p = 0.048 | |||||||
| hsa-miR-454* | Postmortem DLPFC tissues of individuals with schizophrenia (SZ, n= 35) and BD (n = 35) | - | - | - | p = 0.00004 | High throughput | - | [42] |
| hsa-miR-29a | p = 0.00005 | |||||||
| hsa-miR-520c-3p | p = 0.00018 | |||||||
| hsa-miR-140-3p | p = 0.00053 | |||||||
| hsa-miR-767-5p | p = 0.00102 | |||||||
| hsa-miR-874 | p = 0.00181 | |||||||
| hsa-miR-32 | p = 0.00209 | |||||||
| hsa-miR-573 | p = 0.00227 | |||||||
| miR-134 | Plasma sample of drug-free bipolar I patients (14 men and 7 women) and controls (n = 21) | - | Limk1 | - | p = 0.009 | Candidate molecule analysis | miR-134 regulates dendritic spine development though Limk1, that controls synaptic development, maturation and/or plasticity. | [49] |
| miR-346 | DLPFC samples of SZ patients (n = 35), BD (n = 32), normal subjects (n =34) | - | CSF2RA | - | p = 0.086 | Candidate molecule analysis | miR-346 gene lies in intron 2 of the GRID1 gene, which has been proposed to be important in SZ susceptibility. | [50] |
| miR-19b-3p | Blood plasma from 7 UD patients, 7 BD patients, and 6 controls | - | MAPK1, PTEN, and PRKAA1 | mTOR, FoxO, and the PI3-K/Akt signaling pathway | p = 0.0462 | Candidate molecule analysis | MiR-19b-3p is a member of the miR-17/92 cluster, which controls lymphocyte growth, activation and proliferation. | [51] |
| miR-10b-5p | Skin biopsies of 3 control and 3 BP patient | Pluripotent Stem Cell-derived neurons | ANK3, BDNF, CAMK2G, DLGAP2, and NFASC | Axon guidance, Mapk, Ras, Hippo, Neurotrophin and Wnt signaling pathway | p < 0.05 | Candidate molecule analysis(58 miRNAs) | - | [44] |
| miR-10b-3p | - |
| miRNAs | Expression Pattern | Targets/Regulators | p Value | Function/Comments | Ref |
|---|---|---|---|---|---|
| hsa-miR-18a-5p | Up | - | p = 0.010761 | These miRNAs were up-regulated in the Asenapine Group in this study. These findings suggest that candidate miRNAs might participate in the mechanism of function of both antipsychotics in bipolar mania. | [52] |
| hsa-miR-27a-3p | p = 0.000161 | ||||
| hsa-miR-148b-3p | p = 0.005188 | ||||
| hsa-miR-17-3p | p = 0.018034 | ||||
| hsa-miR-106b-5p | p = 0.00445 | ||||
| hsa-miR-106a-5p | p = 0.006898 | ||||
| hsa-miR-20a-5p | p = 0.002247 | ||||
| hsa-miR-17-5p | p =0.011219 | ||||
| hsa-miR-19b-3p | Up | - | p = 0.013057 | These miRNAs were up-regulated in the Asenapine Group. miR-19b, miR145, and miR-339, were formerly shown to be dysregulated in patients with autism spectrum disorder and with Alzheimer’s disease. | [52] |
| hsa-miR-145-5p | p = 0.029543 | ||||
| hsa-miR-339-5p | p = 0.002185 | ||||
| hsa-miR-15a-5p | Up | BDNF | p = 0.002422 | hsa-miR-15a-5p was up-regulated in the Asenapine Group. miR-15a is reported to be involved in an interaction with brain-derived neurotrophic factor. | [52] |
| hsa-miR-30b-5p | Up | - | p = 0.015608 | hsa-miR-30b-5p was up-regulated in the Asenapine Group. MiR-30b is associated with schizophrenia, a psychiatric disorder that has been shown to share common genetic roots with BD. | [52] |
| hsa-miR-210-3p | Up | - | p = 0.005157 | hsa-miR-210-3p was up-regulated in the Asenapine Group. Overexpression of miR-210 induces angiogenesis and neurogenesis. | [52] |
| hsa-miR-92b-5p | Down | - | p = 0.04547 | These miRNAs were down-regulated in the Asenapine Group in this study. | [52] |
| hsa-miR-1343-5p | p = 0.019721 | ||||
| hsa-miR-664b-5p | Down | - | p = 0.035348 | These miRNAs were down-regulated in the Risperidone Group in this study. | [52] |
| hsa-miR-6778-5p | p = 0.047124 | ||||
| hsa-miR-146b-5p | Down | BDNF | p = 0.005919 | hsa-miR-146b-5p was down-regulated in the Risperidone Group. miR-146b partakes in an interaction with brain-derived neurotrophic factor. | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghafouri-Fard, S.; Badrlou, E.; Taheri, M.; Dürsteler, K.M.; Beatrix Brühl, A.; Sadeghi-Bahmani, D.; Brand, S. A Comprehensive Review on the Role of Non-Coding RNAs in the Pathophysiology of Bipolar Disorder. Int. J. Mol. Sci. 2021, 22, 5156. https://doi.org/10.3390/ijms22105156
Ghafouri-Fard S, Badrlou E, Taheri M, Dürsteler KM, Beatrix Brühl A, Sadeghi-Bahmani D, Brand S. A Comprehensive Review on the Role of Non-Coding RNAs in the Pathophysiology of Bipolar Disorder. International Journal of Molecular Sciences. 2021; 22(10):5156. https://doi.org/10.3390/ijms22105156
Chicago/Turabian StyleGhafouri-Fard, Soudeh, Elham Badrlou, Mohammad Taheri, Kenneth M. Dürsteler, Annette Beatrix Brühl, Dena Sadeghi-Bahmani, and Serge Brand. 2021. "A Comprehensive Review on the Role of Non-Coding RNAs in the Pathophysiology of Bipolar Disorder" International Journal of Molecular Sciences 22, no. 10: 5156. https://doi.org/10.3390/ijms22105156
APA StyleGhafouri-Fard, S., Badrlou, E., Taheri, M., Dürsteler, K. M., Beatrix Brühl, A., Sadeghi-Bahmani, D., & Brand, S. (2021). A Comprehensive Review on the Role of Non-Coding RNAs in the Pathophysiology of Bipolar Disorder. International Journal of Molecular Sciences, 22(10), 5156. https://doi.org/10.3390/ijms22105156







