Regeneration in Spinal Disease: Therapeutic Role of Hypoxia-Inducible Factor-1 Alpha in Regeneration of Degenerative Intervertebral Disc
Abstract
:1. Introduction
2. Structure and Function of the IVD
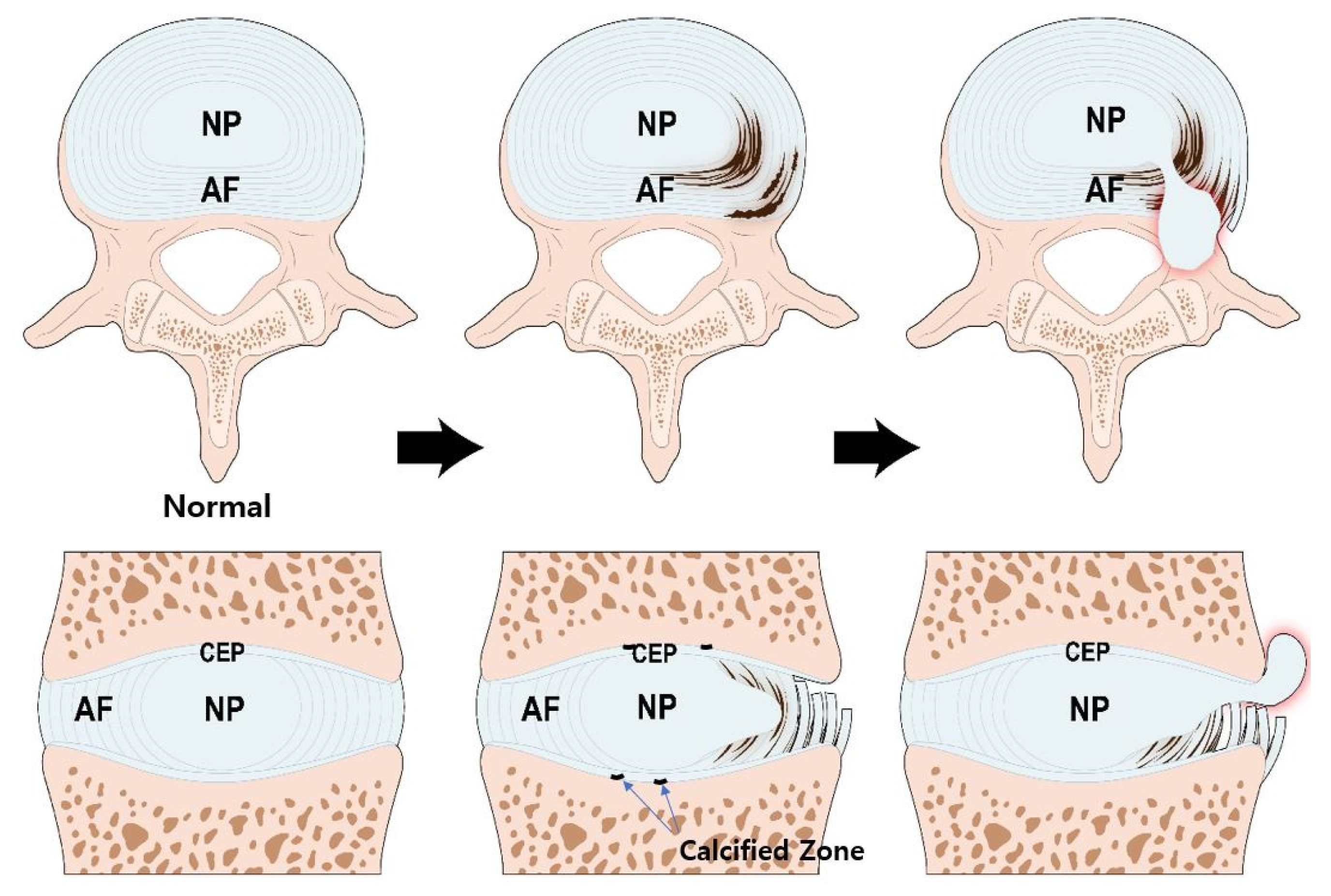
3. Pathogenesis of IVD Degeneration
4. Expression of HIF and Signal Transduction of HIF-1α in IVD
4.1. Expression Patterns of HIF-1α and HIF-2α in IVD
4.2. Signal Transduction Pathway of HIF-1α in IVD
5. Regeneration for IVD Degeneration—Focused on HIF-1α
5.1. Main Roles of HIF-1α in IVD Degeneration
5.1.1. Promotion of Extracellular Matrix in NP Cells
5.1.2. Maintenance of the Metabolic Activity of NP Cells
5.1.3. Regulation of Dystrophic Mineralization in NP Cells
5.1.4. Regulation of Angiogenesis during IVD Degeneration
5.1.5. Autophagy and Apoptosis during IVD Degeneration
5.2. HIF-1α Development Strategies for IVD Regeneration
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IVD | intervertebral disc |
| ATP | adenosine triphosphate |
| HIF-1α | hypoxia-inducible factor-1 alpha |
| NP | nucleus pulposus |
| AF | annulus fibrosus |
| CEP | cartilaginous end plate |
| GAG | anionic glycosaminoglycan |
| PHD | prolyl 4-hydroxylase domain-containing |
| VHL | von Hippel–Lindau tumor suppressor |
| HRE | hypoxia responsive element |
| FIH-1 | factor inhibiting HIF-1 |
| C-TAD | C-transactivation domains |
| FasL | Fas ligand |
| ECM | extracellular matrix |
| GLUT | glucose transporter |
| ANK | ankylosis protein homolog gene |
| VEGF | vascular endothelial growth factor |
| VEGFR | vascular endothelial growth factor receptor |
| AMPK | adenosine 5′-monophosphate-activated protein kinase |
| mTOR | mechanistic target of rapamycin |
References
- Fournier, D.E.; Kiser, P.K.; Shoemaker, J.K.; Battie, M.C.; Seguin, C.A. Vascularization of the human intervertebral disc: A scoping review. JOR Spine 2020, 3, e1123. [Google Scholar] [CrossRef]
- Choi, H.; Tessier, S.; Silagi, E.S.; Kyada, R.; Yousefi, F.; Pleshko, N.; Shapiro, I.M.; Risbud, M.V. A novel mouse model of intervertebral disc degeneration shows altered cell fate and matrix homeostasis. Matrix Biol. 2018, 70, 102–122. [Google Scholar] [CrossRef]
- Tian, Y.; Yuan, W.; Li, J.; Wang, H.; Hunt, M.G.; Liu, C.; Shapiro, I.M.; Risbud, M.V. TGFβ regulates Galectin-3 expression through canonical Smad3 signaling pathway in nucleus pulposus cells: Implications in intervertebral disc degeneration. Matrix Biol. 2016, 50, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Korecki, C.L.; MacLean, J.J.; Iatridis, J.C. Dynamic compression effects on intervertebral disc mechanics and biology. Spine 2008, 33, 1403–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg-Johansen, B.; Fields, A.J.; Liebenberg, E.C.; Li, A.; Lotz, J.C. Structure-function relationships at the human spinal disc-vertebra interface. J. Orthop. Res. 2018, 36, 192–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fields, A.J.; Ballatori, A.; Liebenberg, E.C.; Lotz, J.C. Contribution of the endplates to disc degeneration. Curr. Mol. Biol. Rep. 2018, 4, 151–160. [Google Scholar] [CrossRef]
- Bartels, E.M.; Fairbank, J.C.; Winlove, C.P.; Urban, J.P. Oxygen and lactate concentrations measured in vivo in the intervertebral discs of patients with scoliosis and back pain. Spine 1998, 23, 1–7; discussion 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, J.R. Growth of human intervertebral discs and vertebral bodies. J. Anat. 1975, 120, 49–68. [Google Scholar]
- Fujita, N.; Markova, D.; Anderson, D.G.; Chiba, K.; Toyama, Y.; Shapiro, I.M.; Risbud, M.V. Expression of prolyl hydroxylases (PHDs) is selectively controlled by HIF-1 and HIF-2 proteins in nucleus pulposus cells of the intervertebral disc: Distinct roles of PHD2 and PHD3 proteins in controlling HIF-1alpha activity in hypoxia. J. Biol. Chem. 2012, 287, 16975–16986. [Google Scholar] [CrossRef] [Green Version]
- Rudert, M.; Tillmann, B. Lymph and blood supply of the human intervertebral disc. Cadaver study of correlations to discitis. Acta Orthop. Scand. 1993, 64, 37–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, K.T.; Jacobsen, T.D.; Maidhof, R.; Virojanapa, J.; Overby, C.; Bloom, O.; Quraishi, S.; Levine, M.; Chahine, N.O. Developments in intervertebral disc disease research: Pathophysiology, mechanobiology, and therapeutics. Curr. Rev. Musculoskelet. Med. 2015, 8, 18–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Maitre, C.L.; Pockert, A.; Buttle, D.J.; Freemont, A.J.; Hoyland, J.A. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem. Soc. Trans. 2007, 35, 652–655. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liu, S.; Pan, D.; Xu, B.; Xing, X.; Zhou, H.; Zhang, B.; Zhou, S.; Ning, G.; Feng, S. The potential role and trend of HIF-1α in intervertebral disc degeneration: Friend or foe? (Review). Mol. Med. Rep. 2021, 23. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, C.Z.; Chen, Q.X. Regulatory role of hypoxia inducible factor in the biological behavior of nucleus pulposus cells. Yonsei Med. J. 2013, 54, 807–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urban, J.P.; Smith, S.; Fairbank, J.C. Nutrition of the intervertebral disc. Spine 2004, 29, 2700–2709. [Google Scholar] [CrossRef]
- Urban, J.P. The role of the physicochemical environment in determining disc cell behaviour. Biochem. Soc. Trans. 2002, 30 Pt 6, 858–864. [Google Scholar] [CrossRef]
- Richardson, S.M.; Knowles, R.; Tyler, J.; Mobasheri, A.; Hoyland, J.A. Expression of glucose transporters GLUT-1, GLUT-3, GLUT-9 and HIF-1alpha in normal and degenerate human intervertebral disc. HistoChem. Cell Biol. 2008, 129, 503–511. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-inducible factor 1: Oxygen homeostasis and disease pathophysiology. Trends Mol. Med. 2001, 7, 345–350. [Google Scholar] [CrossRef]
- Carmeliet, P.; Dor, Y.; Herbert, J.M.; Fukumura, D.; Brusselmans, K.; Dewerchin, M.; Neeman, M.; Bono, F.; Abramovitch, R.; Maxwell, P.; et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 1998, 394, 485–490. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, P.; Qin, L.; Pan, X.H. Hypoxia is essential for bone-tendon junction healing: The molecular biological evidence. Int. Orthop. 2011, 35, 925–928. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, A.; Gajghate, S.; Smith, H.; Anderson, D.G.; Albert, T.J.; Shapiro, I.M.; Risbud, M.V. Cited2 modulates hypoxia-inducible factor-dependent expression of vascular endothelial growth factor in nucleus pulposus cells of the rat intervertebral disc. Arthritis Rheum. 2008, 58, 3798–3808. [Google Scholar] [CrossRef] [PubMed]
- Podjarny, E.; Bernheim, J.; Hasdan, G.; Karsh, D.; Rashid, G.; Green, J.; Katz, B.; Bernheim, J. Additive renoprotective effect of candesartan and tetrahydrobiopterin in rats after 5/6 nephrectomy. Nephrol. Dial. Transplant. 2007, 22, 1864–1872. [Google Scholar] [CrossRef] [Green Version]
- Risbud, M.V.; Guttapalli, A.; Stokes, D.G.; Hawkins, D.; Danielson, K.G.; Schaer, T.P.; Albert, T.J.; Shapiro, I.M. Nucleus pulposus cells express HIF-1 alpha under normoxic culture conditions: A metabolic adaptation to the intervertebral disc microenvironment. J. Cell Biochem. 2006, 98, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Rajpurohit, R.; Risbud, M.V.; Ducheyne, P.; Vresilovic, E.J.; Shapiro, I.M. Phenotypic characteristics of the nucleus pulposus: Expression of hypoxia inducing factor-1, glucose transporter-1 and MMP-2. Cell Tissue Res. 2002, 308, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. HIF-1: Mediator of physiological and pathophysiological responses to hypoxia. J. Appl. Physiol. 2000, 88, 1474–1480. [Google Scholar] [CrossRef] [Green Version]
- Semenza, G.L.; Wang, G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell Biol. 1992, 12, 5447–5454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, N.; Chiba, K.; Shapiro, I.M.; Risbud, M.V. HIF-1alpha and HIF-2alpha degradation is differentially regulated in nucleus pulposus cells of the intervertebral disc. J. Bone Miner. Res. 2012, 27, 401–412. [Google Scholar] [CrossRef] [Green Version]
- Gogate, S.S.; Nasser, R.; Shapiro, I.M.; Risbud, M.V. Hypoxic regulation of β-1,3-glucuronyltransferase 1 expression in nucleus pulposus cells of the rat intervertebral disc: Role of hypoxia-inducible factor proteins. Arthritis Rheum. 2011, 63, 1950–1960. [Google Scholar] [CrossRef] [Green Version]
- Skubutyte, R.; Markova, D.; Freeman, T.A.; Anderson, D.G.; Dion, A.S.; Williams, C.J.; Shapiro, I.M.; Risbud, M.V. Hypoxia-inducible factor regulation of ANK expression in nucleus pulposus cells: Possible implications in controlling dystrophic mineralization in the intervertebral disc. Arthritis Rheum. 2010, 62, 2707–2715. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Danielson, K.G.; Albert, T.J.; Shapiro, I.M.; Risbud, M.V. HIF-1 alpha is a regulator of galectin-3 expression in the intervertebral disc. J. Bone Miner. Res. 2007, 22, 1851–1861. [Google Scholar] [CrossRef]
- Agrawal, A.; Guttapalli, A.; Narayan, S.; Albert, T.J.; Shapiro, I.M.; Risbud, M.V. Normoxic stabilization of HIF-1alpha drives glycolytic metabolism and regulates aggrecan gene expression in nucleus pulposus cells of the rat intervertebral disk. Am. J. Physiol. Cell Physiol. 2007, 293, C621–C631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, K.Y.; Koh, I.J.; Kirpalani, P.A.; Kim, Y.Y.; Cho, Y.K.; Khang, G.S.; Han, C.W. The expression of hypoxia inducible factor-1alpha and apoptosis in herniated discs. Spine 2006, 31, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Neucere, J.N.; Godshall, M.A. Effects of base-soluble proteins and methanol-soluble polysaccharides from corn on mycelial growth of Aspergillus flavus. Mycopathologia 1991, 113, 103–108. [Google Scholar] [CrossRef]
- Merceron, C.; Mangiavini, L.; Robling, A.; Wilson, T.L.; Giaccia, A.J.; Shapiro, I.M.; Schipani, E.; Risbud, M.V. Loss of HIF-1alpha in the notochord results in cell death and complete disappearance of the nucleus pulposus. PLoS ONE 2014, 9, e110768. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Zhuang, L.; Wang, J.; Liu, Z.; Wang, Y.; Xiao, D.; Zhang, X. Hypoxia-inducible factor (HIF)-1alpha knockout accelerates intervertebral disc degeneration in mice. Int. J. Clin. Exp. Pathol. 2018, 11, 548–557. [Google Scholar] [PubMed]
- Buckwalter, J.A. Aging and degeneration of the human intervertebral disc. Spine 1995, 20, 1307–1314. [Google Scholar] [CrossRef]
- Adams, P.; Eyre, D.R.; Muir, H. Biochemical aspects of development and ageing of human lumbar intervertebral discs. Rheumatol. Rehabil. 1977, 16, 22–29. [Google Scholar] [CrossRef]
- Walker, M.H.; Anderson, D.G. Molecular basis of intervertebral disc degeneration. Spine J. 2004, 4 (Suppl. S6), 158S–166S. [Google Scholar] [CrossRef]
- Jordao, H.W.; McKenna, G.; McMenamin, U.C.; Kunzmann, A.T.; Murray, L.J.; Coleman, H.G. The association between self-reported poor oral health and gastrointestinal cancer risk in the UK Biobank: A large prospective cohort study. United Eur. Gastroenterol. J. 2019, 7, 1241–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, H.; Danfelter, M.; Stromqvist, B.; Heinegard, D. Extracellular matrix in disc degeneration. J. Bone Jt. Surg. Am. 2006, 88 (Suppl. S2), 25–29. [Google Scholar]
- Marchand, F.; Ahmed, A.M. Investigation of the laminate structure of lumbar disc anulus fibrosus. Spine 1990, 15, 402–410. [Google Scholar] [CrossRef]
- Humzah, M.D.; Soames, R.W. Human intervertebral disc: Structure and function. Anat. Rec. 1988, 220, 337–356. [Google Scholar] [CrossRef] [PubMed]
- Roughley, P.J.; Melching, L.I.; Heathfield, T.F.; Pearce, R.H.; Mort, J.S. The structure and degradation of aggrecan in human intervertebral disc. Eur. Spine J. 2006, 15 (Suppl. S3), S326–S332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hukins, D.W. A simple model for the function of proteoglycans and collagen in the response to compression of the intervertebral disc. Proc. Biol. Sci. 1992, 249, 281–285. [Google Scholar] [PubMed]
- Wade, K.R.; Robertson, P.A.; Broom, N.D. A fresh look at the nucleus-endplate region: New evidence for significant structural integration. Eur. Spine J. 2011, 20, 1225–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Cisewski, S.E.; Wegner, N.; Zhao, S.; Pellegrini, V.D., Jr.; Slate, E.H.; Yao, H. Region and strain-dependent diffusivities of glucose and lactate in healthy human cartilage endplate. J. Biomech. 2016, 49, 2756–2762. [Google Scholar] [CrossRef] [Green Version]
- Maroudas, A.; Stockwell, R.A.; Nachemson, A.; Urban, J. Factors involved in the nutrition of the human lumbar intervertebral disc: Cellularity and diffusion of glucose in vitro. J. Anat. 1975, 120, 113–130. [Google Scholar]
- Nachemson, A.; Lewin, T.; Maroudas, A.; Freeman, M.A. In vitro diffusion of dye through the end-plates and the annulus fibrosus of human lumbar inter-vertebral discs. Acta Orthop. Scand. 1970, 41, 589–607. [Google Scholar] [CrossRef] [Green Version]
- Ogata, K.; Whiteside, L.A. 1980 Volvo award winner in basic science. Nutritional pathways of the intervertebral disc. An experimental study using hydrogen washout technique. Spine 1981, 6, 211–216. [Google Scholar] [CrossRef]
- Grunhagen, T.; Wilde, G.; Soukane, D.M.; Shirazi-Adl, S.A.; Urban, J.P. Nutrient supply and intervertebral disc metabolism. J Bone Jt. Surg. Am. 2006, 88 (Suppl. S2), 30–35. [Google Scholar]
- Ashinsky, B.G.; Gullbrand, S.E.; Wang, C.; Bonnevie, E.D.; Han, L.; Mauck, R.L.; Smith, H.E. Degeneration alters structure-function relationships at multiple length-scales and across interfaces in human intervertebral discs. J. Anat. 2021, 238, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Holm, S.; Maroudas, A.; Urban, J.P.; Selstam, G.; Nachemson, A. Nutrition of the intervertebral disc: Solute transport and metabolism. Connect Tissue Res. 1981, 8, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Bibby, S.R.; Jones, D.A.; Ripley, R.M.; Urban, J.P. Metabolism of the intervertebral disc: Effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine 2005, 30, 487–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishihara, H.; Urban, J.P. Effects of low oxygen concentrations and metabolic inhibitors on proteoglycan and protein synthesis rates in the intervertebral disc. J Orthop. Res. 1999, 17, 829–835. [Google Scholar] [CrossRef]
- Bibby, S.R.; Urban, J.P. Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur. Spine J. 2004, 13, 695–701. [Google Scholar] [CrossRef]
- Ohshima, H.; Urban, J.P. The effect of lactate and pH on proteoglycan and protein synthesis rates in the intervertebral disc. Spine 1992, 17, 1079–1082. [Google Scholar] [CrossRef]
- Razaq, S.; Wilkins, R.J.; Urban, J.P. The effect of extracellular pH on matrix turnover by cells of the bovine nucleus pulposus. Eur. Spine J. 2003, 12, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Bernick, S.; Cailliet, R. Vertebral end-plate changes with aging of human vertebrae. Spine 1982, 7, 97–102. [Google Scholar] [CrossRef]
- Grant, M.P.; Epure, L.M.; Bokhari, R.; Roughley, P.; Antoniou, J.; Mwale, F. Human cartilaginous endplate degeneration is induced by calcium and the extracellular calcium-sensing receptor in the intervertebral disc. Eur. Cell Mater. 2016, 32, 137–151. [Google Scholar] [CrossRef]
- Boos, N.; Weissbach, S.; Rohrbach, H.; Weiler, C.; Spratt, K.F.; Nerlich, A.G. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine 2002, 27, 2631–2644. [Google Scholar] [CrossRef]
- Garfin, S.R.; Eismont, F.J.; Bell, G.R.; Bono, C.M.; Fischgrund, J.S. The Intervertebral Disc: Normal, Aging, and Pathologic. In Rothman-Simeone and Herkowitz’s the Spine, 7th ed.; Olsen, S.A., Kang, J.D., Vo, N.V., Sowa, G.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 2, pp. 79–89. [Google Scholar]
- Eyre, D.R.; Muir, H. Types I and II collagens in intervertebral disc. Interchanging radial distributions in annulus fibrosus. Biochem. J. 1976, 157, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Duance, V.C.; Crean, J.K.; Sims, T.J.; Avery, N.; Smith, S.; Menage, J.; Eisenstein, S.M.; Roberts, S. Changes in collagen cross-linking in degenerative disc disease and scoliosis. Spine 1998, 23, 2545–2551. [Google Scholar] [CrossRef]
- Madhu, V.; Boneski, P.K.; Silagi, E.; Qiu, Y.; Kurland, I.; Guntur, A.R.; Shapiro, I.M.; Risbud, M.V. Hypoxic Regulation of Mitochondrial Metabolism and Mitophagy in Nucleus Pulposus Cells Is Dependent on HIF-1α-BNIP3 Axis. J. Bone Miner. Res. 2020, 35, 1504–1524. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Wu, C.; Tian, W. Elevated expression of hypoxia-inducible factor-2α regulated catabolic factors during intervertebral disc degeneration. Life Sci. 2019, 232, 116565. [Google Scholar] [CrossRef]
- Buckley, D.L.; Van Molle, I.; Gareiss, P.C.; Tae, H.S.; Michel, J.; Noblin, D.J.; Jorgensen, W.L.; Ciulli, A.; Crews, C.M. Targeting the von Hippel-Lindau E3 ubiquitin ligase using small molecules to disrupt the VHL/HIF-1alpha interaction. J. Am. Chem. Soc. 2012, 134, 4465–4468. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lei, Y.; Guo, S.; Zuo, Z. Ensemble-based virtual screening in discovering potent inhibitors targeting Von Hippel-Lindau (VHL) E3 ubiquitin ligase. Life Sci. 2020, 262, 118495. [Google Scholar] [CrossRef]
- Mahon, P.C.; Hirota, K.; Semenza, G.L. FIH-1: A novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001, 15, 2675–2686. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Ren, Z.; Ye, L.C.; Zhou, P.H.; Xu, J.M.; Shi, Q.; Yao, L.Q.; Zhong, Y.S. Factor inhibiting HIF1alpha (FIH-1) functions as a tumor suppressor in human colorectal cancer by repressing HIF1alpha pathway. Cancer Biol. Ther. 2015, 16, 244–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Yu, X.; Pang, M.; Tong, J. Molecular characterization and expression regulation of the factor-inhibiting HIF-1 (FIH-1) gene under hypoxic stress in bighead carp (Aristichthys nobilis). Fish. Physiol. Biochem. 2019, 45, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Chang, H.; Kim, K.W. Expression of Fas ligand and apoptosis of disc cells in herniated lumbar disc tissue. Spine 2001, 26, 618–621. [Google Scholar] [CrossRef]
- Zhu, G.B.; Jiang, X.R.; Xia, C.L.; Sun, Y.J.; Zeng, Q.S.; Wu, X.M.; Li, X.C. Association of FAS and FAS ligand polymorphisms with the susceptibility and severity of lumbar disc degeneration in Chinese Han population. Biomarkers 2011, 16, 485–490. [Google Scholar] [CrossRef]
- Richardson, S.M.; Kalamegam, G.; Pushparaj, P.N.; Matta, C.; Memic, A.; Khademhosseini, A.; Mobasheri, R.; Poletti, F.L.; Hoyland, J.A.; Mobasheri, A. Mesenchymal stem cells in regenerative medicine: Focus on articular cartilage and intervertebral disc regeneration. Methods 2016, 99, 69–80. [Google Scholar] [CrossRef]
- Vasiliadis, E.S.; Pneumaticos, S.G.; Evangelopoulos, D.S.; Papavassiliou, A.G. Biologic treatment of mild and moderate intervertebral disc degeneration. Mol. Med. 2014, 20, 400–409. [Google Scholar] [CrossRef]
- Wang, S.Z.; Chang, Q.; Lu, J.; Wang, C. Growth factors and platelet-rich plasma: Promising biological strategies for early intervertebral disc degeneration. Int. Orthop. 2015, 39, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Fang, X.Q.; Wang, Q.; Wang, S.W.; Hu, Z.J.; Zhou, Z.J.; Xu, W.B.; Wang, J.Y.; Qin, A.; Fan, S.W. PHD/HIF-1 upregulates CA12 to protect against degenerative disc disease: A human sample, in vitro and ex vivo study. Lab. Investig. 2016, 96, 561–569. [Google Scholar] [CrossRef] [Green Version]
- Feng, G.; Li, L.; Hong, Y.; Liu, H.; Song, Y.; Pei, F.; Ma, P.X.; Gong, Q.; Gupte, M.J. Hypoxia promotes nucleus pulposus phenotype in 3D scaffolds in vitro and in vivo: Laboratory investigation. J. Neurosurg. Spine 2014, 21, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Li, L.; Liu, H.; Song, Y.; Huang, F.; Tu, C.; Shen, B.; Gong, Q.; Li, T.; Liu, L.; et al. Hypoxia differentially regulates human nucleus pulposus and annulus fibrosus cell extracellular matrix production in 3D scaffolds. Osteoarthr. Cartil. 2013, 21, 582–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Lv, G.; Li, J.; Wang, B.; Zhang, Q.; Lu, C. LncRNA-RP11-296A18.3/miR-138/HIF1A Pathway Regulates the Proliferation ECM Synthesis of Human Nucleus Pulposus Cells (HNPCs). J. Cell Biochem. 2017, 118, 4862–4871. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, A.; Skubutyte, R.; Markova, D.; Anderson, D.G.; Yadla, S.; Sakai, D.; Mochida, J.; Albert, T.J.; Shapiro, I.M.; Risbud, M.V. Hypoxia activates the notch signaling pathway in cells of the intervertebral disc: Implications in degenerative disc disease. Arthritis Rheum. 2011, 63, 1355–1364. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Li, C.; Meng, X.; Bai, Y.; Qi, J.; Wang, J.; Zhou, Q.; Zhang, W.; Zhang, X. Hypoxia-inducible factor-lalpha mediates aggrecan and collagen Pi expression via NOTCH1 signaling in nucleus pulposus cells during intervertebral disc degeneration. Biochem. Biophys. Res. Commun. 2017, 488, 554–561. [Google Scholar] [CrossRef]
- Tannenbaum, A.; Silverstone, H. Failure to inhibit the formation of mammary carcinoma in mice by intermittent fasting. Cancer Res. 1950, 10, 577–579. [Google Scholar] [PubMed]
- Benita, Y.; Kikuchi, H.; Smith, A.D.; Zhang, M.Q.; Chung, D.C.; Xavier, R.J. An integrative genomics approach identifies Hypoxia Inducible Factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Res. 2009, 37, 4587–4602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papandreou, I.; Cairns, R.A.; Fontana, L.; Lim, A.L.; Denko, N.C. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006, 3, 187–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golub, E.E. Biomineralization and matrix vesicles in biology and pathology. Semin. Immunopathol. 2011, 33, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Kirsch, T. Determinants of pathological mineralization. Curr. Opin. Rheumatol. 2006, 18, 174–180. [Google Scholar] [CrossRef]
- Shao, J.; Yu, M.; Jiang, L.; Wei, F.; Wu, F.; Liu, Z.; Liu, X. Differences in calcification and osteogenic potential of herniated discs according to the severity of degeneration based on Pfirrmann grade: A cross-sectional study. BMC Musculoskelet. Disord. 2016, 17, 191. [Google Scholar] [CrossRef] [Green Version]
- Guiot, B.H.; Fessler, R.G. Molecular biology of degenerative disc disease. Neurosurgery 2000, 47, 1034–1040. [Google Scholar] [CrossRef]
- Pendleton, A.; Johnson, M.D.; Hughes, A.; Gurley, K.A.; Ho, A.M.; Doherty, M.; Dixey, J.; Gillet, P.; Loeuille, D.; McGrath, R.; et al. Mutations in ANKH cause chondrocalcinosis. Am. J. Hum. Genet. 2002, 71, 933–940. [Google Scholar] [CrossRef] [Green Version]
- Williams, C.J.; Pendleton, A.; Bonavita, G.; Reginato, A.J.; Hughes, A.E.; Peariso, S.; Doherty, M.; McCarty, D.J.; Ryan, L.M. Mutations in the amino terminus of ANKH in two US families with calcium pyrophosphate dihydrate crystal deposition disease. Arthritis Rheum. 2003, 48, 2627–2631. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.H.; Sun, C.; Yao, Y.; Fan, X.; Liu, H.; Cui, Y.H.; Bian, X.W.; Huang, B.; Zhou, Y. Matrix stiffness promotes cartilage endplate chondrocyte calcification in disc degeneration via miR-20a targeting ANKH expression. Sci. Rep. 2016, 6, 25401. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S. Vascular endothelial growth factor (VEGF), VEGF receptors and their inhibitors for antiangiogenic tumor therapy. Biol. Pharm. Bull. 2011, 34, 1785–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, I.B.; Ropper, A.E.; Teng, Y.D.; Shin, D.A.; Jeon, Y.J.; Park, H.M.; Shin, D.E.; Park, Y.S.; Kim, K.N.; Kim, N.K. Association between VEGF and eNOS gene polymorphisms and lumbar disc degeneration in a young Korean population. Genet. Mol. Res. 2013, 12, 2294–2305. [Google Scholar] [CrossRef] [PubMed]
- Haro, H.; Kato, T.; Komori, H.; Osada, M.; Shinomiya, K. Vascular endothelial growth factor (VEGF)-induced angiogenesis in herniated disc resorption. J. Orthop. Res. 2002, 20, 409–415. [Google Scholar] [CrossRef]
- Doita, M.; Kanatani, T.; Ozaki, T.; Matsui, N.; Kurosaka, M.; Yoshiya, S. Influence of macrophage infiltration of herniated disc tissue on the production of matrix metalloproteinases leading to disc resorption. Spine 2001, 26, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.K.; Moon, H.J.; Kwon, T.H.; Park, Y.K.; Kim, J.H. The Role of Hypoxia in Angiogenesis and Extracellular Matrix Regulation of Intervertebral Disc Cells During Inflammatory Reactions. Neurosurgery 2017, 81, 867–875. [Google Scholar] [CrossRef]
- Wu, W.J.; Zhang, X.K.; Zheng, X.F.; Yang, Y.H.; Jiang, S.D.; Jiang, L.S. SHH-dependent knockout of HIF-1 alpha accelerates the degenerative process in mouse intervertebral disc. Int. J. Immunopathol. Pharmacol. 2013, 26, 601–609. [Google Scholar] [CrossRef]
- Mazure, N.M.; Pouysségur, J. Hypoxia-induced autophagy: Cell death or cell survival? Curr. Opin. Cell Biol. 2010, 22, 177–180. [Google Scholar] [CrossRef]
- Scherz-Shouval, R.; Elazar, Z. Regulation of autophagy by ROS: Physiology and pathology. Trends Biochem. Sci. 2011, 36, 30–38. [Google Scholar] [CrossRef]
- Tu, J.; Li, W.; Li, S.; Liu, W.; Zhang, Y.; Wu, X.; Luo, R.; Hua, W.; Wang, K.; Song, Y.; et al. Sestrin-Mediated Inhibition of Stress-Induced Intervertebral Disc Degradation Through the Enhancement of Autophagy. Cell Physiol. Biochem. 2018, 45, 1940–1954. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.W.; Ni, B.B.; Zheng, X.F.; Li, B.; Jiang, S.D.; Jiang, L.S. Hypoxia facilitates the survival of nucleus pulposus cells in serum deprivation by down-regulating excessive autophagy through restricting ROS generation. Int. J. Biochem. Cell Biol. 2015, 59, 1–10. [Google Scholar] [CrossRef]
- Ding, F.; Shao, Z.W.; Xiong, L.M. Cell death in intervertebral disc degeneration. Apoptosis 2013, 18, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Gruber, H.E.; Hanley, E.N., Jr. Analysis of aging and degeneration of the human intervertebral disc. Comparison of surgical specimens with normal controls. Spine 1998, 23, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, J.; Teng, J.; Liu, Y.; Zhang, D.; Liu, L.; Zhang, W. microRNA-155-3p attenuates intervertebral disc degeneration via inhibition of KDM3A and HIF1α. Inflamm. Res. 2021, 70, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Deng, G.; Qiu, Y.; Huang, X.; Xi, Y.; Yu, J.; Yang, X.; Ye, X. Transplantation of allogenic nucleus pulposus cells attenuates intervertebral disc degeneration by inhibiting apoptosis and increasing migration. Int. J. Mol. Med. 2018, 41, 2553–2564. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhang, L.; Feng, X.; Chen, T.; Bi, S. A new in vivo method to retard progression of intervertebral disc degeneration through stimulation of endogenous stem cells with simvastatin. Med. Hypotheses 2017, 101, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhang, B.; Mu, K.; Feng, S.Q.; Dong, Z.Y.; Ning, G.Z.; Li, H.R.; Liu, S.; Zhao, L.; Li, Y.; et al. Circular RNA GRB10 as a competitive endogenous RNA regulating nucleus pulposus cells death in degenerative intervertebral disk. Cell Death Dis. 2018, 9, 319. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-W.; Jeon, N.; Shin, D.-E.; Lee, S.-Y.; Kim, M.; Han, D.H.; Shin, J.Y.; Lee, S. Regeneration in Spinal Disease: Therapeutic Role of Hypoxia-Inducible Factor-1 Alpha in Regeneration of Degenerative Intervertebral Disc. Int. J. Mol. Sci. 2021, 22, 5281. https://doi.org/10.3390/ijms22105281
Kim J-W, Jeon N, Shin D-E, Lee S-Y, Kim M, Han DH, Shin JY, Lee S. Regeneration in Spinal Disease: Therapeutic Role of Hypoxia-Inducible Factor-1 Alpha in Regeneration of Degenerative Intervertebral Disc. International Journal of Molecular Sciences. 2021; 22(10):5281. https://doi.org/10.3390/ijms22105281
Chicago/Turabian StyleKim, Jin-Woo, Neunghan Jeon, Dong-Eun Shin, So-Young Lee, Myongwhan Kim, Dong Hun Han, Jae Yeon Shin, and Soonchul Lee. 2021. "Regeneration in Spinal Disease: Therapeutic Role of Hypoxia-Inducible Factor-1 Alpha in Regeneration of Degenerative Intervertebral Disc" International Journal of Molecular Sciences 22, no. 10: 5281. https://doi.org/10.3390/ijms22105281
APA StyleKim, J.-W., Jeon, N., Shin, D.-E., Lee, S.-Y., Kim, M., Han, D. H., Shin, J. Y., & Lee, S. (2021). Regeneration in Spinal Disease: Therapeutic Role of Hypoxia-Inducible Factor-1 Alpha in Regeneration of Degenerative Intervertebral Disc. International Journal of Molecular Sciences, 22(10), 5281. https://doi.org/10.3390/ijms22105281






