Design, Radiosynthesis and Preliminary Biological Evaluation in Mice of a Brain-Penetrant 18F-Labelled σ2 Receptor Ligand
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of Fluorinated Indole and Azaindole Derivatives
2.2. Determination of the In Vitro Affinity
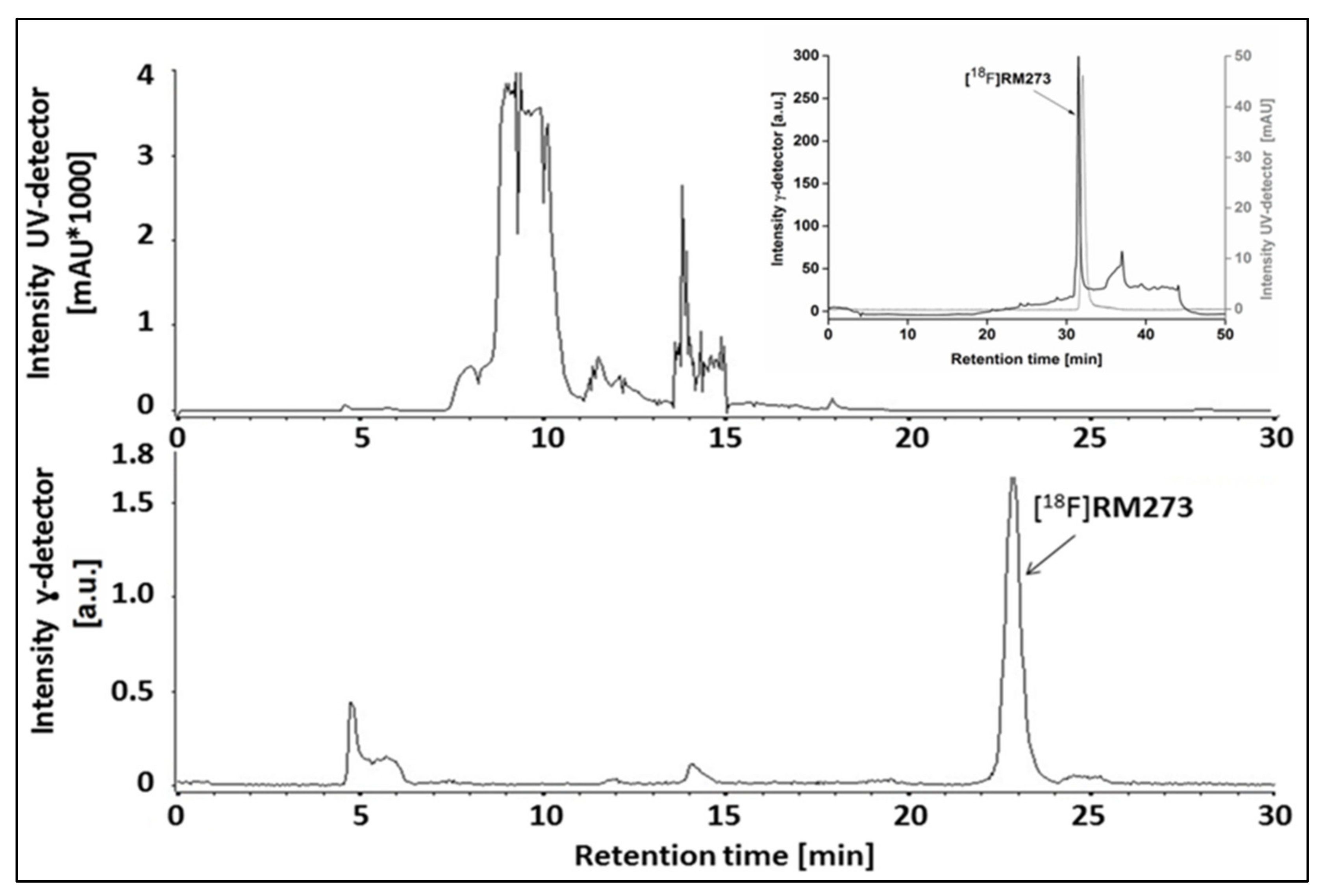
2.3. Automated Radiosynthesis of [18F]RM273
2.4. In Vitro Evaluation of the σ2 Receptor Binding of [18F]RM273 in F98 Rat Glioblastoma
2.5. PET Imaging of [18F]RM273 in CD1 Mice
3. Summary
4. Conclusions
5. Materials and Methods
5.1. Chemistry
5.1.1. General Information
5.1.2. Chemical Synthesis
5.2. Radiochemistry
5.2.1. Automated Radiosynthesis of [18F]RM273
5.2.2. Quality Control
5.2.3. Determination of In Vitro Stability
5.2.4. Determination of Lipophilicity (LogD7.4)
5.3. Biological Experiments
5.3.1. Determination of Binding Affinities by Homogenate Assays
5.3.2. In Vitro Autoradiography
5.3.3. PET Studies
5.3.4. Quantification of Radiometabolites
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Martin, W.R.; Eades, C.G.; Thompson, J.A.; Huppler, R.E.; Gilbert, P.E. Effects of morphine-like and nalorphine-like drugs in nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976, 197, 517–532. [Google Scholar] [PubMed]
- Hellewell, S.B.; Bowen, W.D. A sigma-like binding site in rat pheochromocytoma (PC12) cells—Decreased affinity for (+)-benzomorphans and lower molecular-weightsuggest a different sigma receptor form from that of guinea-pig brain. Brain Res. 1990, 527, 244–253. [Google Scholar] [CrossRef]
- Maurice, T. Bi-phasic dose response in the preclinical and clinical developments of sigma-1 receptor ligands for the treatment of neurodegenerative disorders. Expert Opin. Drug Discov. 2021, 16. [Google Scholar] [CrossRef] [PubMed]
- Soriani, O.; Kourrich, S. The Sigma-1 receptor: When adaptive regulation of cell electrical activity contributes to stimulant addiction and cancer. Front. Neurosci. 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zeng, C.; Chu, W.; Pan, F.; Rothfuss, J.M.; Zhang, F.; Tu, Z.; Zhou, D.; Zeng, D.; Vangveravong, S.; et al. Identification of the PGRMC1 protein complex as the putative sigma-2 receptor binding site. Nat. Commun. 2011, 2. [Google Scholar] [CrossRef]
- Alon, A.; Schmidt, H.R.; Wood, M.D.; Sahn, J.J.; Martin, S.F.; Kruse, A.C. Identification of the gene that codes for the σ2 receptor. Proc. Natl. Acad. Sci. USA 2017, 114, 7160–7165. [Google Scholar] [CrossRef]
- Moebius, F.F.; Striessnig, J.; Glossmann, H. The mysteries of sigma receptors: New family members reveal a role in cholesterol synthesis. Trends Pharmacol. Sci. 1997, 18, 67–70. [Google Scholar] [CrossRef]
- Wilcox, C.B.; Feddes, G.O.; Willett-Brozick, J.E.; Hsu, L.-C.; DeLoia, J.A.; Baysal, B.E. Coordinate up-regulation of TMEM97 and cholesterol biosynthesis genes in normal ovarian surface epithelial cells treated with progesterone: Implications for pathogenesis of ovarian cancer. BMC Cancer 2007, 7. [Google Scholar] [CrossRef]
- Ebrahimi-Fakhari, D.; Wahlster, L.; Bartz, F.; Werenbeck-Ueding, J.; Praggastis, M.; Zhang, J.; Joggerst-Thomalla, B.; Theiss, S.; Grimm, D.; Ory, D.S.; et al. Reduction of TMEM97 increases NPC1 protein levels and restores cholesterol trafficking in Niemann-pick type C1 disease cells. Hum. Mol. Genet. 2016, 25, 3588–3599. [Google Scholar] [CrossRef]
- Cahill, M.A. Progesterone receptor membrane component 1: An integrative review. J. Steroid Biochem. Mol. Biol. 2007, 105, 16–36. [Google Scholar] [CrossRef]
- Riad, A.; Zeng, C.; Weng, C.-C.; Winters, H.; Xu, K.; Makvandi, M.; Metz, T.; Carlin, S.; Mach, R.H. Sigma-2 receptor/TMEM97 and PGRMC-1 increase the rate of internalization of LDL by LDL receptor through the formation of a ternary complex. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Izzo, N.J.; Colom-Cadena, M.; Riad, A.A.; Xu, J.; Singh, M.; Abate, C.; Cahill, M.A.; Spires-Jones, T.L.; Bowen, W.D.; Mach, R.H.; et al. Proceedings from the fourth international symposium on σ-2 receptors: Role in health and disease. Eneuro 2020, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vilner, B.J.; John, C.S.; Bowen, W.D. Sigma-1 and sigma-2 receptors are expressed in a wide variety of human and rodent tumor cell lines. Cancer Res. 1995, 55, 408–413. [Google Scholar] [PubMed]
- John, C.S.; Vilner, B.J.; Gulden, M.E.; Efange, S.M.N.; Langason, R.B.; Moody, T.W.; Bowen, W.D. Synthesis and pharmacological characterization of 4-[125I]-N-(N-Benzylpiperidin-4-yl)-4-iodobenzamide: A high affinity o- receptor ligand for potential imaging of breast cancer. Cancer Res. 1995, 55, 3022–3027. [Google Scholar]
- Mach, R.H.; Smith, C.R.; Al-Nabulsi, I.; Whirrett, B.R.; Childers, S.R.; Wheeler, K.T. σ2 receptors as potential biomarkers of proliferation in breast cancer. Cancer Res. 1997, 57, 156–161. [Google Scholar] [PubMed]
- Zeng, C.; Rothfuss, J.M.; Zhang, J.; Vangveravong, S.; Chu, W.; Li, S.; Tu, Z.; Xu, J.; Mach, R.H. Functional assays to define agonists and antagonists of the sigma-2 receptor. Anal. Biochem. 2014, 448, 68–74. [Google Scholar] [CrossRef]
- Søby, K.K.; Mikkelsen, J.D.; Meier, E.; Thomsen, C. Lu 28-179 labels a σ2-site in rat and human brain. Neuropharmacology 2002, 43, 95–100. [Google Scholar] [CrossRef]
- Nicholson, H.; Mesangeau, C.; McCurdy, C.R.; Bowen, W.D. Sigma-2 receptors play a role in cellular metabolism: Stimulation of glycolytic hallmarks by CM764 in human SK-N-SH neuroblastomas. J. Pharmacol Exp. Ther. 2016, 356, 232–243. [Google Scholar] [CrossRef]
- Yang, K.; Zeng, C.; Wang, C.; Sun, M.; Yin, D.; Sun, T. Sigma-2 receptor-A potential target for cancer/alzheimer’s disease treatment via its regulation of cholesterol homeostasis. Molecules 2020, 25, 5439. [Google Scholar] [CrossRef]
- Van Waarde, A.; Buursma, A.R.; Hospers, G.A.P.; Kawamura, K.; Kobayashi, T.; Ishii, K.; Oda, K.; Ishiwata, K.; Vaalburg, W.; Elsinga, P.H. Tumor imaging with 2 σ-receptor ligands, 18F-FE-SA5845 and 11C-SA4503: A feasibility study. J. Nucl. Med. 2004, 45, 1939–1945. [Google Scholar]
- Kawamura, K.; Kubota, K.; Kobayashi, T.; Elsinga, P.H.; Ono, M.; Ishiwata, K. Evaluation of [11C]SA5845 and [11C]SA4503 for imaging of sigma receptors in tumors by animal PET. Ann. Nucl. Med. 2005, 19, 701–709. [Google Scholar] [PubMed]
- Rowland, D.J.; Tu, Z.; Xu, J.; Ponde, D.; Mach, R.H.; Welch, M.J. Synthesis and in vivo evaluation of 2 high-affinity 76Br-labeled σ2-receptor ligands. J. Nucl. Med. 2006, 47, 1041–1048. [Google Scholar] [PubMed]
- Tu, Z.; Dence, C.S.; Ponde, D.E.; Jones, L.; Wheeler, K.T.; Welch, M.J.; Mach, R.H. Carbon-11 labeled σ2 receptor ligands for imaging breast cancer. Nucl. Med. Biol. 2005, 32, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Abate, C.; Selivanova, S.V.; Müller, A.; Krämer, S.D.; Schibli, R.; Marottoli, R.; Perrone, R.; Berardi, F.; Niso, M.; Ametamey, S.M. Development of 3,4-dihydroisoquinolin-1(2H)-one derivatives for the positron emission tomography (PET) imaging of σ₂ receptors. Eur. J. Med. Chem. 2013, 69, 920–930. [Google Scholar] [CrossRef]
- Tu, Z.; Xu, J.; Jones, L.A.; Li, S.; Dumstorff, C.; Vangveravong, S.; Chen, D.L.; Wheeler, K.T.; Welch, A.M.J.; Mach, R.H. Fluorine-18-labeled benzamide analogues for imaging the σ2 receptor status of solid tumors with positron emission tomography. J. Med. Chem. 2007, 50, 3194–3204. [Google Scholar] [CrossRef]
- Dehdashti, F.; Laforest, R.; Gao, F.; Shoghi, K.I.; Aft, R.L.; Nussenbaum, B.; Kreisel, F.H.; Bartlett, N.L.; Cashen, A.; Wagner-Johnston, N.; et al. Assessment of cellular proliferation in tumors by PET using 18F-ISO-1. J. Nucl. Med. 2013, 54, 350–357. [Google Scholar] [CrossRef]
- Wang, L.; Ye, J.; He, Y.; Deuther-Conrad, W.; Zhang, J.; Zhang, X.; Cui, M.; Steinbach, J.; Huang, Y.; Brust, P.; et al. 18F-labeled indole-based analogs as highly selective radioligands for imaging sigma-2 receptors in the brain. Bioorg. Med. Chem. 2017, 25, 3792–3802. [Google Scholar] [CrossRef]
- Mérour, J.-Y.; Buron, F.; Plé, K.; Bonnet, P.; Routier, S. The azaindole framework in the design of kinase inhibitors. Molecules 2014, 19, 19935–19979. [Google Scholar] [CrossRef]
- Blaazer, A.R.; Lange, J.H.; van der Neut, M.A.; Mulder, A.; Boon, F.S.D.; Werkman, T.R.; Kruse, C.G.; Wadman, W.J. Novel indole and azaindole (pyrrolopyridine) cannabinoid (CB) receptor agonists: Design, synthesis, structure-activity relationships, physicochemical properties and biological activity. Eur. J. Med. Chem. 2011, 46, 5086–5098. [Google Scholar] [CrossRef]
- Zheng, H.; Dong, Y.; Li, L.; Sun, B.; Liu, L.; Yuan, H.; Lou, H. Novel benzo[a]quinolizidine analogs induce cancer cell death through paraptosis and apoptosis. J. Med. Chem. 2016, 59, 5063–5076. [Google Scholar] [CrossRef]
- Ogawa, K.; Shiba, K. In vivo and in vitro characteristics of radiolabeled vesamicol analogs as the vesicular acetylcholine transporter imaging agents. Contrast Media Mol. Imaging 2018, 2018. [Google Scholar] [CrossRef]
- Mach, R.H.; Zeng, C.; Hawkins, W.G. The σ2 receptor: A novel protein for the imaging and treatment of cancer. J. Med. Chem. 2013, 56, 7137–7160. [Google Scholar] [CrossRef] [PubMed]
- Ichiishi, N.; Brooks, A.; Topczewski, J.J.; Rodnick, M.E.; Sanford, M.S.; Scott, P.J.H. Copper-catalyzed [18F]fluorination of (mesityl)(aryl)iodonium salts. Org. Lett. 2014, 16, 3224–3227. [Google Scholar] [CrossRef] [PubMed]
- Tredwell, M.; Preshlock, S.M.; Taylor, N.J.; Gruber, S.; Huiban, M.; Passchier, J.; Mercier, J.; Génicot, C.; Gouverneur, V. A general copper-mediated nucleophilic 18F fluorination of arenes. Angew. Chem. Int. Ed. Eng. 2014, 53, 7751–7755. [Google Scholar] [CrossRef] [PubMed]
- Mossine, A.V.; Brooks, A.; Makaravage, K.J.; Miller, J.M.; Ichiishi, N.; Sanford, M.S.; Scott, P.J.H. Synthesis of [18F]arenes via the copper-mediated [18F]fluorination of boronic acids. Org. Lett. 2015, 17, 5780–5783. [Google Scholar] [CrossRef]
- Makaravage, K.J.; Brooks, A.; Mossine, A.V.; Sanford, M.S.; Scott, P.J.H. Copper-mediated radiofluorination of arylstannanes with [18F]KF. Org. Lett. 2016, 18. [Google Scholar] [CrossRef] [PubMed]
- Preshlock, S.; Calderwood, S.; Verhoog, S.; Tredwell, M.; Huiban, M.; Hienzsch, A.; Gruber, S.; Wilson, T.C.; Taylor, N.J.; Cailly, T.; et al. Enhanced copper-mediated 18F-fluorination of aryl boronic esters provides eight radiotracers for PET applications. Chem. Commun. 2016, 52, 8361–8364. [Google Scholar] [CrossRef]
- Zlatopolskiy, B.D.; Zischler, J.; Krapf, P.; Zarrad, F.; Urusova, E.A.; Kordys, E.; Endepols, H.; Neumaier, B. Copper-mediated aromatic radiofluorination revisited: Efficient production of PET tracers on a preparative scale. Chemistry 2015, 21, 5972–5979. [Google Scholar] [CrossRef]
- Zischler, J.; Kolks, N.; Modemann, D.; Neumaier, B.; Zlatopolskiy, B.D. Alcohol-enhanced Cu-mediated radiofluorination. Chemistry 2017, 23, 3251–3256. [Google Scholar] [CrossRef]
- Ishiyama, T.; Murata, M.; Miyaura, N. Palladium(0)-catalyzed cross-coupling reaction of alkoxydiboron with haloarenes: A direct procedure for arylboronic esters. J. Org. Chem. 1995, 60, 7508–7510. [Google Scholar] [CrossRef]
- Lai, T.; Schröder, S.; Toussaint, M.; Dukić-Stefanović, S.; Kranz, M.; Ludwig, F.-A.; Fischer, S.; Steinbach, J.; Deuther-Conrad, W.; Brust, P.; et al. Development of 18F-labeled radiotracers for PET imaging of the adenosine A2A receptor: Synthesis, radiolabeling and preliminary biological evaluation. Int. J. Mol. Sci. 2021, 22, 2285. [Google Scholar] [CrossRef]
- Clemente, G.S.; Zarganes-Tzitzikas, T.; Dömling, A.; Elsinga, P.H. Late-stage copper-catalyzed radiofluorination of an arylboronic ester derivative of atorvastatin. Molecules 2019, 24, 4210. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, R.N. Determination of lipophilicity and its use as a predictor of blood-brain barrier penetration of molecular imaging agents. Mol. Imaging Biol. 2003, 5, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, P.; Quirion, R. [3H]1,3-di(2-tolyl)guanidine and [3H](+)pentazocine binding sites in the rat brain: Autoradiographic visualization of the putative sigma1 and sigma2 receptor subtypes. Neuroscience 1997, 76, 467–477. [Google Scholar] [CrossRef]
- Guo, Q.; Brady, M.; Gunn, R.N. A biomathematical modeling approach to central nervous system radioligand discovery and development. J. Nucl. Med. 2009, 50, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Bernsen, M.R.; Kooiman, K.; Segbers, M.; van Leeuwen, F.; De Jong, M. Biomarkers in preclinical cancer imaging. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 579–596. [Google Scholar] [CrossRef]
- Bautista-Aguilera, Ó.M.; Budni, J.; Mina, F.; Medeiros, E.B.; Deuther-Conrad, W.; Entrena, J.-M.; Moraleda, I.; Iriepa, I.; López-Muñoz, F.; Marco-Contelles, J. Contilisant, a tetratarget small molecule for alzheimer’s disease therapy combining cholinesterase, monoamine oxidase inhibition, and H3R antagonism with S1R agonism profile. J. Med. Chem. 2018, 61, 6937–6943. [Google Scholar] [CrossRef] [PubMed]







| Compound | Ki σ1 (nM) | Ki σ2 (nM) | Selectivity (Ki σ1/Ki σ2) | |
|---|---|---|---|---|
| 34 |  | 21 a | 14 | 1.5 |
| 35 |  | 116 a | 12 | 9.7 |
| 36 |  | 46 a | 7.1 | 6.5 |
| 37 |  | 80 a | 10 | 8 |
| 38 |  | 80 b | 5.5 | 14 |
| 39 |  | 122 b | 3.8 | 32 |
| 40 |  | 85 b | 1.0 | 85 |
| 41 (RM273)c |  | 691 b | 1.6 | 432 |
| 42 (RM297) |  | 916 b | 2.7 | 339 |
| ISO-1 | n.d. | 10 d | ||
| Haloperidol | 4.9 a,e | 21 e | ||
| Siramesine | 13 a | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moldovan, R.-P.; Gündel, D.; Teodoro, R.; Ludwig, F.-A.; Fischer, S.; Toussaint, M.; Schepmann, D.; Wünsch, B.; Brust, P.; Deuther-Conrad, W. Design, Radiosynthesis and Preliminary Biological Evaluation in Mice of a Brain-Penetrant 18F-Labelled σ2 Receptor Ligand. Int. J. Mol. Sci. 2021, 22, 5447. https://doi.org/10.3390/ijms22115447
Moldovan R-P, Gündel D, Teodoro R, Ludwig F-A, Fischer S, Toussaint M, Schepmann D, Wünsch B, Brust P, Deuther-Conrad W. Design, Radiosynthesis and Preliminary Biological Evaluation in Mice of a Brain-Penetrant 18F-Labelled σ2 Receptor Ligand. International Journal of Molecular Sciences. 2021; 22(11):5447. https://doi.org/10.3390/ijms22115447
Chicago/Turabian StyleMoldovan, Rareş-Petru, Daniel Gündel, Rodrigo Teodoro, Friedrich-Alexander Ludwig, Steffen Fischer, Magali Toussaint, Dirk Schepmann, Bernhard Wünsch, Peter Brust, and Winnie Deuther-Conrad. 2021. "Design, Radiosynthesis and Preliminary Biological Evaluation in Mice of a Brain-Penetrant 18F-Labelled σ2 Receptor Ligand" International Journal of Molecular Sciences 22, no. 11: 5447. https://doi.org/10.3390/ijms22115447
APA StyleMoldovan, R.-P., Gündel, D., Teodoro, R., Ludwig, F.-A., Fischer, S., Toussaint, M., Schepmann, D., Wünsch, B., Brust, P., & Deuther-Conrad, W. (2021). Design, Radiosynthesis and Preliminary Biological Evaluation in Mice of a Brain-Penetrant 18F-Labelled σ2 Receptor Ligand. International Journal of Molecular Sciences, 22(11), 5447. https://doi.org/10.3390/ijms22115447









