Metabolomic Biomarkers in Gestational Diabetes Mellitus: A Review of the Evidence
Abstract
1. Introduction
2. The Aetiology and Pathogenesis of GDM
2.1. Risk Factors for Gestational Diabetes
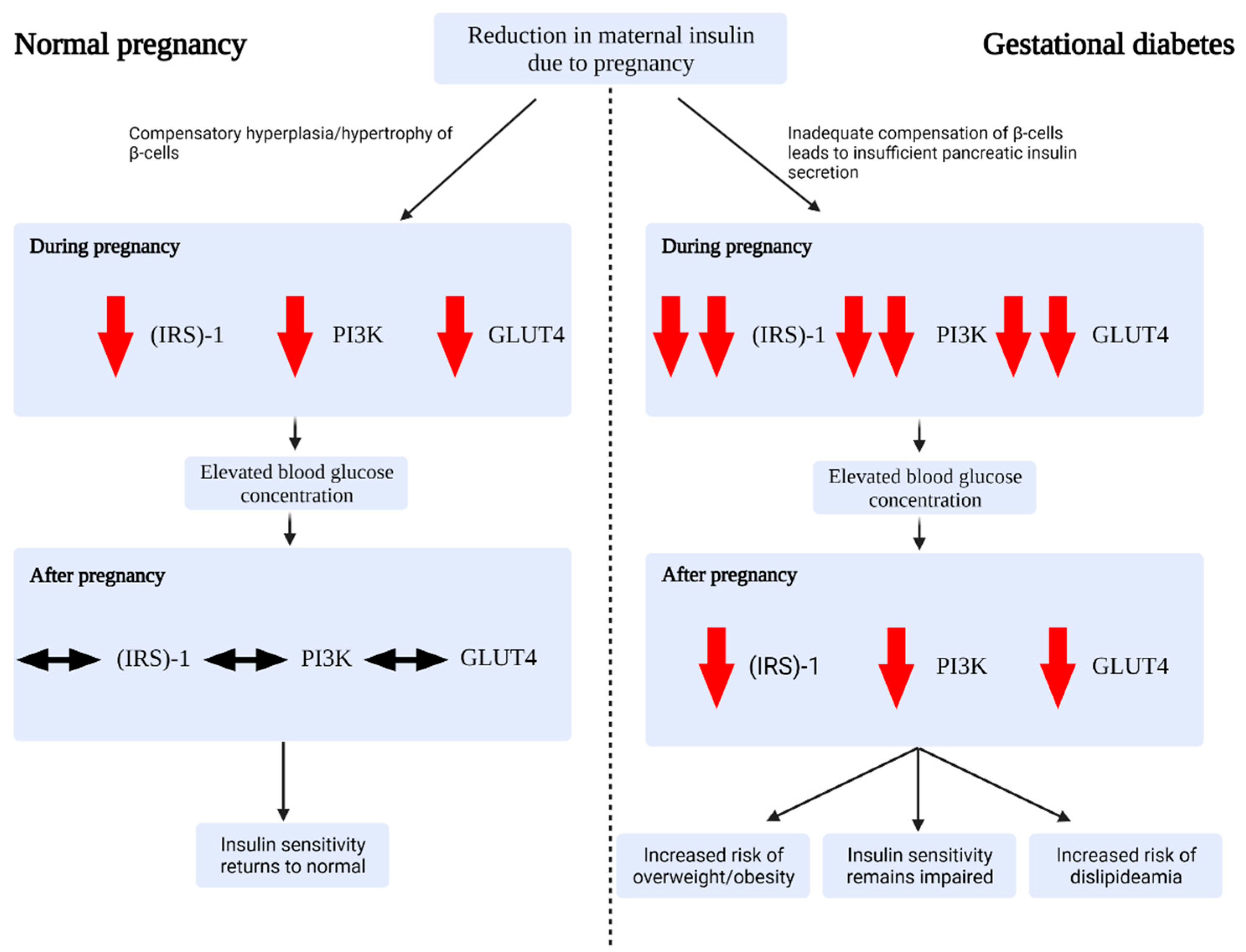
2.2. Glucose Regulation during Healthy Pregnancy
2.3. Alterations in Gestational Diabetes
3. Metabolomics as a Potential Tool to Investigate GDM
4. The Metabolomic Profile of GDM: A Review of the Literature
4.1. Potential Early Screening Diagnostic Markers and Models
4.2. Amino Acid Profile in GDM
4.3. The Carbohydrate Profile in GDM
4.4. The Lipid Profile in GDM
4.5. Prospective Diagnostic Markers for the Likelihood of Developing T2DM Postpartum
5. Limitations and Future Directions
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Laurie, J.G.; McIntyre, H.D. A Review of the Current Status of Gestational Diabetes Mellitus in Australia-The Clinical Impact of Changing Population Demographics and Diagnostic Criteria on Prevalence. Int. J. Environ. Res. Public Health 2020, 17, 9387. [Google Scholar] [CrossRef]
- Cade, T.J.; Polyakov, A.; Brennecke, S.P. Implications of the introduction of new criteria for the diagnosis of gestational diabetes: A health outcome and cost of care analysis. BMJ Open 2019, 9, e023293. [Google Scholar] [CrossRef]
- Bilous, R.W.; Jacklin, P.B.; Maresh, M.J.; Sacks, D.A. Resolving the Gestational Diabetes Diagnosis Conundrum: The Need for a Randomized Controlled Trial of Treatment. Diabetes Care 2021, 44, 858–864. [Google Scholar] [CrossRef]
- Byrn, M.; Penckofer, S. The relationship between gestational diabetes and antenatal depression. J. Obs. Gynecol. Neonatal. Nurs. 2015, 44, 246–255. [Google Scholar] [CrossRef]
- Tan, P.C.; Ling, L.P.; Omar, S.Z. The 50-g glucose challenge test and pregnancy outcome in a multiethnic Asian population at high risk for gestational diabetes. Int. J. Gynaecol Obs. 2009, 105, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Crowther, C.A.; Hiller, J.E.; Moss, J.R.; McPhee, A.J.; Jeffries, W.S.; Robinson, J.S.; Australian Carbohydrate Intolerance Study in Pregnant Women Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N. Engl. J. Med. 2005, 352, 2477–2486. [Google Scholar] [CrossRef] [PubMed]
- Roman, A.S.; Rebarber, A.; Fox, N.S.; Klauser, C.K.; Istwan, N.; Rhea, D.; Saltzman, D. The effect of maternal obesity on pregnancy outcomes in women with gestational diabetes. J. Matern Fetal Neonatal. Med. 2011, 24, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Langer, O.; Yogev, Y.; Most, O.; Xenakis, E.M. Gestational diabetes: The consequences of not treating. Am. J. Obs. Gynecol. 2005, 192, 989–997. [Google Scholar] [CrossRef]
- Blotsky, A.L.; Rahme, E.; Dahhou, M.; Nakhla, M.; Dasgupta, K. Gestational diabetes associated with incident diabetes in childhood and youth: A retrospective cohort study. CMAJ 2019, 191, E410–E417. [Google Scholar] [CrossRef]
- American Diabetes, A. (2) Classification and diagnosis of diabetes. Diabetes Care 2015, 38 (Suppl. 1), S8–S16. [Google Scholar] [CrossRef]
- Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; Leiva, A.; Hod, M.; Kitzmiler, J.L.; et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Pinu, F.R.; Goldansaz, S.A.; Jaine, J. Translational Metabolomics: Current Challenges and Future Opportunities. Metabolites 2019, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Chen, X.; Chen, C.; Zhang, H.; Law, K.P. Metabolomics in gestational diabetes. Clin. Chim. Acta 2017, 475, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Francis, E.; Hu, G.; Chen, L. Metabolomic profiling of women with gestational diabetes mellitus and their offspring: Review of metabolomics studies. J. Diabetes Complicat. 2018, 32, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E.; Buchanan, T.A.; Coustan, D.R.; de Leiva, A.; Dunger, D.B.; Hadden, D.R.; Hod, M.; Kitzmiller, J.L.; Kjos, S.L.; Oats, J.N.; et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007, 30 (Suppl. 2), S251–S260. [Google Scholar] [CrossRef] [PubMed]
- Ben-Haroush, A.; Yogev, Y.; Hod, M. Epidemiology of gestational diabetes mellitus and its association with Type 2 diabetes. Diabet. Med. 2004, 21, 103–113. [Google Scholar] [CrossRef]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [PubMed]
- Anghebem-Oliveira, M.I.; Martins, B.R.; Alberton, D.; Ramos, E.A.S.; Picheth, G.; Rego, F.G.M. Type 2 diabetes-associated genetic variants of FTO, LEPR, PPARg, and TCF7L2 in gestational diabetes in a Brazilian population. Arch. Endocrinol. Metab. 2017, 61, 238–248. [Google Scholar] [CrossRef]
- Durnwald, C. Gestational diabetes: Linking epidemiology, excessive gestational weight gain, adverse pregnancy outcomes, and future metabolic syndrome. Semin. Perinatol. 2015, 39, 254–258. [Google Scholar] [CrossRef]
- Catalano, P.M.; Tyzbir, E.D.; Roman, N.M.; Amini, S.B.; Sims, E.A. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am. J. Obs. Gynecol. 1991, 165, 1667–1672. [Google Scholar] [CrossRef]
- Kampmann, U.; Madsen, L.R.; Skajaa, G.O.; Iversen, D.S.; Moeller, N.; Ovesen, P. Gestational diabetes: A clinical update. World J. Diabetes 2015, 6, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Roman, M.A. Prolactin and lactation as modifiers of diabetes risk in gestational diabetes. Horm. Metab. Res. 2011, 43, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.A.; O’Sullivan, M.J.; Skyler, J.S. Insulin action during pregnancy. Studies with the euglycemic clamp technique. Diabetes 1985, 34, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Kahanovitz, L.; Sluss, P.M.; Russell, S.J. Type 1 Diabetes—A Clinical Perspective. Point Care 2017, 16, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Olokoba, A.B.; Obateru, O.A.; Olokoba, L.B. Type 2 diabetes mellitus: A review of current trends. Oman Med. J. 2012, 27, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Latek, D.; Rutkowska, E.; Niewieczerzal, S.; Cielecka-Piontek, J. Drug-induced diabetes type 2: In silico study involving class B GPCRs. PLoS ONE 2019, 14, e0208892. [Google Scholar] [CrossRef]
- Damm, P.; Kuhl, C.; Buschard, K.; Jakobsen, B.K.; Svejgaard, A.; Sodoyez-Goffaux, F.; Shattock, M.; Bottazzo, G.F.; Molsted-Pedersen, L. Prevalence and predictive value of islet cell antibodies and insulin autoantibodies in women with gestational diabetes. Diabet. Med. 1994, 11, 558–563. [Google Scholar] [CrossRef]
- Friedman, J.E.; Ishizuka, T.; Shao, J.; Huston, L.; Highman, T.; Catalano, P. Impaired glucose transport and insulin receptor tyrosine phosphorylation in skeletal muscle from obese women with gestational diabetes. Diabetes 1999, 48, 1807–1814. [Google Scholar] [CrossRef]
- Catalano, P.M. Trying to understand gestational diabetes. Diabet. Med. 2014, 31, 273–281. [Google Scholar] [CrossRef]
- Friedman, J.E.; Kirwan, J.P.; Jing, M.; Presley, L.; Catalano, P.M. Increased skeletal muscle tumor necrosis factor-alpha and impaired insulin signaling persist in obese women with gestational diabetes mellitus 1 year postpartum. Diabetes 2008, 57, 606–613. [Google Scholar] [CrossRef]
- Hasin, Y.; Seldin, M.; Lusis, A. Multi-omics approaches to disease. Genome Biol. 2017, 18, 83. [Google Scholar] [CrossRef]
- Ni, C.-M.; Huang, W.-L.; Jiang, Y.-M.; Xu, J.; Duan, R.; Zhu, Y.-L.; Zhu, X.-P.; Fan, X.-M.; Luo, G.-A.; Wang, Y.-M.; et al. Improving the accuracy and efficacy of diagnosing polycystic ovary syndrome by integrating metabolomics with clinical characteristics: Study protocol for a randomized controlled trial. Trials 2020, 21, 169. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.D.; Souza, A.L.; Gerszten, R.E.; Clish, C.B. Targeted metabolomics. Curr. Protoc. Mol. Biol. 2012, 30, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Goodacre, R.; Vaidyanathan, S.; Dunn, W.B.; Harrigan, G.G.; Kell, D.B. Metabolomics by numbers: Acquiring and understanding global metabolite data. Trends Biotechnol. 2004, 22, 245–252. [Google Scholar] [CrossRef]
- Rajska, A.; Buszewska-Forajta, M.; Rachon, D.; Markuszewski, M.J. Metabolomic Insight into Polycystic Ovary Syndrome-An Overview. Int. J. Mol. Sci. 2020, 21, 4853. [Google Scholar] [CrossRef]
- Segers, K.; Declerck, S.; Mangelings, D.; Heyden, Y.V.; Eeckhaut, A.V. Analytical techniques for metabolomic studies: A review. Bioanalysis 2019, 11, 2297–2318. [Google Scholar] [CrossRef] [PubMed]
- Pechlaner, R.; Kiechl, S.; Mayr, M. Potential and Caveats of Lipidomics for Cardiovascular Disease. Circulation 2016, 134, 1651–1654. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.L.; Feng, Y.; Fiehn, O.; Tsai, M.Y.; Tekola-Ayele, F.; Zhu, Y.; Zhang, C. Plasma Lipidomics and Gestational Diabetes—A Longitudinal Study in a Multiracial Cohort. Diabetes 2018, 67, 174. [Google Scholar] [CrossRef]
- Griffin, J.L.; Nicholls, A.W.; Daykin, C.A.; Heald, S.; Keun, H.C.; Schuppe-Koistinen, I.; Griffiths, J.R.; Cheng, L.L.; Rocca-Serra, P.; Rubtsov, D.V.; et al. Standard reporting requirements for biological samples in metabolomics experiments: Mammalian/in vivo experiments. Metabolomics 2007, 3, 179–188. [Google Scholar] [CrossRef]
- Pinto, J.; Almeida, L.M.; Martins, A.S.; Duarte, D.; Barros, A.S.; Galhano, E.; Pita, C.; Almeida, M.d.C.; Carreira, I.M.; Gil, A.M. Prediction of Gestational Diabetes through NMR Metabolomics of Maternal Blood. J. Proteome Res. 2015, 14, 2696–2706. [Google Scholar] [CrossRef]
- Hou, W.; Meng, X.; Zhao, A.; Zhao, W.; Pan, J.; Tang, J.; Huang, Y.; Li, H.; Jia, W.; Liu, F.; et al. Development of Multimarker Diagnostic Models from Metabolomics Analysis for Gestational Diabetes Mellitus (GDM). Mol. Cell Proteom. 2018, 17, 431–441. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, K.; Alexander, J.; Azuma, R.; Xiao, R.; Snyder, N.W.; Mesaros, C.A.; Blair, I.A.; Pinney, S.E. Gestational Diabetes Alters the Metabolomic Profile in 2nd Trimester Amniotic Fluid in a Sex-Specific Manner. Int. J. Mol. Sci. 2018, 19, 2696. [Google Scholar] [CrossRef] [PubMed]
- Scholtens, D.M.; Muehlbauer, M.J.; Daya, N.R.; Stevens, R.D.; Dyer, A.R.; Lowe, L.P.; Metzger, B.E.; Newgard, C.B.; Bain, J.R.; Lowe, W.L.; et al. Metabolomics reveals broad-scale metabolic perturbations in hyperglycemic mothers during pregnancy. Diabetes Care 2014, 37, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Gall, W.E.; Beebe, K.; Lawton, K.A.; Adam, K.-P.; Mitchell, M.W.; Nakhle, P.J.; Ryals, J.A.; Milburn, M.V.; Nannipieri, M.; Camastra, S. α-Hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS ONE 2010, 5, e10883. [Google Scholar] [CrossRef]
- Anderson, S.G.; Dunn, W.B.; Banerjee, M.; Brown, M.; Broadhurst, D.I.; Goodacre, R.; Cooper, G.J.S.; Kell, D.B.; Cruickshank, J.K. Evidence that multiple defects in lipid regulation occur before hyperglycemia during the prodrome of type-2 diabetes. PLoS ONE 2014. [Google Scholar] [CrossRef]
- Diaz, S.l.O.; Pinto, J.; Graça, G.a.; Duarte, I.F.; Barros, A.n.S.; Galhano, E.l.; Pita, C.; Almeida, M.d.C.; Goodfellow, B.J.; Carreira, I.M. Metabolic biomarkers of prenatal disorders: An exploratory NMR metabonomics study of second trimester maternal urine and blood plasma. J. Proteome Res. 2011, 10, 3732–3742. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Cani, P.D. Diabetes, obesity and gut microbiota. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Clarke, E.; Cade, T.J.; Brennecke, S. Early Pregnancy Screening for Women at High-Risk of GDM Results in Reduced Neonatal Morbidity and Similar Maternal Outcomes to Routine Screening. J. Pregnancy 2020, 2020, 9083264. [Google Scholar] [CrossRef]
- Van Assche, F.A.; Aerts, L.; De Prins, F.A. The fetal endocrine pancreas. Eur J. Obs. Gynecol. Reprod. Biol. 1984, 18, 267–272. [Google Scholar] [CrossRef]
- Sudharshana Murthy, K.A.; Bhandiwada, A.; Chandan, S.L.; Gowda, S.L.; Sindhusree, G. Evaluation of Oxidative Stress and Proinflammatory Cytokines in Gestational Diabetes Mellitus and Their Correlation with Pregnancy Outcome. Indian J. Endocrinol. Metab. 2018, 22, 79–84. [Google Scholar] [CrossRef]
- Ricart, W.; Lopez, J.; Mozas, J.; Pericot, A.; Sancho, M.A.; Gonzalez, N.; Balsells, M.; Luna, R.; Cortazar, A.; Navarro, P.; et al. Maternal glucose tolerance status influences the risk of macrosomia in male but not in female fetuses. J. Epidemiol. Community Health 2009, 63, 64–68. [Google Scholar] [CrossRef]
- Enquobahrie, D.A.; Denis, M.; Tadesse, M.G.; Gelaye, B.; Ressom, H.W.; Williams, M.A. Maternal Early Pregnancy Serum Metabolites and Risk of Gestational Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2015, 100, 4348–4356. [Google Scholar] [CrossRef]
- Adachi, Y.; De Sousa-Coelho, A.L.; Harata, I.; Aoun, C.; Weimer, S.; Shi, X.; Gonzalez Herrera, K.N.; Takahashi, H.; Doherty, C.; Noguchi, Y.; et al. l-Alanine activates hepatic AMP-activated protein kinase and modulates systemic glucose metabolism. Mol. Metab. 2018, 17, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, N. Metabolomics in diabetes research. J. Endocrinol. 2012, 215, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Rhee, E.P.; Larson, M.G.; Lewis, G.D.; McCabe, E.L.; Shen, D.; Palma, M.J.; Roberts, L.D.; Dejam, A.; Souza, A.L.; et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 2012, 125, 2222–2231. [Google Scholar] [CrossRef] [PubMed]
- Butte, N.F.; Hsu, H.W.; Thotathuchery, M.; Wong, W.W.; Khoury, J.; Reeds, P. Protein metabolism in insulin-treated gestational diabetes. Diabetes Care 1999, 22, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Pappa, K.I.; Vlachos, G.; Theodora, M.; Roubelaki, M.; Angelidou, K.; Antsaklis, A. Intermediate metabolism in association with the amino acid profile during the third trimester of normal pregnancy and diet-controlled gestational diabetes. Am. J. Obs. Gynecol. 2007, 196, 65 e1–5. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E.; Phelps, R.L.; Freinkel, N.; Navickas, I.A. Effects of gestational diabetes on diurnal profiles of plasma glucose, lipids, and individual amino acids. Diabetes Care 1980, 3, 402–409. [Google Scholar] [CrossRef]
- Cetin, I.; de Santis, M.S.; Taricco, E.; Radaelli, T.; Teng, C.; Ronzoni, S.; Spada, E.; Milani, S.; Pardi, G. Maternal and fetal amino acid concentrations in normal pregnancies and in pregnancies with gestational diabetes mellitus. Am. J. Obs. Gynecol. 2005, 192, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Stefan, N.; Yu, Z.; Mühlenbruch, K.; Drogan, D.; Joost, H.G.; Fritsche, A.; Häring, H.U.; Hrabě de Angelis, M.; Peters, A.; et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 2013, 62, 639–648. [Google Scholar] [CrossRef]
- Roberts, L.D.; Koulman, A.; Griffin, J.L. Towards metabolic biomarkers of insulin resistance and type 2 diabetes: Progress from the metabolome. Lancet Diabetes Endocrinol. 2014, 2, 65–75. [Google Scholar] [CrossRef]
- Irving, B.A.; Carter, R.E.; Soop, M.; Weymiller, A.; Syed, H.; Karakelides, H.; Bhagra, S.; Short, K.R.; Tatpati, L.; Barazzoni, R. Effect of insulin sensitizer therapy on amino acids and their metabolites. Metabolism 2015, 64, 720–728. [Google Scholar] [CrossRef]
- Würtz, P.; Soininen, P.; Kangas, A.J.; Rönnemaa, T.; Lehtimäki, T.; Kähönen, M.; Viikari, J.S.; Raitakari, O.T.; Ala-Korpela, M. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care 2013, 36, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Wright, L.A.; Hirsch, I.B.; Gooley, T.A.; Brown, Z. 1,5-Anhydroglucitol and neonatal complications in pregnancy complicated by diabetes. Endocr. Pract. 2015, 21, 725–733. [Google Scholar] [CrossRef] [PubMed]
- San Martin, R.; Sobrevia, L. Gestational diabetes and the adenosine/L-arginine/nitric oxide (ALANO) pathway in human umbilical vein endothelium. Placenta 2006, 27, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Gutierrez, E.; Abarzua, F.; Belmar, C.; Nien, J.K.; Ramirez, M.A.; Arroyo, P.; Salomon, C.; Westermeier, F.; Puebla, C.; Leiva, A.; et al. Functional link between adenosine and insulin: A hypothesis for fetoplacental vascular endothelial dysfunction in gestational diabetes. Curr. Vasc. Pharm. 2011, 9, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Law, K.P.; Han, T.L.; Mao, X.; Zhang, H. Tryptophan and purine metabolites are consistently upregulated in the urinary metabolome of patients diagnosed with gestational diabetes mellitus throughout pregnancy: A longitudinal metabolomics study of Chinese pregnant women part 2. Clin. Chim. Acta 2017, 468, 126–139. [Google Scholar] [CrossRef]
- Catalano, P.M.; Tyzbir, E.D.; Wolfe, R.R.; Calles, J.; Roman, N.M.; Amini, S.B.; Sims, E.A. Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. Am. J. Physiol. 1993, 264, E60–67. [Google Scholar] [CrossRef] [PubMed]
- Spellacy, W.; Buhi, W.; Bradley, B.; Holsinger, K. Maternal, fetal and amniotic fluid levels of glucose, insulin and growth hormone. Obstet. Gynecol. 1973, 41, 323–331. [Google Scholar] [PubMed]
- Graça, G.; Duarte, I.F.; Barros, A.n.S.; Goodfellow, B.J.; Diaz, S.O.; Pinto, J.; Carreira, I.M.; Galhano, E.; Pita, C.; Gil, A.M. Impact of prenatal disorders on the metabolic profile of second trimester amniotic fluid: A nuclear magnetic resonance metabonomic study. J. Proteome Res. 2010, 9, 6016–6024. [Google Scholar] [CrossRef] [PubMed]
- Bogavac, M.; Lakic, N.; Simin, N.; Nikolic, A.; Sudji, J.; Bozin, B. Biomarkers of oxidative stress in amniotic fluid and complications in pregnancy. J. Matern. Fetal Neonatal Med. 2012, 25, 104–108. [Google Scholar] [CrossRef]
- Pramodkumar, T.A.; Jayashri, R.; Gokulakrishnan, K.; Velmurugan, K.; Pradeepa, R.; Venkatesan, U.; Saravanan, P.; Uma, R.; Anjana, R.M.; Mohan, V. 1,5 Anhydroglucitol in gestational diabetes mellitus. J. Diabetes Complicat. 2019, 33, 231–235. [Google Scholar] [CrossRef]
- Pramodkumar, T.A.; Jayashri, R.; Gokulakrishnan, K.; Velmurugan, K.; Pradeepa, R.; Anjana, R.M.; Mohan, V. Relationship of glycemic control markers 1,5 anhydroglucitol, fructosamine, and glycated hemoglobin among Asian Indians with different degrees of glucose intolerance. Indian J. Endocrinol. Metab. 2016, 20, 690–695. [Google Scholar]
- Hashimoto, K.; Koga, M. Indicators of glycemic control in patients with gestational diabetes mellitus and pregnant women with diabetes mellitus. World J. Diabetes 2015, 6, 1045–1056. [Google Scholar] [CrossRef]
- Kahn, B.B.; Flier, J.S. Obesity and insulin resistance. J. Clin. Investig. 2000, 106, 473–481. [Google Scholar] [CrossRef]
- Kursawe, R.; Eszlinger, M.; Narayan, D.; Liu, T.; Bazuine, M.; Cali, A.M.; D’Adamo, E.; Shaw, M.; Pierpont, B.; Shulman, G.I.; et al. Cellularity and adipogenic profile of the abdominal subcutaneous adipose tissue from obese adolescents: Association with insulin resistance and hepatic steatosis. Diabetes 2010, 59, 2288–2296. [Google Scholar] [CrossRef]
- Dudzik, D.; Zorawski, M.; Skotnicki, M.; Zarzycki, W.; Kozlowska, G.; Bibik-Malinowska, K.; Vallejo, M.; Garcia, A.; Barbas, C.; Ramos, M.P. Metabolic fingerprint of Gestational Diabetes Mellitus. J. Proteom. 2014, 103, 57–71. [Google Scholar] [CrossRef]
- Lu, L.; Koulman, A.; Petry, C.J.; Jenkins, B.; Matthews, L.; Hughes, I.A.; Acerini, C.L.; Ong, K.K.; Dunger, D.B. An Unbiased Lipidomics Approach Identifies Early Second Trimester Lipids Predictive of Maternal Glycemic Traits and Gestational Diabetes Mellitus. Diabetes Care 2016, 39, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Allalou, A.; Nalla, A.; Prentice, K.J.; Liu, Y.; Zhang, M.; Dai, F.F.; Ning, X.; Osborne, L.R.; Cox, B.J.; Gunderson, E.P.; et al. A Predictive Metabolic Signature for the Transition from Gestational Diabetes Mellitus to Type 2 Diabetes. Diabetes 2016, 65, 2529–2539. [Google Scholar] [CrossRef] [PubMed]
- Eades, C.E.; Styles, M.; Leese, G.P.; Cheyne, H.; Evans, J.M. Progression from gestational diabetes to type 2 diabetes in one region of Scotland: An observational follow-up study. BMC Pregnancy Childbirth 2015, 15, 11. [Google Scholar] [CrossRef]
- Lai, M.; Liu, Y.; Ronnett, G.V.; Wu, A.; Cox, B.J.; Dai, F.F.; Röst, H.L.; Gunderson, E.P.; Wheeler, M.B. Amino acid and lipid metabolism in post-gestational diabetes and progression to type 2 diabetes: A metabolic profiling study. PLoS Med. 2020, 17, e1003112. [Google Scholar] [CrossRef] [PubMed]
- Unni, S.N.; Lakshman, L.R.; Vaidyanathan, K.; Subhakumari, K.N.; Menon, N.L. Alterations in the levels of plasma amino acids in polycystic ovary syndrome—A pilot study. Indian J. Med. Res. 2015, 142, 549–554. [Google Scholar] [PubMed]
- Batchuluun, B.; Al Rijjal, D.; Prentice, K.J.; Eversley, J.A.; Burdett, E.; Mohan, H.; Bhattacharjee, A.; Gunderson, E.P.; Liu, Y.; Wheeler, M.B. Elevated Medium-Chain Acylcarnitines Are Associated with Gestational Diabetes Mellitus and Early Progression to Type 2 Diabetes and Induce Pancreatic β-Cell Dysfunction. Diabetes 2018, 67, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Lappas, M.; Mundra, P.A.; Wong, G.; Huynh, K.; Jinks, D.; Georgiou, H.M.; Permezel, M.; Meikle, P.J. The prediction of type 2 diabetes in women with previous gestational diabetes mellitus using lipidomics. Diabetologia 2015, 58, 1436–1442. [Google Scholar] [CrossRef]
- Johnson, C.H.; Gonzalez, F.J. Challenges and opportunities of metabolomics. J. Cell Physiol. 2012, 227, 2975–2981. [Google Scholar] [CrossRef]
| Author, Year [Ref] | Population | Metabolomic Platform(s) | Metabolic Medium | Main Altered Metabolites in GDM |
|---|---|---|---|---|
| Potential Early Screening Markers | ||||
| Pinto, et al., 2015 [40] | Pre-diagnosis GDM (2–21 weeks gestation) who later developed GDM (n = 41–93) | NMR | Maternal plasma and lipid extracts | Pre-diagnosis GDM showed increases in plasma valine and pyruvate, with decreases in proline and urea |
| Hou, et al., 2018 [41] | n = 131 women with GDM and 138 controls | UHPLC-MS, GC, NMR | Maternal serum | Perturbations in free fatty acids, branched chain amino acids, and organooxygen compounds in the GDM group |
| Amino Acids | ||||
| O’Neill, et al., 2018 [42] | n = 20 women with second trimester GDM diagnosis | GC-MS | Amniotic fluid | Glutathione was increased, which may be related to increased lipid peroxidation in GDM |
| Scholtens, et al., 2014 [43] | n = 67 high FPG; n = 50 low FPG at ~28 weeks gestation | GC-MS | Fasting serum | Alanine, valine, and serine were most commonly deranged |
| Carbohydrates | ||||
| Gall, et al., 2010 [44] | n = 399 non-diabetic pregnant women with varying degrees of insulin sensitivity | UHPLC/ GC-MS | Fasting plasma samples | Increases in 2-hydroxybutyrate (AHB), and decreases in 1,5-anhydroglucitol and lactate were associated with reduced insulin sensitivity |
| Lipids | ||||
| Rahman, et al., 2018 [38] | n = 107 women with GDM, and 214 without GDM | GC-MS | Plasma | Mid-to-long carbon chain glycerolipids were positively related to GDM |
| Anderson, et al., 2014 [45] | Women with overt GDM (n = 18); hyperglycaemia (n = 45); or healthy controls (n = 43) | UPLC-MS | Fasting serum | Phosphatidylcholines and lysophosphatidylcholines had strong positive relationships with GDM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alesi, S.; Ghelani, D.; Rassie, K.; Mousa, A. Metabolomic Biomarkers in Gestational Diabetes Mellitus: A Review of the Evidence. Int. J. Mol. Sci. 2021, 22, 5512. https://doi.org/10.3390/ijms22115512
Alesi S, Ghelani D, Rassie K, Mousa A. Metabolomic Biomarkers in Gestational Diabetes Mellitus: A Review of the Evidence. International Journal of Molecular Sciences. 2021; 22(11):5512. https://doi.org/10.3390/ijms22115512
Chicago/Turabian StyleAlesi, Simon, Drishti Ghelani, Kate Rassie, and Aya Mousa. 2021. "Metabolomic Biomarkers in Gestational Diabetes Mellitus: A Review of the Evidence" International Journal of Molecular Sciences 22, no. 11: 5512. https://doi.org/10.3390/ijms22115512
APA StyleAlesi, S., Ghelani, D., Rassie, K., & Mousa, A. (2021). Metabolomic Biomarkers in Gestational Diabetes Mellitus: A Review of the Evidence. International Journal of Molecular Sciences, 22(11), 5512. https://doi.org/10.3390/ijms22115512







