Orientated Immobilization of FAD-Dependent Glucose Dehydrogenase on Electrode by Carbohydrate-Binding Module Fusion for Efficient Glucose Assay
Abstract
1. Introduction
2. Results and Discussion
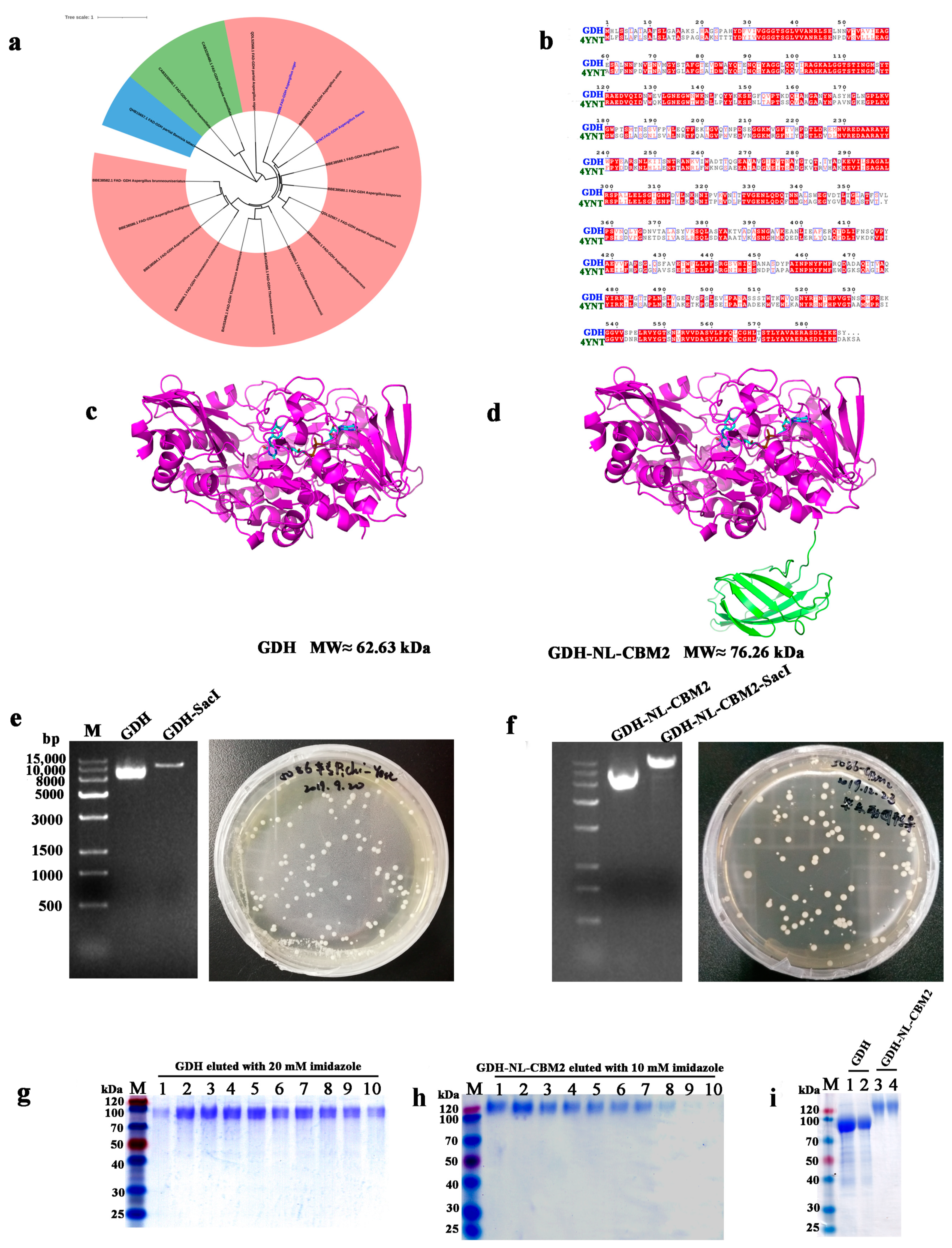
2.1. Heterologous Expression and Purification of GDH and GDH-NL-CBM2
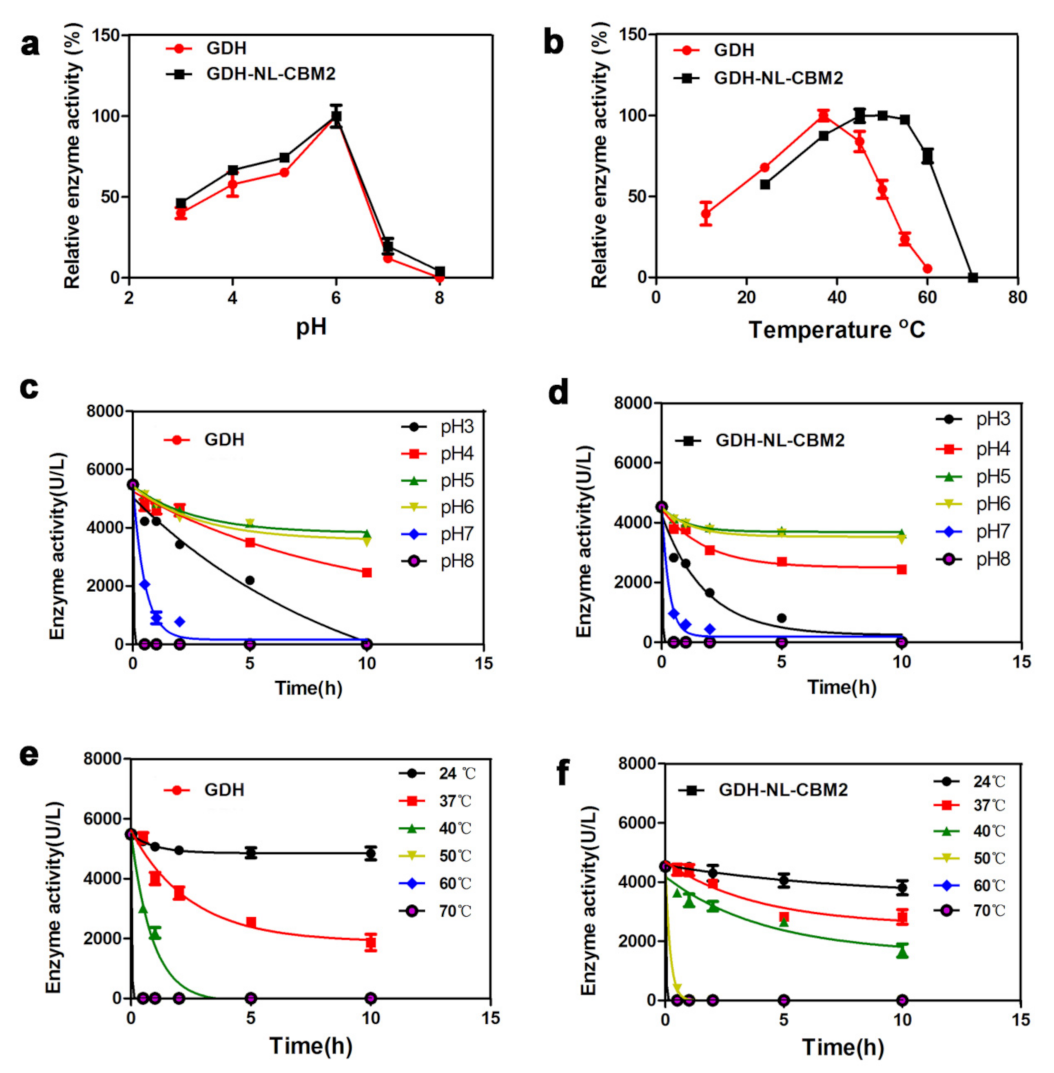
2.2. Analysis of the Enzyme Activity of GDH and GDH-NL-CBM2
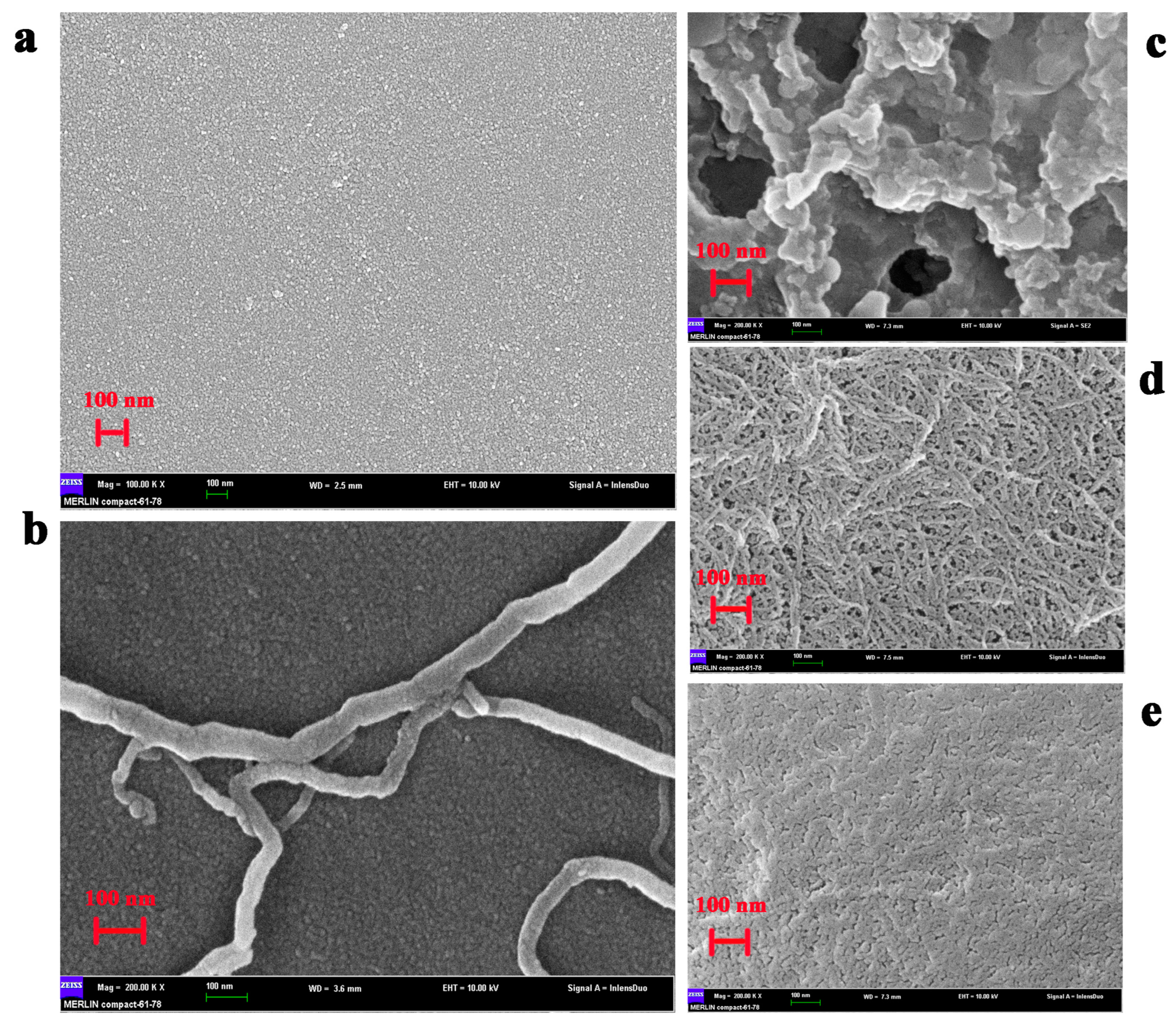
2.3. Morphological Characterization of the Modified Electrode Surface
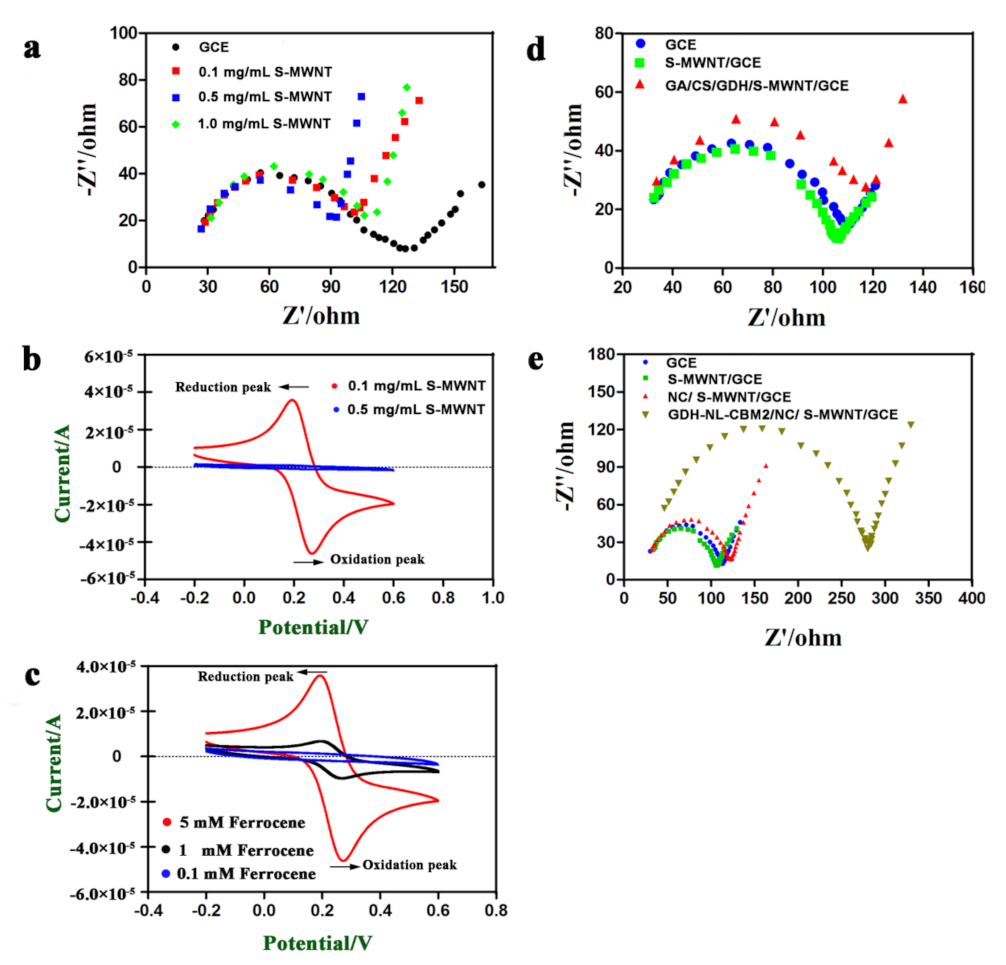
2.4. Electrochemical Characterization of the Electrodes Prepared by Two Different Methods
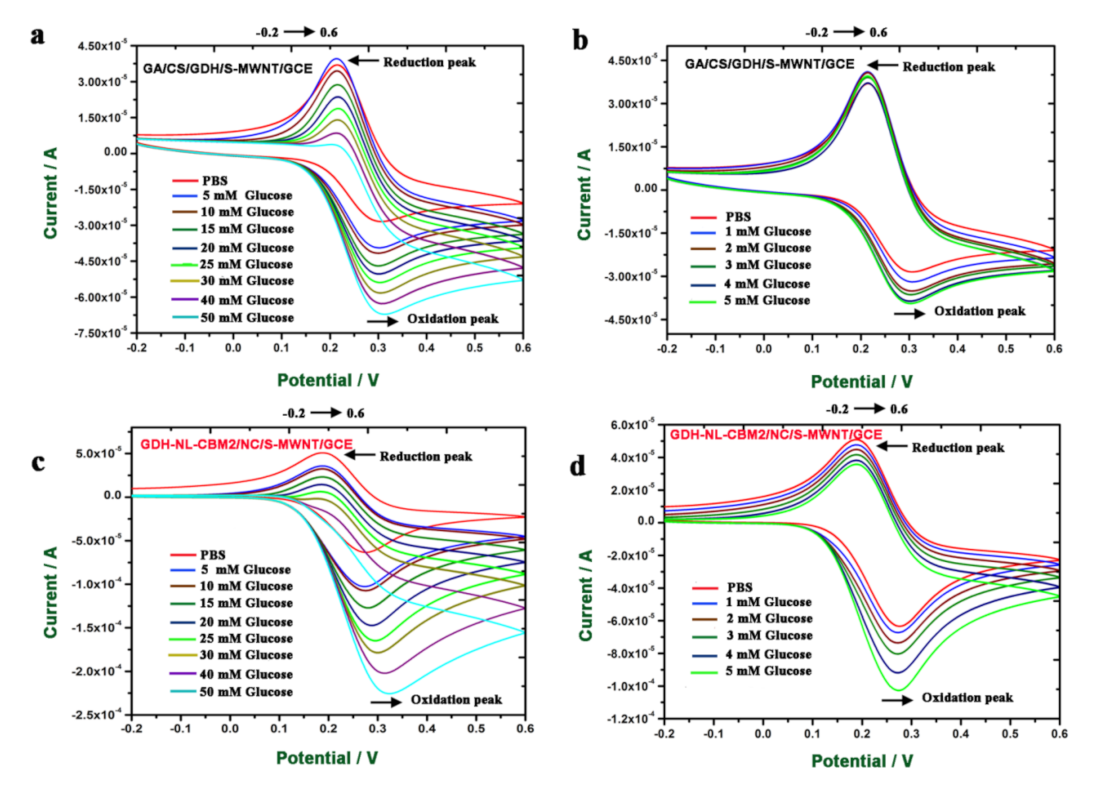
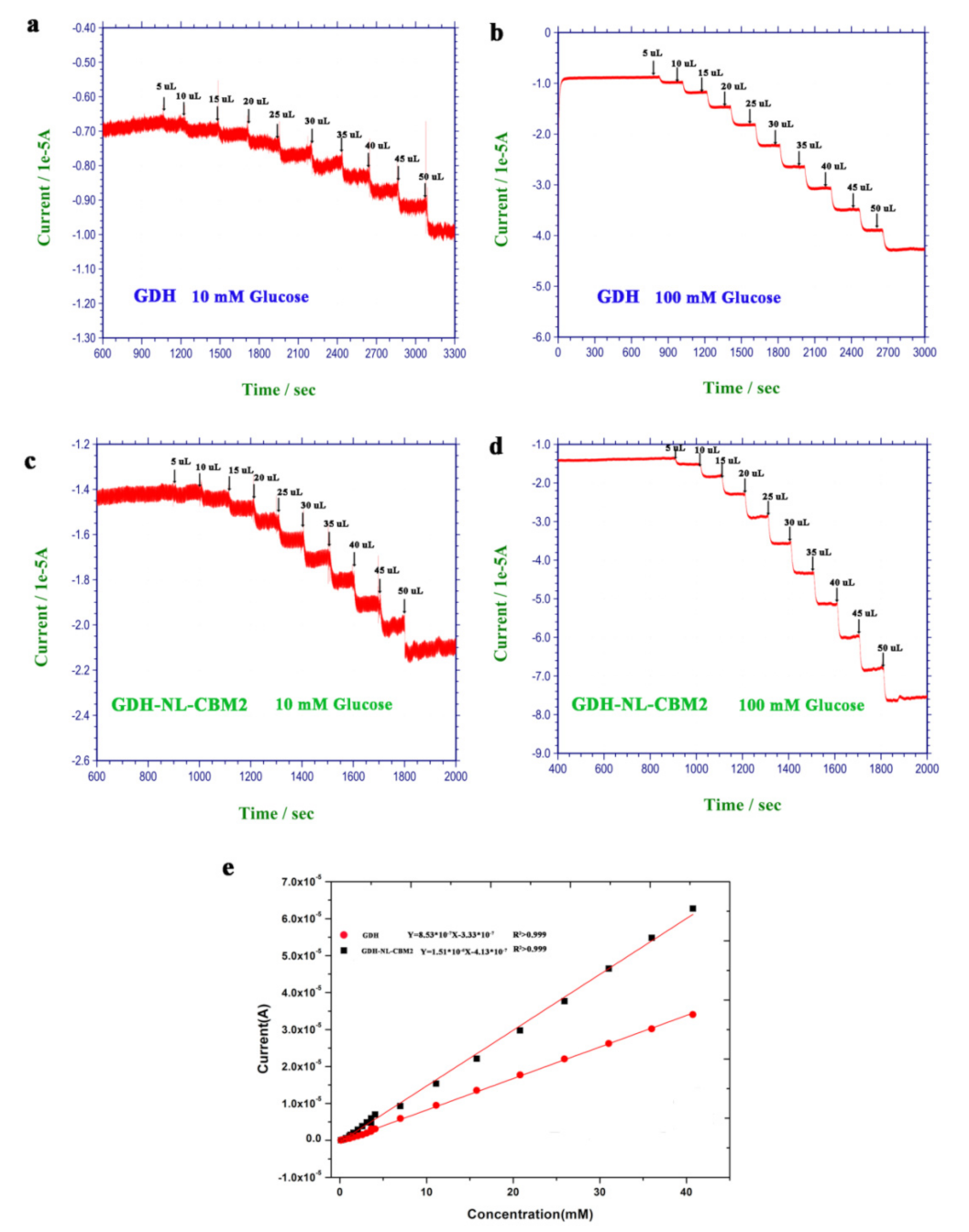
2.5. Electrochemical Behavior of the Electrodes Prepared by Two Methods to Glucose
2.6. Specificity and Anti-Interference Studies
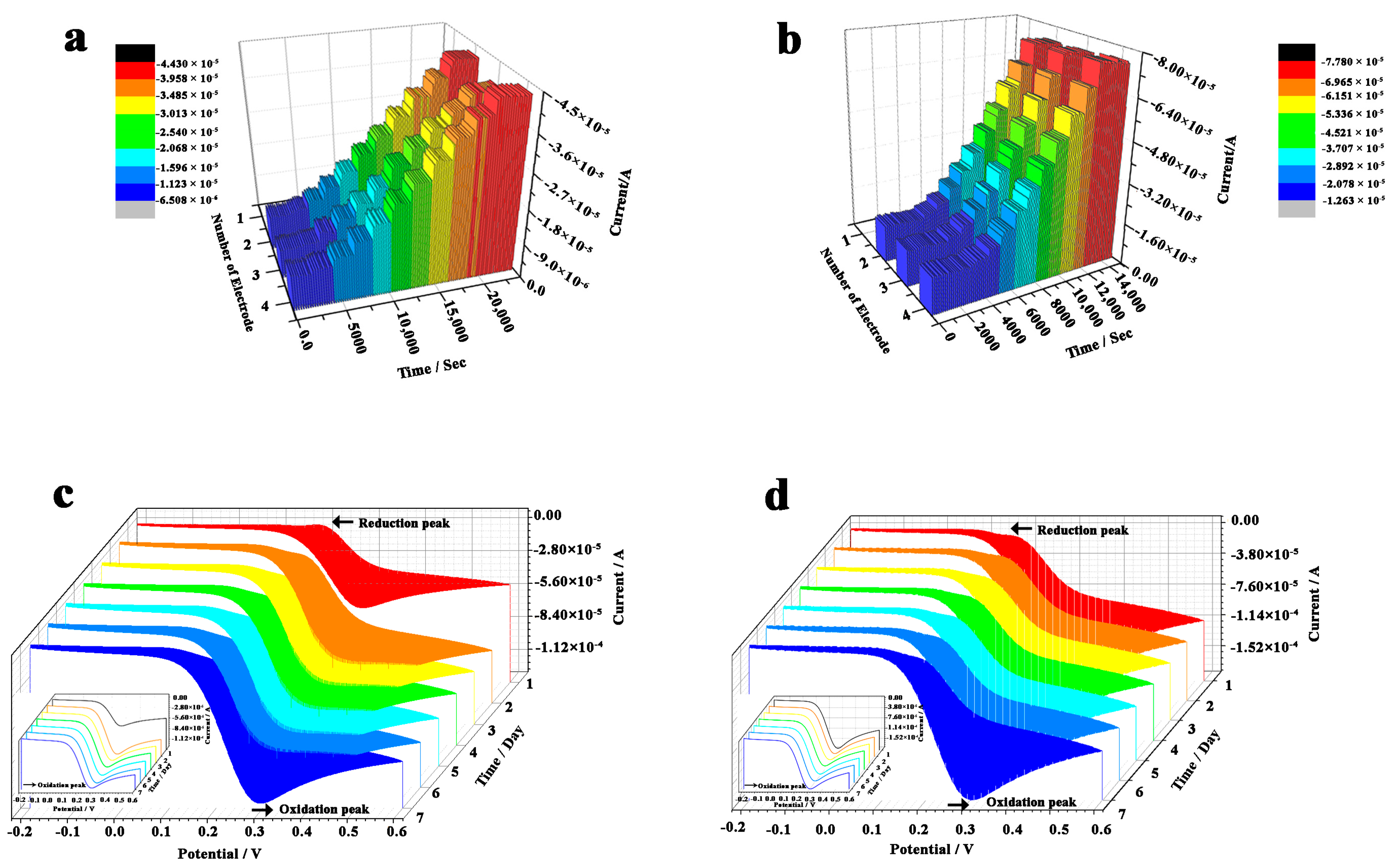
2.7. Stability and Reproducibility of the Electrodes
2.8. Determination of Glucose in Rat Serum Samples and Glucose Drinks
3. Materials and Methods
3.1. Sequencing and Phylogenetic Analysis
3.2. Materials and Chemical Reagents
3.3. Construction of Recombinant Plasmid
3.4. Heterologous Expression of GDH and GDH-NL-CBM2 in Pichia pastoris
3.5. Purification of GDH and GDH-NL-CBM2
3.6. Determination of Optimum Temperature and pH Value
3.7. Preparation of GA/CS/GDH/S-MWNT/GCE and GDH-NL-CBM2/NC/S-MWNT/GCE
3.8. Electrochemical Measurements
3.9. Detection of Glucose in Rat Serum and Drink Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, S.X.; Liu, L.P.; Fan, M.Y.; Jia, Y.R.; Zhou, P. A facile strategy for immobilizing GOD and HRP onto pollen grain and its application to visual detection of glucose. Int. J. Mol. Sci. 2020, 21, 9529. [Google Scholar] [CrossRef] [PubMed]
- Sode, K.; Loew, N.; Ohnishi, Y.; Tsuruta, H.; Mori, K.; Kojima, K.; Tsugawa, W.; LaBelle, J.T.; Klonoff, D.C. Novel fungal FAD glucose dehydrogenase derived from Aspergillus niger for glucose enzyme sensor strips. Biosens. Bioelectron. 2017, 87, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Paolo, B.; Lo, G. Enzyme based amperometric biosensors. Curr. Opin. Electrochem. 2018, 10, 157–173. [Google Scholar]
- Zafar, M.N.; Beden, N.; Leech, D.; Sygmund, C.; Ludwig, R.; Gorton, L. Characterization of different FAD-dependent glucose dehydrogenases for possible use in glucose-based biosensors and biofuel cells. Anal. Bioanal. Chem. 2012, 402, 2069–2077. [Google Scholar] [CrossRef]
- Okurita, M.; Suzuki, N.; Loew, N.; Yoshida, H.; Tsugawa, W.; Mori, K.; Kojima, K.; Klonoff, D.C.; Sode, K. Engineered fungus derived FAD-dependent glucose dehydrogenase with acquired ability to utilize hexaammineruthenium(III) as an electron acceptor. Bioelectrochemistry 2018, 123, 62–71. [Google Scholar] [CrossRef]
- Shimazaki, J.O.; Yoshida, H.; Sode, K. FAD dependent glucose dehydrogenases–Discovery and engineering of representative glucose sensing enzymes. Bioelectrochemistry 2020, 132, 1074–1084. [Google Scholar]
- Iwasa, H.; Ozawa, K.; Sasaki, N.; Kinoshita, N.; Yokoyama, K.; Hiratsuka, A. Fungal FAD-dependent glucose dehydrogenases concerning high activity, affinity, and thermostability for maltose-insensitive blood glucose sensor. Biochem. Eng. J. 2018, 140, 115–122. [Google Scholar] [CrossRef]
- Loew, N.; Tsugawa, W.; Nagae, D.; Kojima, K.; Sode, K. Mediator Preference of Two Different FAD-Dependent Glucose Dehydrogenases Employed in Disposable Enzyme Glucose Sensors. Sensors 2017, 17, 2636. [Google Scholar] [CrossRef] [PubMed]
- Sassolas, A.; Blum, L.J.; Bouvier, B.D.L. Immobilization strategies to develop enzymatic biosensors. Biotechnol. Adv. 2012, 30, 489–511. [Google Scholar] [CrossRef]
- Hernandez, K.; Lafuente, R.F. Control of protein immobilization: Coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance. Enzyme Microb. Technol. 2011, 48, 107–122. [Google Scholar] [CrossRef]
- Hitaishi, V.P.; Clement, R.; Bourassin, N.; Baaden, M.; Poulpiquet, A.D.; Mora, S.S.; Ciaccafava, A.; Lojou, E. Controlling redox enzyme orientation at planar electrodes. Catalysts 2018, 8, 192. [Google Scholar] [CrossRef]
- Greta, F. From Protein Features to Sensing Surfaces. Sensors 2018, 18, 1204–1214. [Google Scholar]
- Liu, Y.; Yu, J. Oriented immobilization of proteins on solid supports for use in biosensors and biochips: A review. Microchim. Acta 2016, 183, 1–19. [Google Scholar] [CrossRef]
- Oliveira, C.; Carvalho, V.; Domingues, L.; Gama, F.M. Recombinant CBM-fusion technology—Applications overview. Biotechnol. Adv. 2015, 33, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cui, G.Z.; Song, X.F.; Feng, Y.G.; Cui, Q. Efficiency and Stability Enhancement of Cis-epoxysuccinic Acid Hydrolase by Fusion with a Carbohydrate Binding Module and Immobilization onto Cellulose. Appl. Biochem. Biotechnol. 2012, 168, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.M.; Epting, K.L.; Kelly, R.M. N-terminal fusion of a hyperthermophilic chitin-binding domain to xylose isomerase from Thermotoga neapolitana enhances kinetics and thermostability of both free and immobilized enzymes. Biotechnol. Prog. 2010, 26, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Myung, S.; Zhang, X.Z.; Zhang, Y.H. Ultra-stable phosphoglucose isomerase through immobilization of cellulose-binding module-tagged thermophilic enzyme on low-cost high-capacity cellulosic adsorbent. Biotechnol. Prog. 2011, 27, 969–975. [Google Scholar] [CrossRef]
- Rodriguez, B.; Kavoosi, M.; Koska, J.; Creagh, A.L.; Kilburn, D.G.; Haynes, C.A. Inexpensive and Generic Affinity Purification of Recombinant Proteins Using a Family 2a CBM Fusion Tag. Biotechnol. Prog. 2010, 20, 1479–1489. [Google Scholar] [CrossRef]
- Fu, J.P.; Li, D.W.; Li, G.H.; Huang, F.L.; Wei, Q.F. Carboxymethyl cellulose assisted immobilization of silver nanoparticles onto cellulose nanofibers for the detection of catechol. J. Electroanal. Chem. 2015, 738, 92–99. [Google Scholar] [CrossRef]
- Yoshida, H.; Sakai, G.; Mori, K.; Kojima, K.; Kamitori, S.; Sode, K. Structural analysis of fungus-derived FAD glucose dehydrogenase. Sci. Rep. 2015, 5, 13498–13507. [Google Scholar] [CrossRef]
- Sygmund, C.; Staudigl, P.; Klausberger, M.; Pinotsis, N.; Carugo, K.D.; Gorton, L.; Haltrich, D.; Ludwig, R. Heterologous overexpression of Glomerella cingulata FAD-dependent glucose dehydrogenase in Escherichia coli and Pichia pastoris. Microb. Cell. Fact. 2011, 10, 1–9. [Google Scholar] [CrossRef]
- Yu, K.; Liu, C.C.; Kim, B.G.; Lee, D.Y. Synthetic fusion protein design and applications. Biotechnol. Adv. 2015, 33, 155–164. [Google Scholar] [CrossRef]
- Wu, X.Y.; Zhang, Q.; Zhang, L.Z.; Liu, S.J.; Chen, G.J.; Zhang, H.Q.; Wang, L.S. Insights into the role of exposed surface charged residues in the alkali-tolerance of GH11 xylanase. Front. Microbiol. 2020, 11, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.J.; Gao, P.X.; Yu, W.G.; Lu, X.Z. Thermostability enhancement of chitosanase CsnA by fusion a family 5 carbohydrate-binding module. Biotechnol. Lett. 2017, 39, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Qin, Z.; Chen, Q.M.; Fan, L.Q.; Zhou, J.C.; Zhao, L.M. Efficient Immobilization of Bacterial GH Family 46 Chitosanase by Carbohydrate-Binding Module Fusion for the Controllable Preparation of Chitooligosaccharides. J. Agric. Food. Chem. 2019, 67, 6847–6855. [Google Scholar] [CrossRef] [PubMed]
- Muguruma, H.; Iwasa, H.; Hidaka, H.; Hiratsuka, A. Mediatorless direct electron transfer between flavin adenine dinucleotide-dependent glucose dehydrogenase and single-walled carbon nanotubes. ACS. Catal. 2017, 7, 725–734. [Google Scholar] [CrossRef]
- Wang, J.; Musameh, M.; Lin, Y.H. Solubilization of carbon nanotubes by Nafion toward the preparation of amperometric biosensors. J. Am. Chem. Soc. 2003, 125, 2408–2409. [Google Scholar] [CrossRef]
- Masakari, Y.; Hara, C.; Araki, Y.; Gomi, K.; Ito, K. Improvement in the thermal stability of Mucor prainii-derived FAD-dependent glucose dehydrogenase via protein chimerization. Enzyme Microb. Technol. 2020, 132, 109387–109397. [Google Scholar] [CrossRef]
- Wang, J. Analytical Electrochemistry, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 58–63. [Google Scholar]
- Jiang, Z.P.; Shangguan, Y.G.; Zheng, Q. Ferrocene-Modified Polyelectrolyte Film-Coated Electrode and Its Application in Glucose Detection. Polymers 2019, 11, 551. [Google Scholar] [CrossRef]
- Ghica, M.E.; Brett, C.M.A. Development of a carbon film electrode ferrocene-mediated glucose biosensor. Anal. Lett. 2005, 38, 907–920. [Google Scholar] [CrossRef]
- Ofir, K.; Berdichevsky, Y.; Benhar, I.; Azriel, R.R.; Lamed, R.; Barak, Y.; Bayer, E.A.; Morag, E. Versatile protein microarray based on carbohydrate-binding modules. Proteomics 2005, 5, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Cheng, J.S.; Liu, X.F.; Bai, H.T.; Jiang, J.H. Palladium nanoparticle/chitosan-grafted graphene nanocomposites for construction of a glucose biosensor. Biosens. Bioelectron. 2011, 26, 3456–3463. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Liu, S.L.; Bao, J.C.; Dai, Z.H. Pd nanoparticle assemblies-As the substitute of HRP, in their biosensing applications for H2O2 and glucose. Biosens. Bioelectron. 2012, 31, 151–156. [Google Scholar] [CrossRef]
- Razmi, H.; Rezaei, R.M. Graphene quantum dots as a new substrate for immobilization and direct electrochemistry of glucose oxidase: Application to sensitive glucose determination. Biosens. Bioelectron. 2013, 41, 498–504. [Google Scholar] [CrossRef]
- Unnikrishnan, B.; Palanisamy, S.; Chen, S.M. A simple electrochemical approach to fabricate a glucose biosensor based on graphene–glucose oxidase biocomposite. Biosens. Bioelectron. 2013, 39, 70–75. [Google Scholar] [CrossRef]
- Velmurugan, M.; Sakthinathan, S.; Chen, S.; Karuppiah, C. Direct Electron Transfer of Glucose Oxidase and Electrocatalysis of Glucose Based on Gold Nanoparticles/Electroactivated Graphite Nanocomposite. Int. J. Electrochem. Sci. 2015, 10, 1–12. [Google Scholar]
- Zhao, R.X.; Liu, X.Q.; Zhang, J.M.; Zhu, J.; Wong, D.K.Y. Enhancing Direct Electron Transfer of Glucose Oxidase Using a Gold Nanoparticle Titanate Nanotube Nanocomposite on A Biosensor. Electrochim. Acta 2015, 163, 64–70. [Google Scholar] [CrossRef]
- Li, Z.J.; Sheng, L.Y.; Meng, A.; Xie, C.C.; Zhao, K. A glassy carbon electrode modified with a composite consisting of reduced graphene oxide, zinc oxide and silver nanoparticles in a chitosan matrix for studying the direct electron transfer of glucose oxidase and for enzymatic sensing of glucose. Microchim. Acta 2016, 183, 1625–1632. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.S.; Reginald, S.S.; Baek, S.; Lee, E.M.; Choi, I.G.; Chang, I.S. Biosensing and electrochemical properties of flavin adenine dinucleotide (FAD)-Dependent glucose dehydrogenase (GDH) fused to a gold binding peptide. Biosens. Bioelectron. 2020, 165, 112427–112439. [Google Scholar] [CrossRef]
- Ravenna, Y.; Xia, L.; Gun, J.; Mikhaylov, A.A.; Medvedev, A.G.; Lev, O.; Alfonta, L. Biocomposite Based on Reduced Graphene Oxide Film Modified with Phenothiazone and Flavin Adenine Dinucleotide-Dependent Glucose Dehydrogenase for Glucose Sensing and Biofuel Cell Applications. Anal. Chem. 2015, 87, 9567–9571. [Google Scholar] [CrossRef]
- Monošík, R.; Stredànský, M.; Lušpai, K.; Magdolend, P.; Šturdík, E. Amperometric glucose biosensor utilizing FAD-dependent glucose dehydrogenase immobilized on nanocomposite electrode. Enzyme Microb. Technol. 2012, 50, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, S.; Kojima, S.; Kano, K.; Ikeda, T.; Sato, M.; Sanada, H.; Omura, H.B. Novel FAD-Dependent Glucose Dehydrogenase for a Dioxygen-Insensitive Glucose Biosensor. Biosci. Biotechnol. Biochem. 2006, 70, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.L.; Cheng, Z.; Zhang, H.Q.; Liu, L.; Gao, P.J.; Wang, L.S. Draft genome sequence of Aspergillus niger strain An76. Genome Announc. 2016, 4, e01700–e01715. [Google Scholar] [CrossRef] [PubMed]
- Tóth, Á.; Barna, T.; Szabó, E.; Elek, R.; Hubert, Á.; Nagy, I.; Nagy, I.; Kriszt, B.; Táncsics, A.; Kukolya, J. Cloning, expression and biochemical characterization of endomannanases from Thermobifida species isolated from different niches. PLoS ONE 2016, 11, e0155769. [Google Scholar] [CrossRef]
- Kang, Z.P.; Jiao, K.L.; Yu, C.; Dong, J. Direct electrochemistry and bioelectrocatalysis of glucose oxidase in CS/CNC film and its application in glucose biosensing and biofuel cells. RSC Adv. 2017, 7, 4572–4579. [Google Scholar] [CrossRef]

| Modified Electrode | Linear Range (mM) | Sensitivity (A/(M*cm2)) | Detection Limit (µM) | Response Time (s) | RSD | Ref |
|---|---|---|---|---|---|---|
| GOx/PdNPs/CS-GR [a] | 0.001–1.0 | 3.12 × 10−2 | 0.2 | ≈10 | 5.1% | [33] |
| GOx/Pd NPAs/GCE [b] | 0.04–22 | -- | 6.1 | -- | 5.1% | [34] |
| GOx–GQD/CCE [c] | 0.005–1.27 | 8.5 × 10−2 | 1.73 | ~3 | 5% | [35] |
| GOx/rGO/GCE [d] | 0.1–27 | 1.85 × 10−3 | -- | <5 | 4.9% | [36] |
| GOx/AuNPs-EGr/SPCE [e] | 0.05–1.6 | 2.55 × 10−1 | 2.5 | -- | 3.8% | [37] |
| TNT-GNP/[Demin]Br/ Nafion/GOx/GCE [f] | 0.01–1.2 | 5.1 × 10−3 | -- | -- | -- | [38] |
| GOx/rGO-Zn-Ag/GCE | 0.1–12.0 | -- | 10.6 | -- | 6.7% | [39] |
| GDH-GBP/Au [g] | 3–30 | 1.33 × 10−3 | 3410 | 5–30 | <10% | [40] |
| PPF/GDH/SWCNT-SC/PPF/Au [h] | 0.05–3.2 | 1.10 × 10−1 | 0.83 | 7 | -- | [26] |
| rGO/PTZ-O/GDH/GCE [i] | 0.5–12 | 4.2 × 10−2 | -- | -- | -- | [41] |
| CS/GDH/MWCNT [j] | 0.07–0.62 | 2.05 × 10−2 | 4.2 | 60 | -- | [42] |
| DM/GDH/GCE [k] | 5–30 | 5.3 × 10−3 | 10 | 200 | 5% | [43] |
| GA/CS/GDH/S-MWNT/GCE | 0.12–40.7 | 1.2067 × 10−2 | 81 | ~40 | <10% | This work |
| Nafion/GDH-NL-CBM2/S-MWNT/GCE | 0.12–40.7 | 2.1362 × 10−2 | 51 | ~18 | <5% | This work |
| Concentrations of Glucose (mM) (RSD, n = 3) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Samples | SBA Glucose Biosensor | Reducing Sugar Analyzer | GA/CS/GDH/S-MWNT/GCE | GDH-NL-CBM2/NC/S-MWNT/GCE | ||||
| Average Value | RSD /% | Average Value | RSD /% | Average Value | RSD /% | Average Value | RSD /% | |
| Glucose drink (≈2777.8 mM) | 2882.7 | 1.51 | 2793.6 | 1.5 | 2917.3 | 2.64 | 2832.1 | 1.59 |
| Pregnant mouse serum | 9.5 | 1.95 | 9.35 | 1.62 | 9.14 | 2.93 | 9.29 | 1.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Q.; Gong, W.; Zhang, Z.; Wang, L.; Wang, B.; Cai, L.; Meng, Q.; Li, Y.; Liu, Q.; Yang, Y.; et al. Orientated Immobilization of FAD-Dependent Glucose Dehydrogenase on Electrode by Carbohydrate-Binding Module Fusion for Efficient Glucose Assay. Int. J. Mol. Sci. 2021, 22, 5529. https://doi.org/10.3390/ijms22115529
Han Q, Gong W, Zhang Z, Wang L, Wang B, Cai L, Meng Q, Li Y, Liu Q, Yang Y, et al. Orientated Immobilization of FAD-Dependent Glucose Dehydrogenase on Electrode by Carbohydrate-Binding Module Fusion for Efficient Glucose Assay. International Journal of Molecular Sciences. 2021; 22(11):5529. https://doi.org/10.3390/ijms22115529
Chicago/Turabian StyleHan, Qingye, Weili Gong, Zhenyu Zhang, Lushan Wang, Binglian Wang, Lei Cai, Qingjun Meng, Yiwei Li, Qingai Liu, Yan Yang, and et al. 2021. "Orientated Immobilization of FAD-Dependent Glucose Dehydrogenase on Electrode by Carbohydrate-Binding Module Fusion for Efficient Glucose Assay" International Journal of Molecular Sciences 22, no. 11: 5529. https://doi.org/10.3390/ijms22115529
APA StyleHan, Q., Gong, W., Zhang, Z., Wang, L., Wang, B., Cai, L., Meng, Q., Li, Y., Liu, Q., Yang, Y., Zheng, L., & Ma, Y. (2021). Orientated Immobilization of FAD-Dependent Glucose Dehydrogenase on Electrode by Carbohydrate-Binding Module Fusion for Efficient Glucose Assay. International Journal of Molecular Sciences, 22(11), 5529. https://doi.org/10.3390/ijms22115529







