Additive Benefits of Radium-223 Dichloride and Bortezomib Combination in a Systemic Multiple Myeloma Mouse Model
Abstract
:1. Introduction
2. Results
2.1. Radium-223 Inhibits the Proliferation of Various MM Cell Lines and Shows Additive Activity in Combination with Bortezomib in 5TGM1 Cells In Vitro
2.2. Radium-223 and Bortezomib Combination Treatment Is Well Tolerated and Delays Disease Progression in the 5TGM1 Mouse Myeloma Model
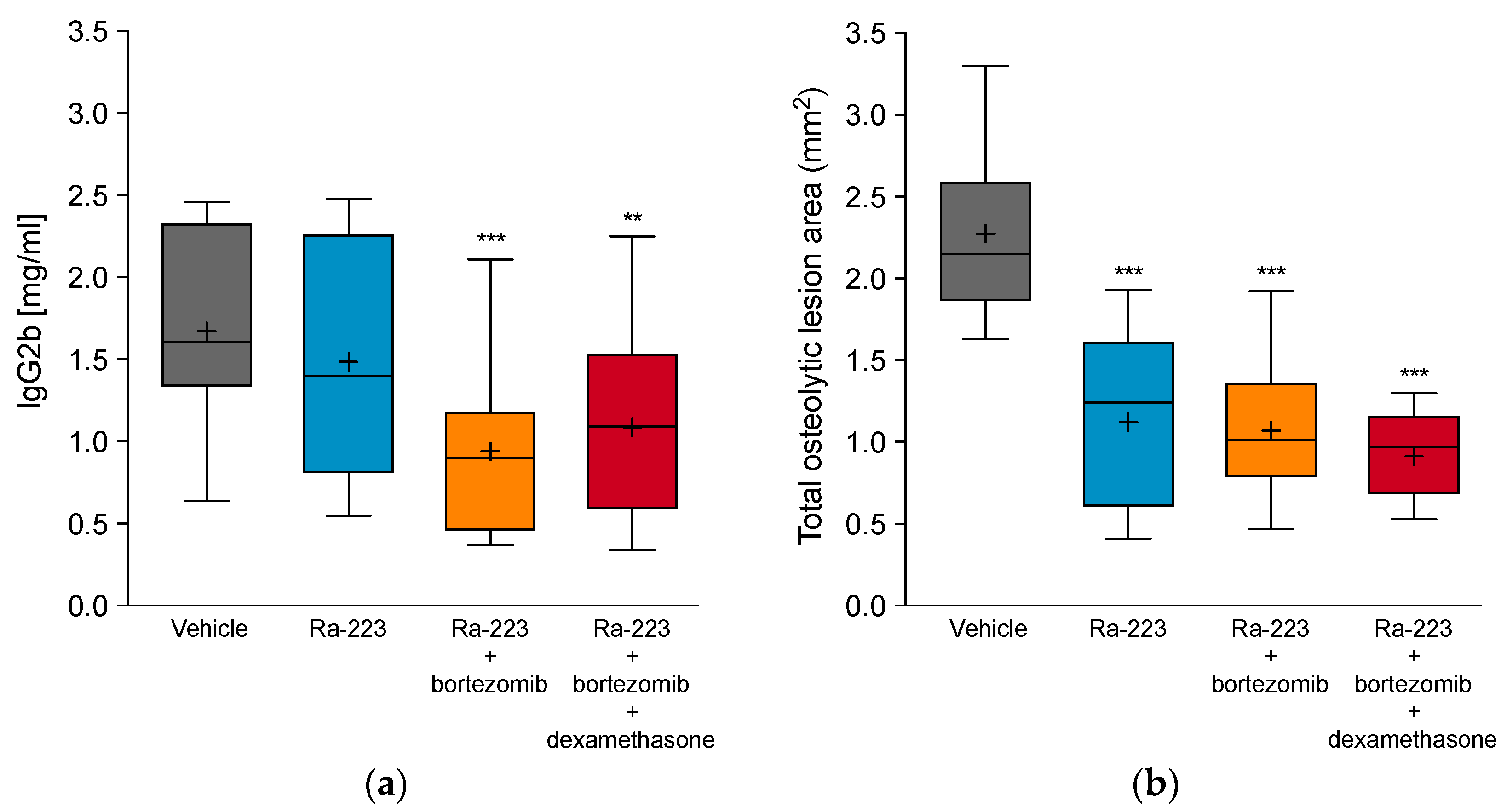
2.3. Combination Effect of Radium-223 and Bortezomib Is Not Altered by Dexamethasone
2.4. Radium-223 and Bortezomib Combination Treatment Results in Tumor Lesion Necrosis
2.5. Radium-223 and Bortezomib Combination Treatment Results in Higher Radium-223 Incorporation into Bone
3. Discussion
4. Materials and Methods
4.1. Cells and Compounds
4.2. In Vitro Proliferation Assays
4.3. Animals
4.4. In Vivo Studies in the Syngeneic 5TGM1 Mouse Multiple Myeloma Model
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Becker, N. Epidemiology of multiple myeloma. Recent Results Cancer Res. 2011, 183, 25–35. [Google Scholar] [CrossRef]
- Kyle, R.A.; Gertz, M.A.; Witzig, T.E.; Lust, J.A.; Lacy, M.Q.; Dispenzieri, A.; Fonseca, R.; Rajkumar, S.V.; Offord, J.R.; Larson, D.R.; et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin. Proc. 2003, 78, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Morgan, G.; Dimopoulos, M.A.; Drake, M.T.; Lentzsch, S.; Raje, N.; Sezer, O.; Garcia-Sanz, R.; Shimizu, K.; Turesson, I.; et al. International Myeloma Working Group recommendations for the treatment of multiple myeloma-related bone disease. J. Clin. Oncol. 2013, 31, 2347–2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Gavriatopoulou, M.; Dimopoulos, M.A. Pathogenesis of bone disease in multiple myeloma: From bench to bedside. Blood Cancer J. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Driscoll, J.J.; Burris, J.; Annunziata, C.M. Targeting the proteasome with bortezomib in multiple myeloma: Update on therapeutic benefit as an upfront single agent, induction regimen for stem-cell transplantation and as maintenance therapy. Am. J. Ther. 2012, 19, 133–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreau, P.; Richardson, P.G.; Cavo, M.; Orlowski, R.Z.; San Miguel, J.F.; Palumbo, A.; Harousseau, J.L. Proteasome inhibitors in multiple myeloma: 10 years later. Blood 2012, 120, 947–959. [Google Scholar] [CrossRef] [Green Version]
- Yong, K.; Gonzalez-McQuire, S.; Szabo, Z.; Schoen, P.; Hajek, R. The start of a new wave: Developments in proteasome inhibition in multiple myeloma. Eur. J. Haematol. 2018. [Google Scholar] [CrossRef]
- Field-Smith, A.; Morgan, G.J.; Davies, F.E. Bortezomib (Velcadetrade mark) in the Treatment of Multiple Myeloma. Ther. Clin. Risk Manag. 2006, 2, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Heider, U.; Kaiser, M.; Muller, C.; Jakob, C.; Zavrski, I.; Schulz, C.O.; Fleissner, C.; Hecht, M.; Sezer, O. Bortezomib increases osteoblast activity in myeloma patients irrespective of response to treatment. Eur. J. Haematol. 2006, 77, 233–238. [Google Scholar] [CrossRef]
- Zangari, M.; Terpos, E.; Zhan, F.; Tricot, G. Impact of bortezomib on bone health in myeloma: A review of current evidence. Cancer Treat. Rev. 2012, 38, 968–980. [Google Scholar] [CrossRef]
- Coleman, R. Treatment of Metastatic Bone Disease and the Emerging Role of Radium-223. Semin. Nucl. Med. 2016, 46, 99–104. [Google Scholar] [CrossRef]
- Makvandi, M.; Dupis, E.; Engle, J.W.; Nortier, F.M.; Fassbender, M.E.; Simon, S.; Birnbaum, E.R.; Atcher, R.W.; John, K.D.; Rixe, O.; et al. Alpha-Emitters and Targeted Alpha Therapy in Oncology: From Basic Science to Clinical Investigations. Target. Oncol. 2018, 13, 189–203. [Google Scholar] [CrossRef]
- Parker, C.; Sartor, O. Radium-223 in prostate cancer. N. Engl. J. Med. 2013, 369, 1659–1660. [Google Scholar] [CrossRef] [Green Version]
- Suominen, M.I.; Wilson, T.; Kakonen, S.M.; Scholz, A. The Mode-of-Action of Targeted Alpha Therapy Radium-223 as an Enabler for Novel Combinations to Treat Patients with Bone Metastasis. Int. J. Mol. Sci. 2019, 20, 3899. [Google Scholar] [CrossRef] [Green Version]
- Neuman, W.F.; Hursh, J.B.; Boyd, J.; Hodge, H.C. On the mechanism of skeletal fixation of radium. Ann. N. Y. Acad. Sci. 1955, 62, 125–136. [Google Scholar] [CrossRef]
- Suominen, M.I.; Fagerlund, K.M.; Rissanen, J.P.; Konkol, Y.M.; Morko, J.P.; Peng, Z.; Alhoniemi, E.J.; Laine, S.K.; Corey, E.; Mumberg, D.; et al. Radium-223 Inhibits Osseous Prostate Cancer Growth by Dual Targeting of Cancer Cells and Bone Microenvironment in Mouse Models. Clin. Cancer Res. 2017, 23, 4335–4346. [Google Scholar] [CrossRef] [Green Version]
- Suominen, M.I.; Rissanen, J.P.; Kakonen, R.; Fagerlund, K.M.; Alhoniemi, E.; Mumberg, D.; Ziegelbauer, K.; Halleen, J.M.; Kakonen, S.M.; Scholz, A. Survival benefit with radium-223 dichloride in a mouse model of breast cancer bone metastasis. J. Natl. Cancer Inst. 2013, 105, 908–916. [Google Scholar] [CrossRef] [Green Version]
- Coleman, R.; Aksnes, A.K.; Naume, B.; Garcia, C.; Jerusalem, G.; Piccart, M.; Vobecky, N.; Thuresson, M.; Flamen, P. A phase IIa, nonrandomized study of radium-223 dichloride in advanced breast cancer patients with bone-dominant disease. Breast Cancer Res. Treat. 2014, 145, 411–418. [Google Scholar] [CrossRef]
- Costa, R.P.; Tripoli, V.; Princiotta, A.; Murabito, A.; Licari, M.; Piazza, D.; Verderame, F.; Pinto, A. Therapeutic effect of RA223 in the management of breast cancer bone metastases. Clin. Ter. 2019, 170, e1–e3. [Google Scholar] [CrossRef]
- Takalkar, A.; Paryani, B.; Adams, S.; Subbiah, V. Radium-223 dichloride therapy in breast cancer with osseous metastases. BMJ Case Rep. 2015, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asosingh, K.; Radl, J.; Van Riet, I.; Van Camp, B.; Vanderkerken, K. The 5TMM series: A useful in vivo mouse model of human multiple myeloma. Hematol. J. 2000, 1, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Garrett, I.R.; Dallas, S.; Radl, J.; Mundy, G.R. A murine model of human myeloma bone disease. Bone 1997, 20, 515–520. [Google Scholar] [CrossRef]
- Manning, L.S.; Berger, J.D.; O’Donoghue, H.L.; Sheridan, G.N.; Claringbold, P.G.; Turner, J.H. A model of multiple myeloma: Culture of 5T33 murine myeloma cells and evaluation of tumorigenicity in the C57BL/KaLwRij mouse. Br. J. Cancer 1992, 66, 1088–1093. [Google Scholar] [CrossRef] [Green Version]
- Radl, J.; Croese, J.W.; Zurcher, C.; Van den Enden-Vieveen, M.H.; de Leeuw, A.M. Animal model of human disease. Multiple myeloma. Am. J. Pathol. 1988, 132, 593–597. [Google Scholar]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Dimopoulos, M.A. Myeloma bone disease: From biology findings to treatment approaches. Blood 2019, 133, 1534–1539. [Google Scholar] [CrossRef] [Green Version]
- Anderson, K.C. Progress and Paradigms in Multiple Myeloma. Clin. Cancer Res. 2016, 22, 5419–5427. [Google Scholar] [CrossRef] [Green Version]
- Naymagon, L.; Abdul-Hay, M. Novel agents in the treatment of multiple myeloma: A review about the future. J. Hematol. Oncol. 2016, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Alanazi, F.; Kwa, F.A.A.; Burchall, G.; Jackson, D.E. New generation drugs for treatment of multiple myeloma. Drug Discov. Today 2020, 25, 367–379. [Google Scholar] [CrossRef]
- Chari, A.; Romanus, D.; Palumbo, A.; Blazer, M.; Farrelly, E.; Raju, A.; Huang, H.; Richardson, P. Randomized Clinical Trial Representativeness and Outcomes in Real-World Patients: Comparison of 6 Hallmark Randomized Clinical Trials of Relapsed/Refractory Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2020, 17, 8–17.e16. [Google Scholar] [CrossRef] [Green Version]
- Durer, C.; Durer, S.; Lee, S.; Chakraborty, R.; Malik, M.N.; Rafae, A.; Zar, M.A.; Kamal, A.; Rosko, N.; Samaras, C.; et al. Treatment of relapsed multiple myeloma: Evidence-based recommendations. Blood Rev. 2020, 39, 100616. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2020, 95, 548–567. [Google Scholar] [CrossRef] [Green Version]
- Moreau, P.; Hulin, C.; Macro, M.; Caillot, D.; Chaleteix, C.; Roussel, M.; Garderet, L.; Royer, B.; Brechignac, S.; Tiab, M.; et al. VTD is superior to VCD prior to intensive therapy in multiple myeloma: Results of the prospective IFM2013-04 trial. Blood 2016, 127, 2569–2574. [Google Scholar] [CrossRef]
- Orlowski, R.Z.; Lonial, S. Integration of Novel Agents into the Care of Patients with Multiple Myeloma. Clin. Cancer Res. 2016, 22, 5443–5452. [Google Scholar] [CrossRef] [Green Version]
- Richardson, P.G.; Weller, E.; Lonial, S.; Jakubowiak, A.J.; Jagannath, S.; Raje, N.S.; Avigan, D.E.; Xie, W.; Ghobrial, I.M.; Schlossman, R.L.; et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood 2010, 116, 679–686. [Google Scholar] [CrossRef] [Green Version]
- Kane, R.C.; Bross, P.F.; Farrell, A.T.; Pazdur, R. Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncology 2003, 8, 508–513. [Google Scholar] [CrossRef]
- Kane, R.C.; Farrell, A.T.; Sridhara, R.; Pazdur, R. United States Food and Drug Administration approval summary: Bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin. Cancer Res. 2006, 12, 2955–2960. [Google Scholar] [CrossRef] [Green Version]
- Niewerth, D.; Jansen, G.; Assaraf, Y.G.; Zweegman, S.; Kaspers, G.J.; Cloos, J. Molecular basis of resistance to proteasome inhibitors in hematological malignancies. Drug Resist. Updates 2015, 18, 18–35. [Google Scholar] [CrossRef]
- Accardi, F.; Toscani, D.; Bolzoni, M.; Dalla Palma, B.; Aversa, F.; Giuliani, N. Mechanism of Action of Bortezomib and the New Proteasome Inhibitors on Myeloma Cells and the Bone Microenvironment: Impact on Myeloma-Induced Alterations of Bone Remodeling. Biomed. Res. Int. 2015, 2015, 172458. [Google Scholar] [CrossRef] [Green Version]
- Qiang, Y.W.; Heuck, C.J.; Shaughnessy, J.D., Jr.; Barlogie, B.; Epstein, J. Proteasome inhibitors and bone disease. Semin. Hematol. 2012, 49, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Zangari, M.; Suva, L.J. The effects of proteasome inhibitors on bone remodeling in multiple myeloma. Bone 2016, 86, 131–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruland, O.S.; Nilsson, S.; Fisher, D.R.; Larsen, R.H. High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: Adjuvant or alternative to conventional modalities? Clin. Cancer Res. 2006, 12, 6250s–6257s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henriksen, G.; Fisher, D.R.; Roeske, J.C.; Bruland, O.S.; Larsen, R.H. Targeting of osseous sites with alpha-emitting 223Ra: Comparison with the beta-emitter 89Sr in mice. J. Nucl. Med. 2003, 44, 252–259. [Google Scholar] [PubMed]
- Parker, C.; Heidenreich, A.; Nilsson, S.; Shore, N. Current approaches to incorporation of radium-223 in clinical practice. Prostate Cancer Prostatic Dis. 2018, 21, 37–47. [Google Scholar] [CrossRef]
- Oyajobi, B.O.; Munoz, S.; Kakonen, R.; Williams, P.J.; Gupta, A.; Wideman, C.L.; Story, B.; Grubbs, B.; Armstrong, A.; Dougall, W.C.; et al. Detection of myeloma in skeleton of mice by whole-body optical fluorescence imaging. Mol. Cancer 2007, 6, 1701–1708. [Google Scholar] [CrossRef] [Green Version]
- Mitra, R. Adverse effects of corticosteroids on bone metabolism: A review. PM&R 2011, 3, 466–471. [Google Scholar] [CrossRef]
- Weinstein, R.S. Glucocorticoid-induced osteoporosis and osteonecrosis. Endocrinol. Metab. Clin. N. Am. 2012, 41, 595–611. [Google Scholar] [CrossRef] [Green Version]
- Dou, Q.P.; Zonder, J.A. Overview of proteasome inhibitor-based anti-cancer therapies: Perspective on bortezomib and second generation proteasome inhibitors versus future generation inhibitors of ubiquitin-proteasome system. Curr. Cancer Drug Targets 2014, 14, 517–536. [Google Scholar] [CrossRef] [Green Version]
- Edelman, M.J. The potential role of bortezomib in combination with chemotherapy and radiation in non-small-cell lung cancer. Clin. Lung Cancer 2005, 7 (Suppl. S2), S64–S66. [Google Scholar] [CrossRef]
- Hideshima, T.; Richardson, P.; Chauhan, D.; Palombella, V.J.; Elliott, P.J.; Adams, J.; Anderson, K.C. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001, 61, 3071–3076. [Google Scholar]
- Mitsiades, N.; Mitsiades, C.S.; Richardson, P.G.; Poulaki, V.; Tai, Y.T.; Chauhan, D.; Fanourakis, G.; Gu, X.; Bailey, C.; Joseph, M.; et al. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: Therapeutic applications. Blood 2003, 101, 2377–2380. [Google Scholar] [CrossRef]
- Henriksen, G.; Hoff, P.; Alstad, J.; Larsen, R.H. 223Ra for endoradiotherapeutic applications prepared from an immobilized 227Ac/227Th source. Radiochim. Acta 2001, 89, 661–666. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Zimmerman, B.E.; Bergeron, D.E.; Cessna, J.T.; Fitzgerald, R.; Pibida, L. Revision of the NIST Standard for (223)Ra: New Measurements and Review of 2008 Data. J. Res. Natl. Inst. Stand. Technol. 2015, 120, 37–57. [Google Scholar] [CrossRef]
- Rossi, M.; Botta, C.; Arbitrio, M.; Grembiale, R.D.; Tagliaferri, P.; Tassone, P. Mouse models of multiple myeloma: Technologic platforms and perspectives. Oncotarget 2018, 9, 20119–20133. [Google Scholar] [CrossRef] [Green Version]






| Cell Line | Radium-223 800 Bq/mL | Radium-223 200 Bq/mL | Bortezomib 25 nM | Bortezomib 2.5 nM | Ra-223, 200 Bq/mL Bortezomib, 2.5 nM |
|---|---|---|---|---|---|
| 5TGM1 | 16.7% *** | 0% | 99.3% *** | 4.1% | 9.5% ***, ###, §§§ |
| JJN-3 | 26.5% *** | 0% | 99.5% *** | 11.0% *** | 29.1% ***, ###, §§§ |
| LP-1 | 20.0% *** | 0% | 99.3% *** | 6.2% | 11.6% ***, ###, §§§ |
| MOLP-8 | 73.6% *** | 22.2% ** | 93.7% *** | 19.0% | 41.3% ***, §§ |
| RPMI-8226 | 46.8% *** | 13.5% | 91.1% *** | 0% | 13.1% |
| OPM-2 | 52.3% *** | 13.3% | 99.5% *** | 18.1% * | 31.0% ***, ##, §§ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suominen, M.I.; Mäki-Jouppila, J.; Huhtinen, A.; Sjöholm, B.; Rissanen, J.P.; Luostarinen, A.; Fagerlund, K.M.; Alhoniemi, E.; Siemeister, G.; Mumberg, D.; et al. Additive Benefits of Radium-223 Dichloride and Bortezomib Combination in a Systemic Multiple Myeloma Mouse Model. Int. J. Mol. Sci. 2021, 22, 5570. https://doi.org/10.3390/ijms22115570
Suominen MI, Mäki-Jouppila J, Huhtinen A, Sjöholm B, Rissanen JP, Luostarinen A, Fagerlund KM, Alhoniemi E, Siemeister G, Mumberg D, et al. Additive Benefits of Radium-223 Dichloride and Bortezomib Combination in a Systemic Multiple Myeloma Mouse Model. International Journal of Molecular Sciences. 2021; 22(11):5570. https://doi.org/10.3390/ijms22115570
Chicago/Turabian StyleSuominen, Mari I., Jenni Mäki-Jouppila, Anna Huhtinen, Birgitta Sjöholm, Jukka P. Rissanen, Anniina Luostarinen, Katja M. Fagerlund, Esa Alhoniemi, Gerhard Siemeister, Dominik Mumberg, and et al. 2021. "Additive Benefits of Radium-223 Dichloride and Bortezomib Combination in a Systemic Multiple Myeloma Mouse Model" International Journal of Molecular Sciences 22, no. 11: 5570. https://doi.org/10.3390/ijms22115570
APA StyleSuominen, M. I., Mäki-Jouppila, J., Huhtinen, A., Sjöholm, B., Rissanen, J. P., Luostarinen, A., Fagerlund, K. M., Alhoniemi, E., Siemeister, G., Mumberg, D., Käkönen, S. -M., & Scholz, A. (2021). Additive Benefits of Radium-223 Dichloride and Bortezomib Combination in a Systemic Multiple Myeloma Mouse Model. International Journal of Molecular Sciences, 22(11), 5570. https://doi.org/10.3390/ijms22115570






